Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus
Abstract
1. Introduction
2. Materials and Methods
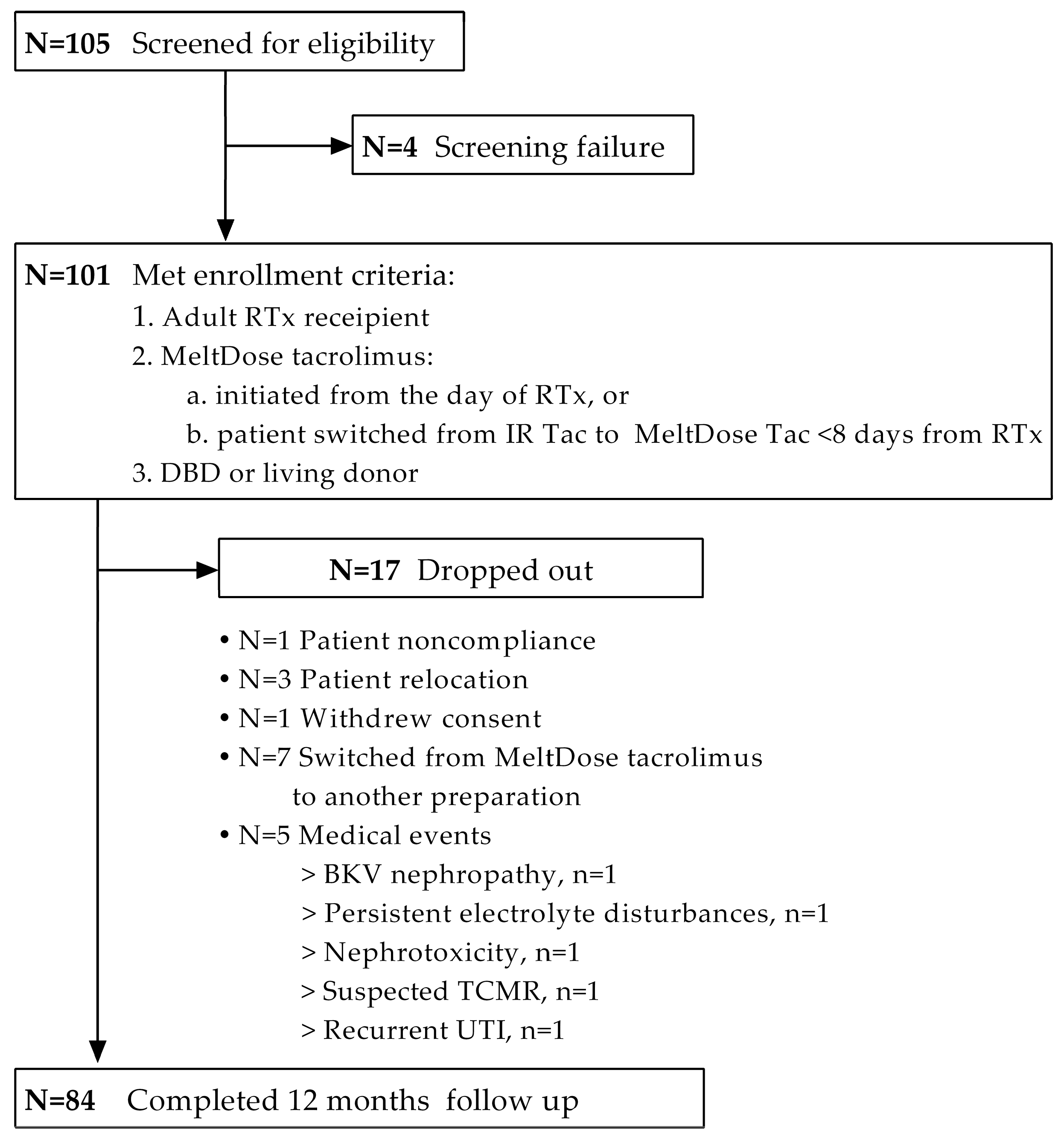
2.1. Study Design and Patient Enrollment
2.2. Follow-Up and Study Outcomes
2.3. Statistical Analysis
3. Results
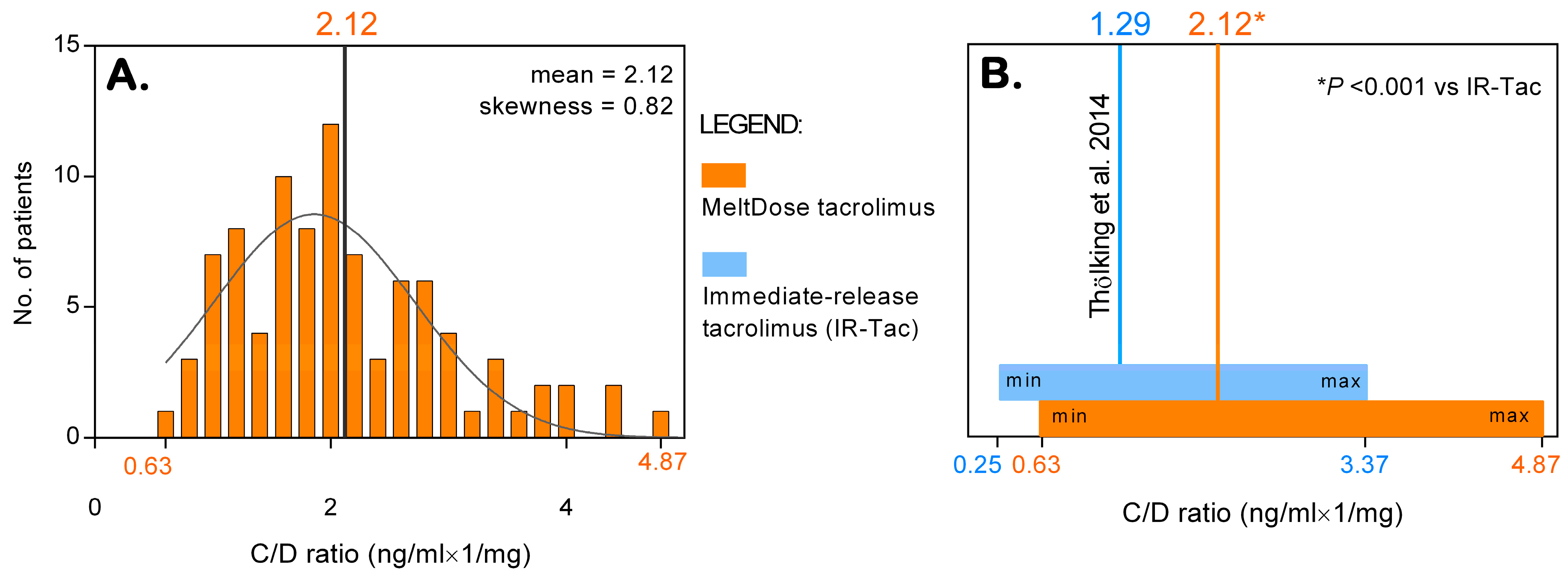
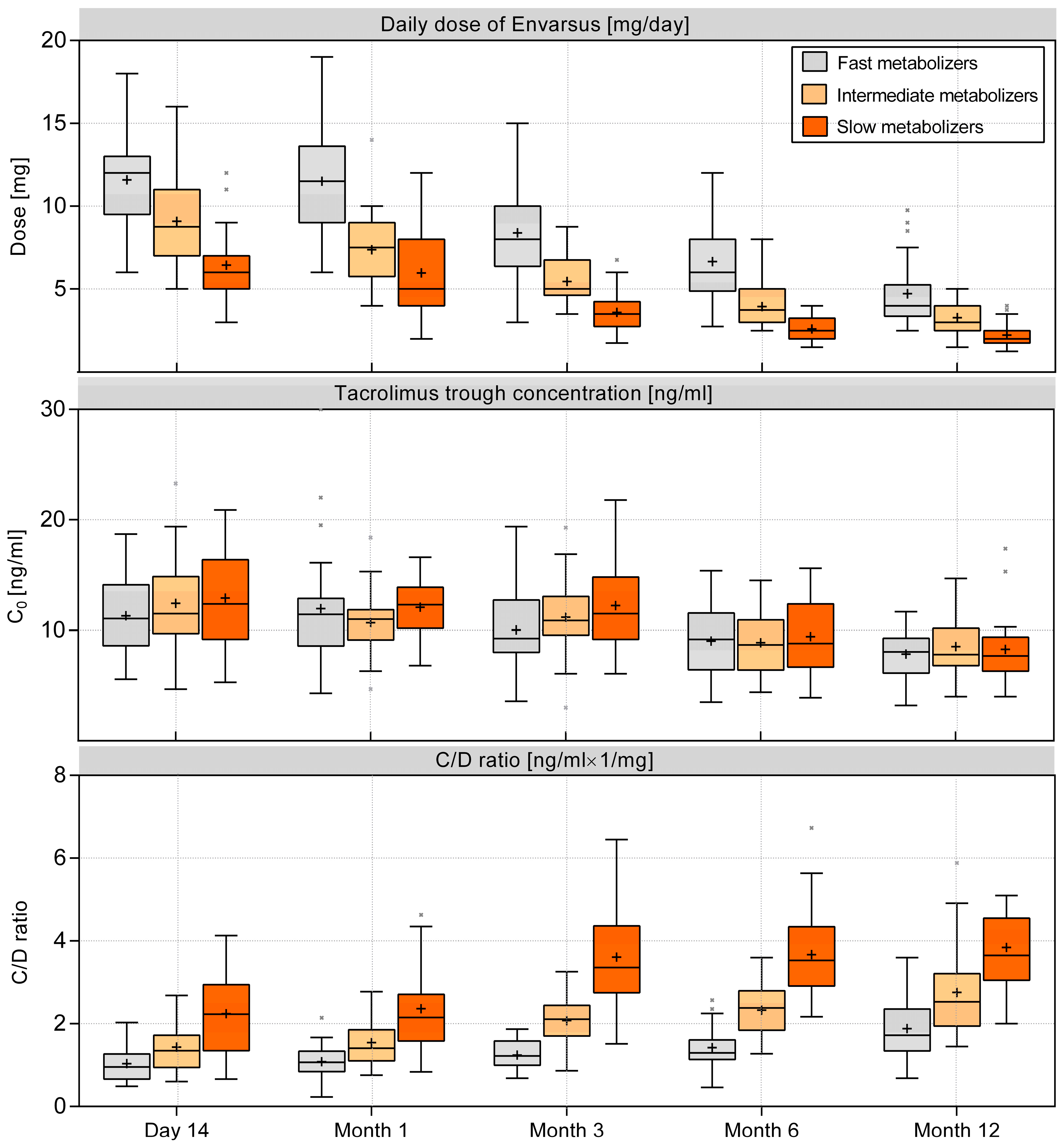
3.1. Metabolizer Classification and the C/D Ratio
3.2. Tacrolimus Dose and Trough Concentration
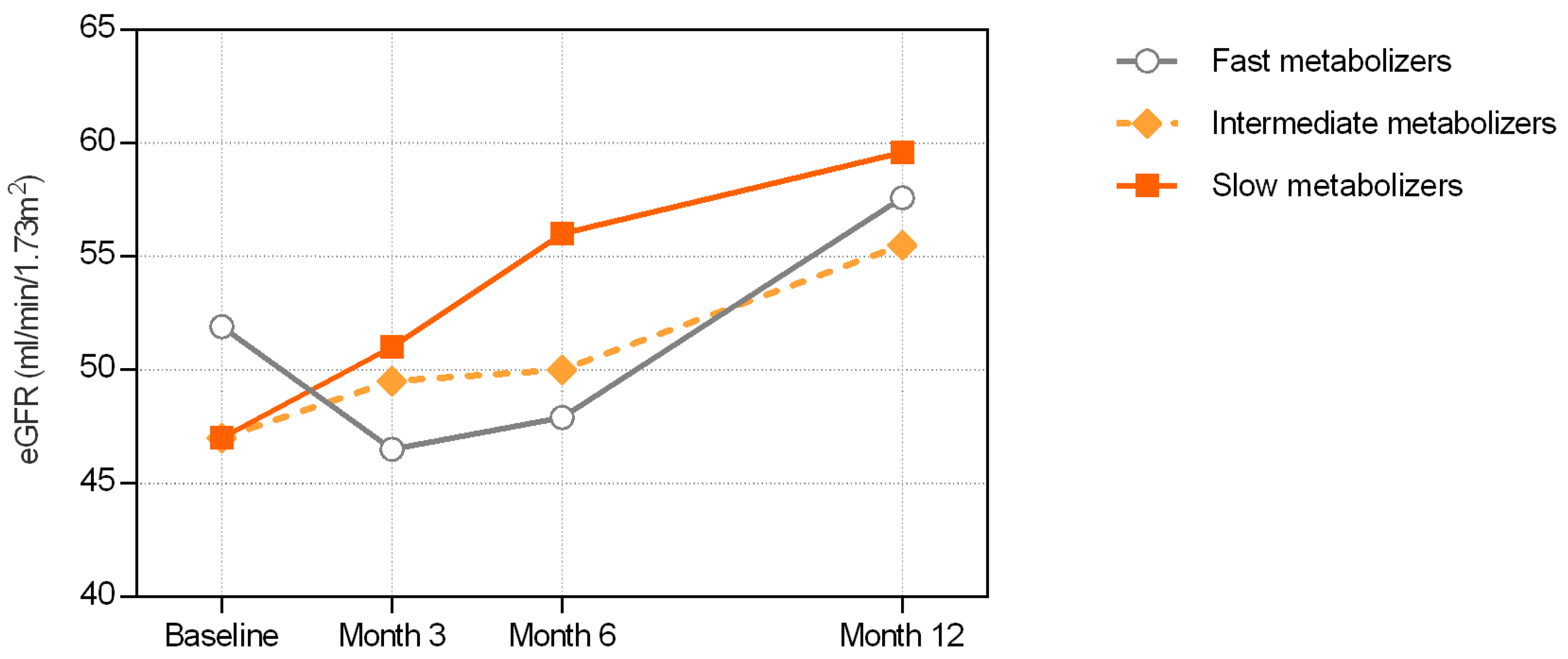
3.3. Renal Function Outcomes
3.4. Comparison of Renal Function Recovery between Fast and Slow Metabolizers
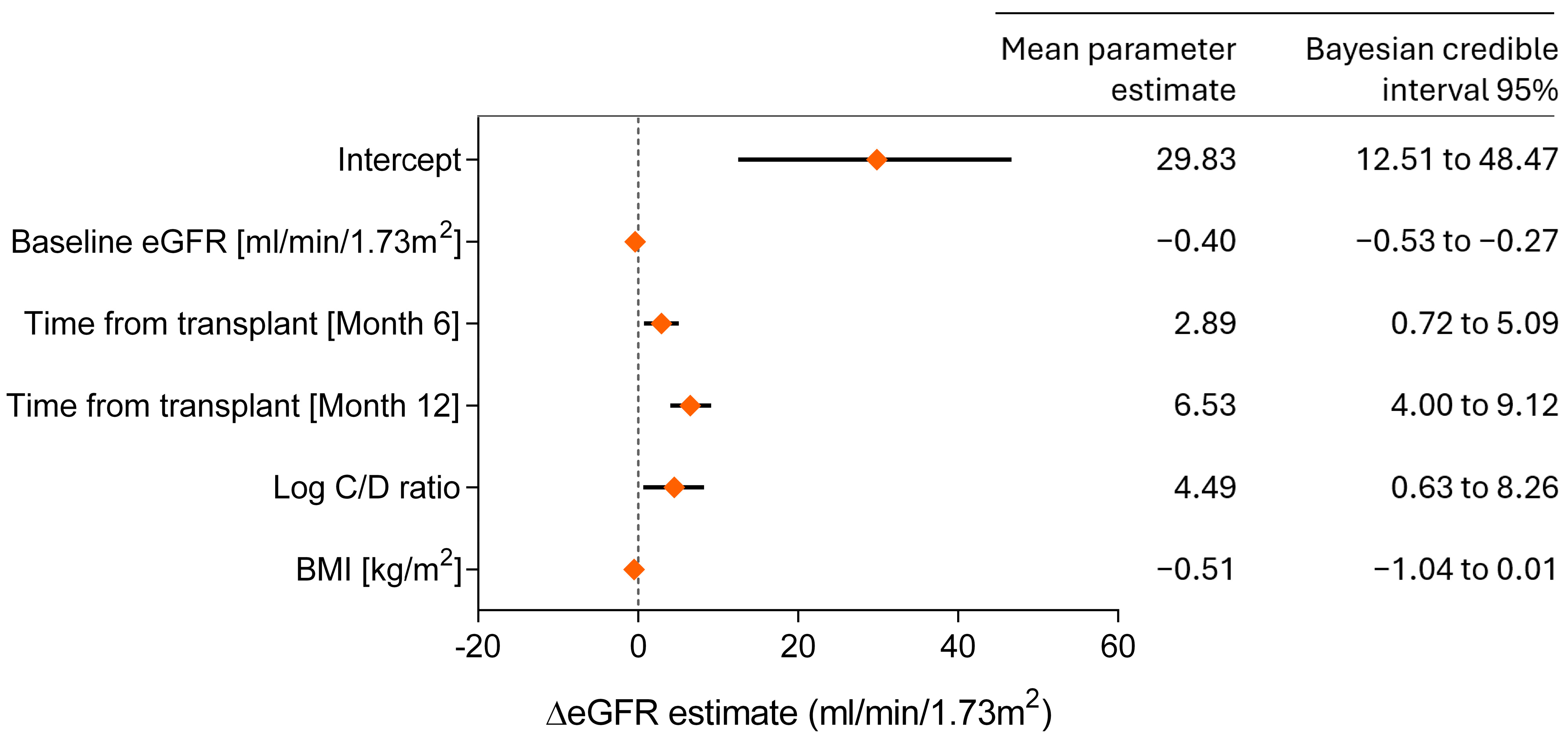
3.5. Bayesian Model for Predicting Renal Function
3.6. Graft Rejections and Viral Infections
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Han, A.; Ahn, S.; Min, S.K.; Ha, J.; Min, S. Association of high intra-patient variability in tacrolimus exposure with calcineurin inhibitor nephrotoxicity in kidney transplantation. Sci. Rep. 2023, 13, 16502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gijsen, V.M.; Madadi, P.; Dube, M.P.; Hesselink, D.A.; Koren, G.; de Wildt, S.N. Tacrolimus-induced nephrotoxicity and genetic variability: A review. Ann. Transplant. 2012, 17, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Thölking, G.; Fortmann, C.; Koch, R.; Gerth, H.U.; Pabst, D.; Pavenstädt, H.; Kabar, I.; Hüsing, A.; Wolters, H.; Reuter, S.; et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS ONE 2014, 9, e111128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tremblay, S.; Nigro, V.; Weinberg, J.; Woodle, E.S.; Alloway, R.R. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am. J. Transplant. 2017, 17, 432–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giral, M.; Grimbert, P.; Morin, B.; Bouvier, N.; Buchler, M.; Dantal, J.; Garrigue, V.; Bertrand, D.; Kamar, N.; Malvezzi, P.; et al. Impact of Switching From Immediate- or Prolonged-Release to Once-Daily Extended-Release Tacrolimus (LCPT) on Tremor in Stable Kidney Transplant Recipients: The Observational ELIT Study. Transpl. Int. 2024, 37, 11571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thölking, G.; Tosun-Koç, F.; Jehn, U.; Koch, R.; Pavenstädt, H.; Suwelack, B.; Reuter, S. Improved Kidney Allograft Function after Early Conversion of Fast IR-Tac Metabolizers to LCP-Tac. J. Clin. Med. 2022, 11, 1290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez Fructuoso, A.; Ruiz, J.C.; Franco, A.; Diekmann, F.; Redondo, D.; Calviño, J.; Serra, N.; Aladrén, M.J.; Cigarrán, S.; Manonelles, A.; et al. Effectiveness and safety of the conversion to MeltDose® extended-release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: A retrospective study. Clin. Transplant. 2020, 34, e13767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Czarnecka, P.; Czarnecka, K.; Baczkowska, T.; Lagiewska, B.; Durlik, M. Real-life comparison of efficacy and safety profiles of two prolonged-release tacrolimus formulations in de novo kidney transplant recipients: 24 months of follow-up. PLoS ONE 2023, 18, e0278894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glander, P.; Waiser, J.; Kasbohm, S.; Friedersdorff, F.; Peters, R.; Rudolph, B.; Wu, K.; Budde, K.; Liefeldt, L. Bioavailability and costs of once-daily and twice-daily tacrolimus formulations in de novo kidney transplantation. Clin. Transplant. 2018, 32, e13311. [Google Scholar] [CrossRef] [PubMed]
- Durlik, M.; Danielewicz, R. (Eds.) Zalecenia Dotyczące Leczenia Immunosupresyjnego po Przeszczepieniu Narządów Unaczynionych [Recommendations for Immunosuppressive Treatment after Transplantation of Vascularized Organs]. Fundacja Zjednoczeni dla Transplantacji: Warszawa, Polska, 2021. [Google Scholar]
- Carpenter, J.; Bithell, J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef] [PubMed]
- Suwelack, B.; Bunnapradist, S.; Meier-Kriesche, U.; Stevens, D.R.; Procaccianti, C.; Morganti, R.; Budde, K. Effect of Concentration/Dose Ratio in De Novo Kidney Transplant Recipients Receiving LCP-Tacrolimus or Immediate-Release Tacrolimus: Post Hoc Analysis of a Phase 3 Clinical Trial. Ann. Transplant. 2020, 25, e923278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandez Rivera, C.; Calvo Rodríguez, M.; Poveda, J.L.; Pascual, J.; Crespo, M.; Gomez, G.; Cabello Pelegrin, S.; Paul, J.; Lauzurica, R.; Perez Mir, M.; et al. Better study. Bioavailability of once-daily tacrolimus formulations used in clinical practice in the management of De Novo kidney transplant recipients: The better study. Clin. Transplant. 2022, 36, e14550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thölking, G.; Filensky, B.; Jehn, U.; Schütte-Nütgen, K.; Koch, R.; Kurschat, C.; Pavenstädt, H.; Suwelack, B.; Reuter, S.; Kuypers, D. Increased renal function decline in fast metabolizers using extended-release tacrolimus after kidney transplantation. Sci. Rep. 2021, 11, 15606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowicka, M.; Górska, M.; Nowicka, Z.; Edyko, K.; Edyko, P.; Wiślicki, S.; Zawiasa-Bryszewska, A.; Strzelczyk, J.; Matych, J.; Kurnatowska, I. Tacrolimus: Influence of the Posttransplant Concentration/Dose Ratio on Kidney Graft Function in a Two-Year Follow-Up. Kidney Blood Press Res. 2019, 44, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Kwiatkowski, S.; Wahler, F.; Gryczman, M.; Domańki, L.; Marchelk-Myśliwiec, M.; Ciechanowski, K.; Drozd-Dabrowska, M. C/D Ratio in Long-Term Renal Function. Transplant Proc. 2019, 51, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Alloway, R.R. Clinical Evaluation of Modified Release and Immediate Release Tacrolimus Formulations. AAPS J. 2017, 19, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Thölking, G.; Schulte, C.; Jehn, U.; Schütte-Nütgen, K.; Pavenstädt, H.; Suwelack, B.; Reuter, S. The Tacrolimus Metabolism Rate and Dyslipidemia after Kidney Transplantation. J. Clin. Med. 2021, 10, 3066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jouve, T.; Fonrose, X.; Noble, J.; Janbon, B.; Fiard, G.; Malvezzi, P.; Stanke-Labesque, F.; Rostaing, L. The TOMATO Study (Tacrolimus Metabolization in Kidney Transplantation): Impact of the Concentration-Dose Ratio on Death-censored Graft Survival. Transplantation 2020, 104, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Marco, D.N.; Molina, M.; Guio, A.M.; Julian, J.; Fortuna, V.; Fabregat-Zaragoza, V.L.; Salas, M.Q.; Monge-Escartín, I.; Riu-Viladoms, G.; Carcelero, E.; et al. Effects of CYP3A5 Genotype on Tacrolimus Pharmacokinetics and Graft-versus-Host Disease Incidence in Allogeneic Hematopoietic Stem Cell Transplantation. Pharmaceuticals 2024, 17, 553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, W.; Wang, X.; Zhang, D.; Zuo, X. Genotype-Guided Model for Prediction of Tacrolimus Initial Dosing After Lung Transplantation. J. Clin. Pharmacol. 2024, 64, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ke, M.; Wang, H.; Wu, W.; Lin, R.; Huang, P.; Lin, C. Physiologically based pharmacokinetic model combined with reverse dose method to study the nephrotoxic tolerance dose of tacrolimus. Arch. Toxicol. 2023, 97, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.M.; Hemani, R.J.; Solanki, N.D.; Shete, N.B.; Gang, S.D.; Konnur, A.M.; Srivastava, R.; Pandey, S.N. A systematic review and meta-analysis recite the efficacy of Tacrolimus treatment in renal transplant patients in association with genetic variants of CYP3A5 gene. Am. J. Clin. Exp. Urol. 2023, 11, 275–292. [Google Scholar] [PubMed]
- van Gelder, T.; Meziyerh, S.; Swen, J.J.; de Vries, A.P.J.; Moes, D.J.A.R. The Clinical Impact of the C0/D Ratio and the CYP3A5 Genotype on Outcome in Tacrolimus Treated Kidney Transplant Recipients. Front Pharmacol. 2020, 11, 1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pallet, N.; Etienne, I.; Buchler, M.; Bailly, E.; Hurault de Ligny, B.; Choukroun, G.; Colosio, C.; Thierry, A.; Vigneau, C.; Moulin, B.; et al. Long-Term Clinical Impact of Adaptation of Initial Tacrolimus Dosing to CYP3A5 Genotype. Am. J. Transplant. 2016, 16, 2670–2695. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, T. Drug interactions with tacrolimus. Drug. Saf. 2002, 25, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Anglicheau, D.; Flamant, M.; Schlageter, M.H.; Martinez, F.; Cassinat, B.; Beaune, P.; Legendre, C.; Thervet, E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol. Dial. Transplant. 2003, 18, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Thölking, G.; Siats, L.; Fortmann, C.; Koch, R.; Hüsing, A.; Cicinnati, V.R.; Gerth, H.U.; Wolters, H.H.; Anthoni, C.; Pavenstädt, H.; et al. Tacrolimus Concentration/Dose Ratio is Associated with Renal Function After Liver Transplantation. Ann. Transplant. 2016, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- von Samson-Himmelstjerna, F.A.; Messtorff, M.L.; Kakavand, N.; Eisenberger, U.; Korth, J.; Lange, U.; Kolbrink, B.; Aldag, L.; Schulze Dieckhoff, T.; Feldkamp, T.; et al. The Tacrolimus Concentration/Dose Ratio Does Not Predict Early Complications After Kidney Transplantation. Transpl. Int. 2023, 36, 11027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirsch, H.H.; Yakhontova, K.; Lu, M.; Manzetti, J. BK Polyomavirus Replication in Renal Tubular Epithelial Cells Is Inhibited by Sirolimus, but Activated by Tacrolimus Through a Pathway Involving FKBP-12. Am. J. Transplant. 2016, 16, 821–832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| All Patients | A. Fast Metabolizers | B. Intermediate Metabolizers | C. Slow Metabolizers | p-Value A vs. B vs. C | p-Value A vs. C | |
|---|---|---|---|---|---|---|
| N | 101 | 30/101 | 31/101 | 32/101 | — | — |
| % | (100%) | (29.7%) | (30.7%) | (31.7%) | ||
| Demographics and allograft function | ||||||
| Age, years | 46.8 ± 12.3 | 44.9 ± 11.7 | 45.9 ± 11 | 49.4 ± 14 | 0.344 | 0.197 |
| Sex (male) | 50 (49.5%) | 14 (46.7%) | 16 (51.6%) | 16 (50%) | 0.925 | 0.994 |
| BMI (kg/m2) | 25.3 ± 4 | 25.1 ± 4.1 | 25.9 ± 4.2 | 24.8 ± 3.8 | 0.791 | 0.844 |
| Serum creatinine (mg/dL) | 1.6 ± 0.6 | 1.6 ± 0.6 | 1.6 ± 0.6 | 1.5 ± 0.4 | 0.662 | 0.893 |
| eGFR (mL/min/1.73 m2) | 47.3 (38–61.6) | 51.9 (38.3–64.5) | 47 (35.6–60.8) | 47 (41–55.2) | 0.896 | 0.690 |
| Significant comorbidity | ||||||
| Arterial hypertension | 69 (68.3%) | 23 (76.7%) | 22 (71%) | 16 (50%) | 0.065 | 0.056 |
| Ischemic heart disease | 14 (13.8%) | 2 (6.7%) | 5 (16.1%) | 7 (21.9%) | 0.217 | 0.197 |
| Type 2 diabetes mellitus | 11 (10.9%) | 3 (10%) | 2 (6.5%) | 6 (18.8%) | 0.340 | 0.475 |
| Type 1 diabetes mellitus | 3 (3%) | 1 (3.3%) | 2 (6.5%) | 0 (0%) | — | — |
| Etiologies of end-stage renal disease | ||||||
| Glomerulonephritis | 43 (42.6%) | 8 (26.6%) | 16 (51.6%) | 15 (46.8%) | 0.074 | 0.236 |
| Polycystic kidney disease | 14 (13.9%) | 7 (23.3%) | 3 (9.7%) | 4 (12.5%) | 0.336 | 0.329 |
| Tubulointerstitial nephritis | 9 (8.9%) | 3 (10%) | 3 (9.7%) | 1 (3.1%) | 0.556 | 0.346 |
| Hypertensive nephropathy | 8 (7.9%) | 3 (10%) | 0 (0%) | 5 (15.6%) | 0.066 | 0.709 |
| Diabetic nephropathy | 7 (6.9%) | 2 (6.7%) | 3 (9.7%) | 2 (6.2%) | 0.894 | 1.000 |
| Congenital urinary abnormalities | 3 (3%) | 0 (0%) | 1 (3.2%) | 0 (0%) | — | — |
| Other | 23 (22.8%) | 9 (30%) | 6 (19.4% | 6 (18.8%) | 0.497 | 0.461 |
| Previous transplants | 13 (12.9%) | 3 (10%) | 4 (12.9%) | 5 (15.6) | 0.926 | 0.709 |
| PRA (%) | 5.4 ± 12.4 | 5.7 ± 12.2 | 3.7 ± 8.1 | 6.5 ± 16.1 | 0.278 | 0.943 |
| PRA <5% | 80 (79%) | 24 (80%) | 25 (81%) | 25 (78%) | 0.967 | 1.000 |
| Donor characteristics | ||||||
| Age | 48.6 ± 13.2 | 47.8 ± 11.5 | 47.1 ± 13.4 | 48.6 ± 15.1 | 0.839 | 0.800 |
| Sex (male) | 65 (64.4%) | 19 (63.3%) | 21 (67.7%) | 20 (62.5) | 0.898 | 1.000 |
| Living donor | 6 (5.9%) | 1 (3.3%) | 3 (9.7%) | 2 (6.2) | 0.778 | 1.000 |
| Transplant characteristics | ||||||
| HLA antigen mismatches | 3 (3–4) | 3 (2–4) | 3 (3–4) | 3 (3–4) | 0.344 | 0.211 |
| Cold ischemia time (h) | 14.8 (10.4–21.2) | 14.5 (11.3–19.8) | 14.3 (10.2–20.8) | 14.3 (10.3–19.7) | 0.875 | 0.683 |
| Warm ischemia time (min) | 28 (24–33) | 30 (25–34) | 27 (22–30) | 28 (24–35) | 0.153 | 0.498 |
| Delayed graft function | 24 (23.8%) | 10 (30%) | 7 (22.6) | 5 (15.6) | 0.282 | 0.141 |
| Induction therapy (basiliximab or thymoglobulin) | 82 (81.2%) | 21 (70%) | 25 (80.6%) | 28 (87.5%) | 0.229 | 0.123 |
| Maintenance immunosuppression | 0.350 | 0.254 | ||||
| Tac + MMF + methylprednisolone | 68 (67.3%) | 22 (73.3%) | 21 (67.7%) | 21 (67.7%) | ||
| Tac + MMF + prednisone | 32 (31.7%) | 8 (26.7%) | 10 (32.3%) | 10 (32.3%) | ||
| Tac + MMF + deflazacort | 1 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębska-Ślizień, A.; Kuźmiuk-Glembin, I.; Hożejowski, R.; Kamińska, D.; Krajewska, M.; Zawiasa-Bryszewska, A.; Kurnatowska, I.; Smykał-Jankowiak, K.; Głyda, M.; Kozioł, L.; et al. Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus. J. Clin. Med. 2024, 13, 6241. https://doi.org/10.3390/jcm13206241
Dębska-Ślizień A, Kuźmiuk-Glembin I, Hożejowski R, Kamińska D, Krajewska M, Zawiasa-Bryszewska A, Kurnatowska I, Smykał-Jankowiak K, Głyda M, Kozioł L, et al. Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus. Journal of Clinical Medicine. 2024; 13(20):6241. https://doi.org/10.3390/jcm13206241
Chicago/Turabian StyleDębska-Ślizień, Alicja, Izabella Kuźmiuk-Glembin, Roman Hożejowski, Dorota Kamińska, Magdalena Krajewska, Anna Zawiasa-Bryszewska, Ilona Kurnatowska, Katarzyna Smykał-Jankowiak, Maciej Głyda, Lidia Kozioł, and et al. 2024. "Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus" Journal of Clinical Medicine 13, no. 20: 6241. https://doi.org/10.3390/jcm13206241
APA StyleDębska-Ślizień, A., Kuźmiuk-Glembin, I., Hożejowski, R., Kamińska, D., Krajewska, M., Zawiasa-Bryszewska, A., Kurnatowska, I., Smykał-Jankowiak, K., Głyda, M., Kozioł, L., Karczewski, M., Ciechanowski, K., & Kwiatkowska, E. (2024). Renal Allograft Function and the Tacrolimus C/D Ratio: Insights from a Prospective Study on MeltDose Tacrolimus. Journal of Clinical Medicine, 13(20), 6241. https://doi.org/10.3390/jcm13206241







