Risks of Cervical Cancer Recurrence After Fertility-Sparing Surgery and the Role of Human Papillomavirus Infection Types
Abstract
1. Introduction
2. Material and Methods
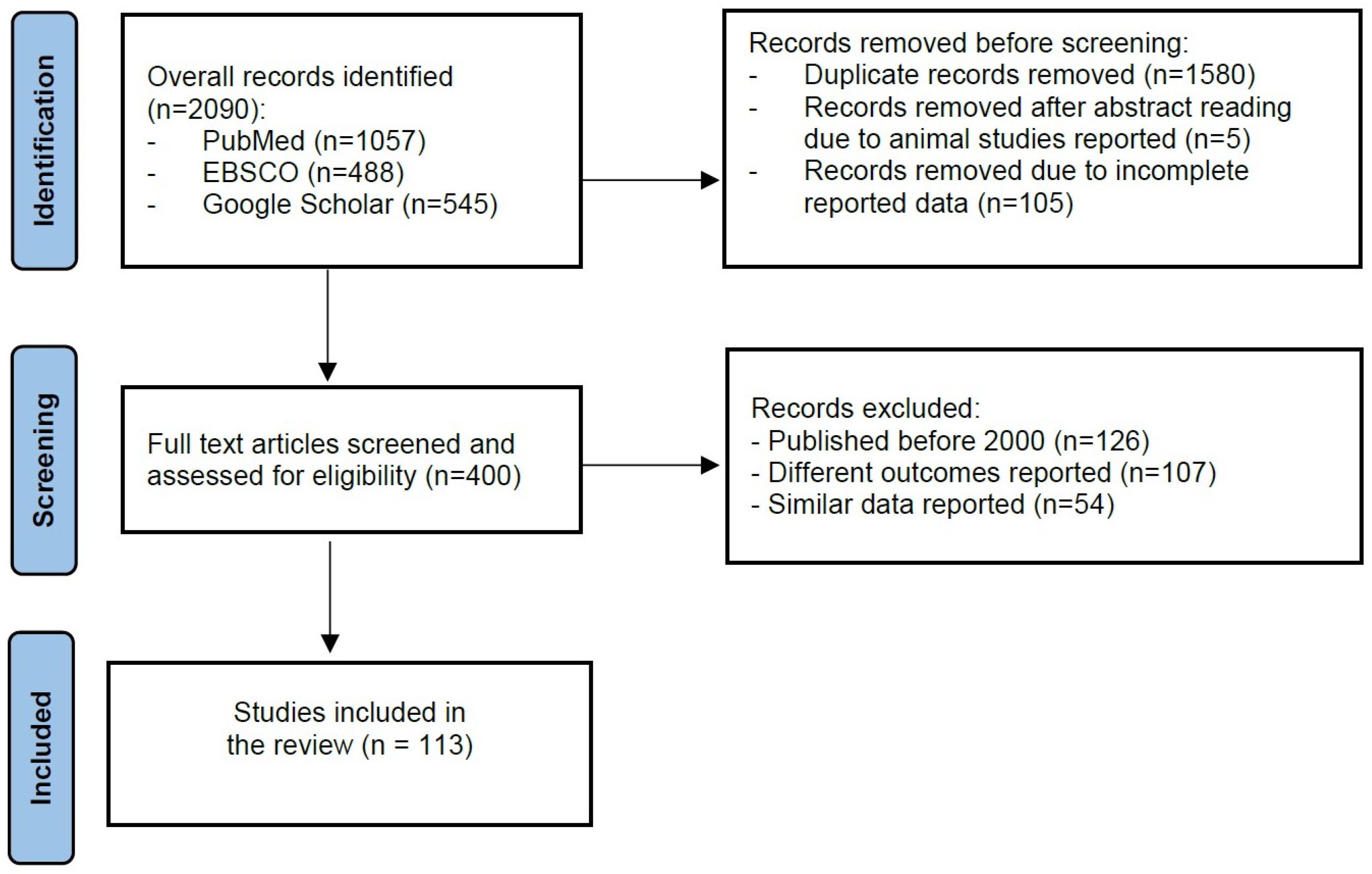
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
3. Results and Discussion
3.1. Epidemiology of Cervical Cancer
3.2. Cervical Cancer and Human Papillomavirus
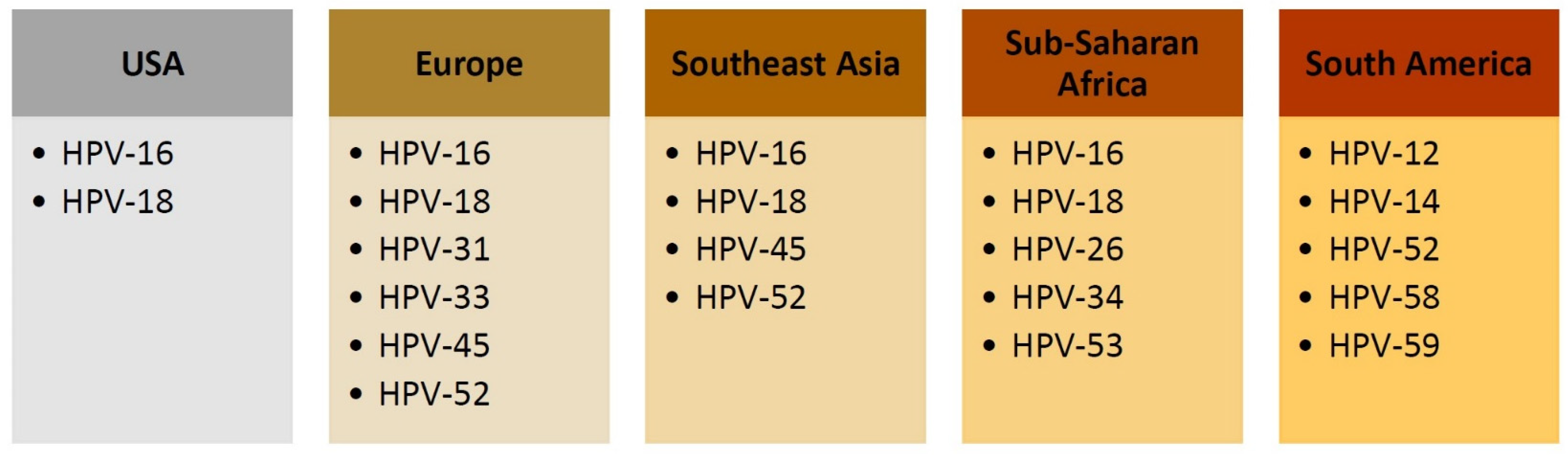
Prevalence of HR-HPV in Cervical Specimens
3.3. Fertility-Sparing Surgery for Women with Cervical Cancer
3.3.1. Fertility-Sparing Surgical Procedures
3.3.2. Success Rates and Outcomes of Fertility-Sparing Surgery
3.4. Cervical Cancer Recurrence After Fertility-Sparing Management
3.4.1. Cervical Cancer Recurrence After Fertility-Sparing Surgery
3.4.2. Cervical Cancer Recurrence After Neoadjuvant Chemotherapy and Fertility-Sparing Surgery
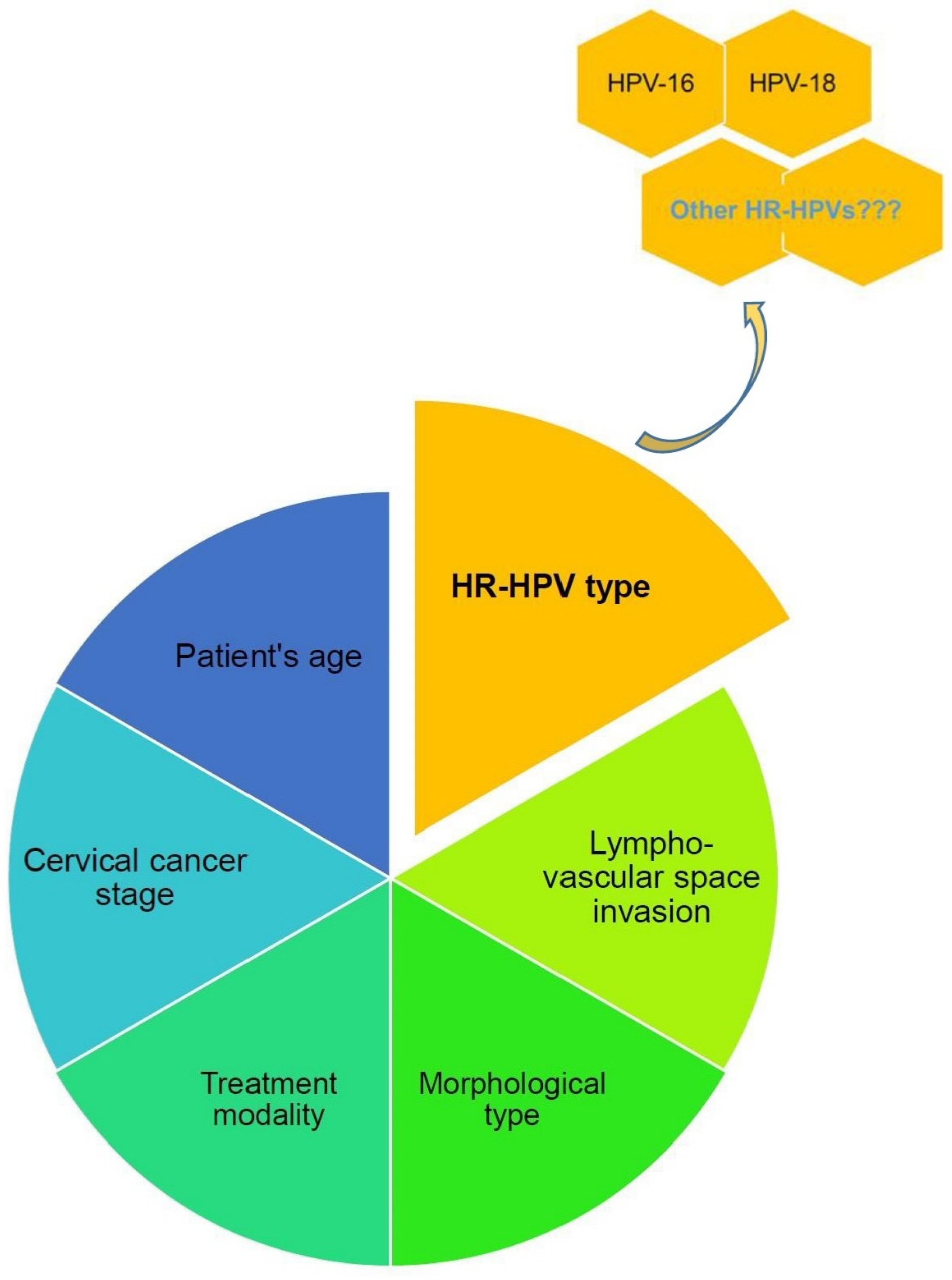
3.5. Risk Factors for Cervical Cancer Recurrence After Fertility-Sparing Management
3.6. Role of HPV Type in Cervical Cancer Recurrence After Fertility-Sparing Surgery
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, C.; Kahesa, C.; Mwaiselage, J.; West, J.T.; Wood, C.; Angeletti, P.C. How the Cervical Microbiota Contributes to Cervical Cancer Risk in Sub-Saharan Africa. Front. Cell Infect. Microbiol. 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 5 September 2023).
- Huiyun, J.; Jie, D.; Huan, W.; Yuebo, Y.; Xiaomao, L. Prevalence and characteristics of cervical human papillomavirus genotypes and cervical lesions among 58,630 women from Guangzhou, China. J. Infect. Public Health 2023, 16, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Park, Y.; Baik, S.; Ho, C.; Lin, C.Y.; Chung, S.H. Progesterone Receptor Is a Haploinsufficient Tumor-Suppressor Gene in Cervical Cancer. Mol. Cancer Res. 2021, 19, 42–47. [Google Scholar] [CrossRef]
- Bowden, S.J.; Bodinier, B.; Kalliala, I.; Zuber, V.; Vuckovic, D.; Doulgeraki, T.; Whitaker, M.D.; Wielscher, M.; Cartwright, R.; Tsilidis, K.K.; et al. Genetic variation in cervical preinvasive and invasive disease: A genome-wide association study. Lancet Oncol. 2021, 22, 548–557. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Frąszczak, K.; Barczyński, B.; Kondracka, A. Does Lactobacillus Exert a Protective Effect on the Development of Cervical and Endometrial Cancer in Women? Cancers 2022, 14, 4909. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Moscicki, A.B. Vaginal microbiome and cervical cancer. Semin. Cancer Biol. 2022, 86 Pt 3, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Fracella, M.; Oliveto, G.; Sorrentino, L.; Roberto, P.; Cinti, L.; Viscido, A.; Di Lella, F.M.; Giuffrè, F.; Gentile, M.; Pietropaolo, V.; et al. Common Microbial Genital Infections and Their Impact on the Innate Immune Response to HPV in Cervical Cells. Pathogens 2022, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahpour-Naghibi, A.; Bagheri, K.; Almukhtar, M.; Taha, S.R.; Zadeh, M.S.; Moghadam, K.B.; Tadi, M.J.; Rouholamin, S.; Razavi, M.; Sepidarkish, M.; et al. Trichomonas vaginalis infection and risk of cervical neoplasia: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0288443. [Google Scholar] [CrossRef] [PubMed]
- Hamar, B.; Teutsch, B.; Hoffmann, E.; Hegyi, P.; Váradi, A.; Nyirády, P.; Hunka, Z.; Ács, N.; Lintner, B.; Hermánné, R.J.; et al. Trichomonas vaginalis infection is associated with increased risk of cervical carcinogenesis: A systematic review and meta-analysis of 470,000 patients [published online ahead of print, 2023 Apr 3]. Int. J. Gynaecol. Obstet. 2023, 163, 31–43. [Google Scholar] [CrossRef]
- Baik, S.; Mehta, F.F.; Unsal, E.; Park, Y.; Chung, S.H. Estrogen Inhibits Epithelial Progesterone Receptor-Dependent Progestin Therapy Efficacy in a Mouse Model of Cervical Cancer. Am. J. Pathol. 2022, 192, 353–360. [Google Scholar] [CrossRef]
- Asthana, S.; Busa, V.; Labani, S. Oral contraceptives use and risk of cervical cancer-A systematic review & meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 163–175. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Fruzzetti, F. Estro-progestin Contraceptives and Risk of Cervical Cancer: A Debated Issue. Anticancer. Res. 2020, 40, 5995–6002. [Google Scholar] [CrossRef]
- Kamani, M.; Akgor, U.; Gültekin, M. Review of the literature on combined oral contraceptives and cancer. Ecancermedicalscience 2022, 16, 1416. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, H.; Yang, X.; Wen, W.; Song, K.; Yu, X.; Qu, X. Screening of cervical cancer-related hub genes based on comprehensive bioinformatics analysis. Cancer Biomark. 2021, 32, 303–315. [Google Scholar] [CrossRef]
- Gong, J.M.; Shen, Y.; Shan, W.W.; He, Y.X. The association between MTHFR polymorphism and cervical cancer. Sci. Rep. 2018, 8, 7244. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Park, B.; Shin, H.-J.; Joo, J.; Yoon, K.-A.; Kim, Y.M.; Hayashi, T.; Tokunaga, K.; Kong, S.-Y.; Kim, J.-Y. Protective association of HLA-DRB1*13:02, HLA-DRB1*04:06, and HLA-DQB1*06:04 alleles with cervical cancer in a Korean population. Hum. Immunol. 2019, 80, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Pruski, D.; Millert-Kalińska, S.; Łagiedo, M.; Sikora, J.; Jach, R.; Przybylski, M. Effect of HPV Vaccination on Virus Disappearance in Cervical Samples of a Cohort of HPV-Positive Polish Patients. J. Clin. Med. 2023, 12, 7592. [Google Scholar] [CrossRef] [PubMed]
- Ferrall, L.; Lin, K.Y.; Roden, R.B.S.; Hung, C.F.; Wu, T.C. Cervical Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2021, 27, 4953–4973. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Wolswinkel, J.T.; Eikelder, M.L.G.T.; Verhoef, C.G.; Zusterzeel, P.L.M. High- or Intermediate-Risk Histologic Features in Patients with Clinical Early-Stage Cervical Cancer Planned for Fertility-Sparing Surgery: A Systematic Review. Cancers 2023, 15, 3920. [Google Scholar] [CrossRef]
- Moreira, A.S.L.; Cunha, T.M.; Esteves, S. Cervical cancer recurrence-can we predict the type of recurrence? Diagn. Interv. Radiol. 2020, 26, 403–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slama, J.; Runnebaum, I.B.; Scambia, G.; Angeles, M.A.; Bahrehmand, K.; Kommoss, S.; Fagotti, A.; Narducci, F.; Matylevich, O.; Holly, J.; et al. Analysis of risk factors for recurrence in cervical cancer patients after fertility-sparing treatment: The FERTIlity Sparing Surgery retrospective multicenter study. Am. J. Obstet. Gynecol. 2023, 228, 443.e1–443.e10. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef]
- Issanov, A.; Karim, M.E.; Aimagambetova, G.; Dummer, T.J.B. Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018). Vaccines 2022, 10, 2113. [Google Scholar] [CrossRef]
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.-B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–2099: A modelling study. Lancet Oncol. 2019, 20, 394–407. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Engholm, G.; Lönnberg, S.; Khan, S.; Bray, F. 50 years of screening in the Nordic countries: Quantifying the effects on cervical cancer incidence. Br. J. Cancer. 2014, 111, 965–969. [Google Scholar] [CrossRef]
- Petersen, L.M.; Fenton, J.M.; Kennedy, L.S.; LaRochelle, E.P.M.; Bejarano, S.; Tsongalis, G.J. HPV, vaccines, and cervical cancer in a low- and middle-income country. Curr. Probl. Cancer 2020, 44, 100605. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Azizan, A. Epidemiology of HPV Infection and HPV-Related Cancers in Kazakhstan: A Review. Asian Pac. J. Cancer Prev. 2018, 19, 1175–1180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aimagambetova, G.; Chan, C.K.; Ukybassova, T.; Imankulova, B.; Balykov, A.; Kongrtay, K.; Azizan, A. Cervical cancer screening and prevention in Kazakhstan and Central Asia. J. Med. Screen. 2021, 28, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; de Sanjosé, S. The epidemiology of human papillomavirus infection and cervical cancer. Dis. Markers 2007, 23, 213–227. [Google Scholar] [CrossRef]
- Bosch, F.X.; Qiao, Y.L.; Castellsagué, X. CHAPTER 2 The epidemiology of human papillomavirus infection and its association with cervical cancer. Int. J. Gynaecol. Obstet. 2006, 94 (Suppl. S1), S8–S21. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/hpv/index.html (accessed on 6 October 2023).
- Castellsagué, X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol. Oncol. 2008, 110 (Suppl. S2), S4–S7. [Google Scholar] [CrossRef]
- Burd, E.M.; Dean, C.L. Human Papillomavirus. Microbiol. Spectr. 2016, 4, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 590, Erratum in J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, X.; Shen, F.; Ma, F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021, 497, 243–254. [Google Scholar] [CrossRef]
- Xia, C.; Li, S.; Long, T.; Chen, Z.; Chan, P.K.S.; Boon, S.S. Current Updates on Cancer-Causing Types of Human Papillomaviruses (HPVs) in East, Southeast, and South Asia. Cancers 2021, 13, 2691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cairns, M.; Cuschieri, K.S.; Cubie, H.A.; Cruickshank, M.E. High-risk HPV genotyping in the follow-up of women treated conservatively for microinvasive cervical cancer. Int. J. Gynecol. Cancer. 2010, 20, 154–157. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Xiao, W.; Pickard, R.K.L.; Kahle, L.; Gillison, M.L. Prevalence of Oral HPV Infection in Unvaccinated Men and Women in the United States, 2009–2016. JAMA 2019, 322, 977–979, Erratum in JAMA 2019, 322, 1925; Erratum in JAMA 2020, 323, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagheden, C.; Eklund, C.; Lamin, H.; Kleppe, S.N.; Lei, J.; Elfström, K.M.; Sundström, K.; Andrae, B.; Sparén, P.; Dillner, J. Nationwide comprehensive human papillomavirus (HPV) genotyping of invasive cervical cancer. Br. J. Cancer. 2018, 118, 1377–1381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Przybylski, M.; Pruski, D.; Wszołek, K.; de Mezer, M.; Żurawski, J.; Jach, R.; Millert-Kalińska, S. Prevalence of HPV and Assessing Type-Specific HPV Testing in Cervical High-Grade Squamous Intraepithelial Lesions in Poland. Pathogens 2023, 12, 350. [Google Scholar] [CrossRef]
- Quek, S.C.; Lim, B.K.; Domingo, E.; Soon, R.; Park, J.-S.; Vu, T.N.; Tay, E.H.; Le, Q.T.; Kim, Y.-T.; Vu, B.Q.; et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical intraepithelial neoplasia across 5 countries in Asia. Int. J. Gynecol. Cancer 2013, 23, 148–156. [Google Scholar] [CrossRef]
- Farahmand, Z.; Soleimanjahi, H.; Garshasbi, M.; Hasanzadeh, M.; Zafari, E. Distribution of the most common types of HPV in Iranian women with and without cervical cancer. Women Health 2021, 61, 73–82. [Google Scholar] [CrossRef]
- Grover, S.; Seckar, T.; Gao, L.; Bhatia, R.; Lin, X.; Zetola, N.; Ramogola-Masire, D.; Robertson, E. Characterization of HPV subtypes in invasive cervical cancer in Botswana patients using a pan-pathogen microarray technology. Tumour Virus Res. 2023, 15, 200262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundström, K.; Ploner, A.; Arnheim-Dahlström, L.; Eloranta, S.; Palmgren, J.; Adami, H.-O.; Helm, N.Y.; Sparén, P.; Dillner, J. Interactions Between High- and Low-Risk HPV Types Reduce the Risk of Squamous Cervical Cancer. J. Natl. Cancer Inst. 2015, 107, djv185. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Terzic, S.; Laganà, A.S.; Bapayeva, G.; la Fleur, P.; Terzic, M. Contemporary Fertility-Sparing Management Options of Early Stage Endometrioid Endometrial Cancer in Young Nulliparous Patients. J. Clin. Med. 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- IARC. Cervical Cancer Screening. In IARC Handbooks of Cancer Prevention; IARC: Lyon, France, 2022; Volume 18, ISBN -13. [Google Scholar]
- Zaccarini, F.; Sanson, C.; Maulard, A.; Schérier, S.; Leary, A.; Pautier, P.; Chargari, C.; Genestie, C.; Gouy, S.; Morice, P. Cervical Cancer and Fertility-Sparing Treatment. J. Clin. Med. 2021, 10, 4825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gwacham, N.I.; McKenzie, N.D.; Fitzgerald, E.R.; Ahmad, S.; Holloway, R.W. Neoadjuvant chemotherapy followed by fertility sparing surgery in cervical cancers size 2–4 cm; emerging data and future perspectives. Gynecol. Oncol. 2021, 162, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.; Campbell, S.; Frumovitz, M.; Ramirez, P.T.; McKenzie, L.J. Fertility considerations prior to conservative management of gynecologic cancers. Int. J. Gynecol. Cancer 2021, 31, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.R.; Katebi Kashi, P.; Acosta-Torres, S.; Beavis, A.L.; Christianson, M.S. Fertility-sparing Surgery for Patients with Cervical, Endometrial, and Ovarian Cancers. J. Minim. Invasive Gynecol. 2021, 28, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, C.; Roman, R.A.; Rambhatla, A.; Nezhat, F. Reproductive and oncologic outcomes after fertility-sparing surgery for early stage cervical cancer: A systematic review. Fertil. Steril. 2020, 113, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Sarría-Santamera, A.; Bapayeva, G.; Utepova, G.; Krstic, J.; Terzic, S.; Aimagambetova, G.; Shauyen, F.; Terzic, M. Women’s Knowledge and Awareness of the Effect of Age on Fertility in Kazakhstan. Sexes 2020, 1, 60–71. [Google Scholar] [CrossRef]
- Silvestris, E.; Paradiso, A.V.; Minoia, C.; Daniele, A.; Cormio, G.; Tinelli, R.; D’Oronzo, S.; Cafforio, P.; Loizzi, V.; Dellino, M. Fertility preservation techniques in cervical carcinoma. Medicine 2022, 101, e29163. [Google Scholar] [CrossRef]
- Terzic, M.; Makhadiyeva, D.; Bila, J.; Andjic, M.; Dotlic, J.; Aimagambetova, G.; Sarria-Santamera, A.; Laganà, A.S.; Chiantera, V.; Vukovic, I.; et al. Reproductive and Obstetric Outcomes after Fertility-Sparing Treatments for Cervical Cancer: Current Approach and Future Directions. J. Clin. Med. 2023, 12, 2614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morice, P.; Maulard, A.; Scherier, S.; Sanson, C.; Zarokian, J.; Zaccarini, F.; Espenel, S.; Pautier, P.; Leary, A.; Genestie, C.; et al. Oncologic results of fertility sparing surgery of cervical cancer: An updated systematic review. Gynecol. Oncol. 2022, 165, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Furey, K.B.; Anderson, Z.S.; Kuznicki, M.L.; Klar, M.; Roman, L.D.; Wright, J.D.; Matsuo, K. Increasing trends of cervical conization with lymph node evaluation for fertility-sparing surgery in early cervical cancer. Gynecol. Oncol. 2023, 173, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Veroniki, A.A.; Efthimiou, O.; Kalliala, I.; Naci, H.; Bowden, S.; Paraskevaidi, M.; Arbyn, M.; Lyons, D.; Martin-Hirsch, P.; et al. Comparative effectiveness and risk of preterm birth of local treatments for cervical intraepithelial neoplasia and stage IA1 cervical cancer: A systematic review and network meta-analysis. Lancet Oncol. 2022, 23, 1097–1108, Erratum in Lancet Oncol. 2022, 23, e370. [Google Scholar] [CrossRef]
- D’Amato, A.; Riemma, G.; Agrifoglio, V.; Chiantera, V.; Laganà, A.S.; Mikuš, M.; Dellino, M.; Maglione, A.; Faioli, R.; Giannini, A.; et al. Reproductive Outcomes in Young Women with Early-Stage Cervical Cancer Greater than 2 cm Undergoing Fertility-Sparing Treatment: A Systematic Review. Medicina 2024, 60, 608. [Google Scholar] [CrossRef]
- Floyd, J.L.; Campbell, S.; Rauh-Hain, J.A.; Woodard, T. Fertility preservation in women with early-stage gynecologic cancer: Optimizing oncologic and reproductive outcomes. Int. J. Gynecol. Cancer. 2021, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; Van Driel, W.; Tidy, J.; Williamson, K.; et al. Simple versus Radical Hysterectomy in Women with Low-Risk Cervical Cancer. N. Engl. J. Med. 2024, 390, 819–829. [Google Scholar] [CrossRef]
- Takekuma, M. Challenges and perspectives on less invasive surgery for early-stage cervical cancer: A critical analysis of the SHAPE trial and its implications. J. Gynecol. Oncol. 2024, 35, e48. [Google Scholar] [CrossRef]
- Willows, K.; Lennox, G.; Covens, A. Fertility-sparing management in cervical cancer: Balancing oncologic outcomes with reproductive success. Gynecol. Oncol. Res. Pract. 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šimják, P.; Cibula, D.; Pařízek, A.; Sláma, J. Management of pregnancy after fertility-sparing surgery for cervical cancer. Acta Obstet. Gynecol. Scand. 2020, 99, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Piątek, S.; Szymusik, I.; Bidziński, M. Reproductive Results in Cancer Survivors after Fertility Sparing Management: The Need for the Standardization of Definitions. Cancers 2023, 15, 3569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robova, H.; Rob, L.; Halaska, M.J.; Drozenova, J.; Pichlik, T.; Drochytek, V.; Hruda, M. Twenty years of experience with less radical fertility-sparing surgery in early-stage cervical cancer: Pregnancy outcomes. Gynecol. Oncol. 2023, 174, 76–79. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, R.C.J.; Bos, A.M.E.; Jürgenliemk-Schulz, I.M.; Gerestein, C.G.; Zweemer, R.P. Fertility-sparing surgery and fertility preservation in cervical cancer: The desire for parenthood, reproductive and obstetric outcomes. Gynecol. Oncol. 2021, 163, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Kuznicki, M.L.; Chambers, L.M.; Morton, M.; Son, J.; Horowitz, M.; Crean-Tate, K.K.; Hackett, L.; Rose, P.G. Fertility-Sparing Surgery for Early-Stage Cervical Cancer: A Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2021, 28, 513–526.e1. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Solazzo, M.C.; Molitierno, R.; De Franciscis, P.; Pasanisi, F.; Cobellis, L.; Colacurci, N. Fertility-Sparing Treatment for Early-Stage Cervical Cancer ≥ 2 cm: Can One Still Effectively Become a Mother? A Systematic Review of Fertility Outcomes. Ann. Surg. Oncol. 2023, 30, 5587–5596. [Google Scholar] [CrossRef]
- Somigliana, E.; Mangili, G.; Martinelli, F.; Noli, S.; Filippi, F.; Bergamini, A.; Bocciolone, L.; Buonomo, B.; Peccatori, F. Fertility preservation in women with cervical cancer. Crit. Rev. Oncol. Hematol. 2020, 154, 103092. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, S.; Kuriakose, S.; Damodaran, B.; Ravishankar, N.; Arunkumar, G. Human papillomavirus (HPV) DNA detection in uterine cervix cancer after radiation indicating recurrence: A systematic review and meta-analysis. J. Gynecol. Oncol. 2020, 31, e20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adiga, D.; Eswaran, S.; Pandey, D.; Sharan, K.; Kabekkodu, S.P. Molecular landscape of recurrent cervical cancer. Crit. Rev. Oncol. Hematol. 2021, 157, 103178. [Google Scholar] [CrossRef] [PubMed]
- Aisagbonhi, O.; Zare, S.Y.; Hasteh, F.; Binder, P.; Roma, A.A.; Fadare, O. PTEN Loss and ARID1A Mutation in an HPV-positive Metastatic Adenocarcinoma Diagnosed Almost 18 yr After an Intact Cone Excision for Endocervical Adenocarcinoma In Situ. Int. J. Gynecol. Pathol. 2022, 41, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Y.; Wu, J. Prognosis of Early Stage Cervical Cancer according to Patterns of Recurrence. Cancer Manag. Res. 2021, 13, 8131–8136. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.Y.; Kim, D.Y.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Long-term outcomes after fertility-sparing laparoscopic radical trachelectomy in young women with early-stage cervical cancer: An Asian Gynecologic Cancer Group (AGCG) study. J. Surg. Oncol. 2014, 110, 252–257. [Google Scholar]
- Plaikner, A.; Siegler, K.; Hertel, H.; Jacob, A.; Petzel, A.; Schubert, M.; Blohmer, J.U.; Böhmer, G.; Marnitz, S.; Ragosch, V.; et al. Fertility sparing therapy in women with lymph node negative cervical cancer > 2cm—Oncologic and fertility outcomes of neoadjuvant chemotherapy followed by radical vaginal trachelectomy. Int. J. Gynecol. Cancer 2023, 33, 1542–1547. [Google Scholar] [CrossRef]
- Zusterzeel, P.L.; Pol, F.J.; van Ham, M.; Zweemer, R.P.; Bekkers, R.L.; Massuger, L.F.; Verheijen, R.H. Vaginal Radical Trachelectomy for Early-Stage Cervical Cancer: Increased Recurrence Risk for Adenocarcinoma. Int. J. Gynecol. Cancer. 2016, 26, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, T.; Zilver, S.; Samuels, S.; Schats, W.; Amant, F.; van Trommel, N.; Lok, C. Fertility-Sparing Surgery in Gynecologic Cancer: A Systematic Review. Cancers 2021, 13, 1008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tesfai, F.M.; Kroep, J.R.; Gaarenstroom, K.; De Kroon, C.; Van Loenhout, R.; Smit, V.; Trimbos, B.; Nout, R.A.; van Poelgeest, M.I.E.; Beltman, J.J. Fertility-sparing surgery of cervical cancer > 2 cm (International Federation of Gynecology and Obstetrics 2009 stage IB1-IIA) after neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2020, 30, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Z.; Lu, J.; Chen, X.; Ge, H.; Wu, X.; Li, J. Neoadjuvant chemotherapy followed by radical trachelectomy versus upfront abdominal radical trachelectomy for patients with FIGO 2018 stage IB2 cervical cancer. Gynecol. Oncol. 2023, 169, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Solazzo, M.C.; Bizzarri, N.; Ambrosio, D.; La Verde, M.; Torella, M.; Carotenuto, R.M.; Cobellis, L.; Colacurci, N.; De Franciscis, P. Fertility-Sparing Treatment for Early-Stage Cervical Cancer ≥ 2 cm: A Problem with a Thousand Nuances-A Systematic Review of Oncological Outcomes. Ann. Surg. Oncol. 2022, 29, 8346–8358. [Google Scholar] [CrossRef]
- Viveros-Carreño, D.; Rodriguez, J.; Rendon Pereira, G.J.; Slama, J.; Halaska, M.J.; Robova, H.; Pareja, R. Fertility-sparing surgery after neo-adjuvant chemotherapy in women with cervical cancer larger than 4 cm: A systematic review. Int. J. Gynecol. Cancer 2022, 32, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.; Tsunoda, A.T.; Guitmann, G.; Vieira, M.A.; Zanvettor, P.H.; Silvestre, J.B.C.H.; Santos, M.H.; Sacramento, R.M.M.; de Araujo, E.O.; Lopes, R.H.; et al. Brazilian Society of Surgical Oncology consensus on fertility-sparing surgery for cervical cancer. J. Surg. Oncol. 2022, 126, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hruda, M.; Robova, H.; Rob, L.; Halaska, M.J.; Drozenova, J.; Pichlik, T.; Malikova, H. Twenty years of experience with less radical fertility-sparing surgery in early-stage cervical cancer: Oncological outcomes. Gynecol. Oncol. 2021, 163, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Anchora, L.P.; Bruni, S.; Sperduti, I.; Certelli, C.; Chiofalo, B.; Giannini, A.; D’Oria, O.; Bizzarri, N.; Legge, F.; et al. Patterns of recurrence in FIGO 2018 stage IB1-IB2 cervical cancer: Comparison between minimally invasive and abdominal radical hysterectomy. Eur. J. Surg. Oncol. 2023, 49, 107047. [Google Scholar] [CrossRef]
- Eriksen, D.O.; Jensen, P.T.; Schroll, J.B.; Hammer, A. Human papillomavirus vaccination in women undergoing excisional treatment for cervical intraepithelial neoplasia and subsequent risk of recurrence: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2022, 101, 597–607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lukic, A.; Rossi, S.; Frega, A.; Ruscito, I.; Bianchi, P.; Nobili, F.; Caserta, D.; Vecchione, A. Prognostic role of immunohistochemical overexpression of the p16 protein in women under the age of 35 and diagnosed with HSIL (CIN2) subjected to “cervix sparing” excision. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, E.; Latouche, A.; Bonneau, C.; Calméjane, M.A.; Beaufort, C.; Ruigrok-Ritstier, K.; Bataillon, G.; Larbi Chérif, L.; Dupain, C.; Lecerf, C.; et al. Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer. Clin. Cancer Res. 2021, 27, 5869–5877. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harima, Y.; Sawada, S.; Nagata, K.; Sougawa, M.; Ohnishi, T. Human papilloma virus (HPV) DNA associated with prognosis of cervical cancer after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Kim, J.Y.; Lee, S.K.; Lim, H.S.; Lim, M.C.; Seo, S.S.; Kang, S.; Lee, D.O.; Park, S.Y. Persistent human papillomavirus DNA is associated with local recurrence after radiotherapy of uterine cervical cancer. Int. J. Cancer 2011, 129, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Noventa, M.; Ancona, E.; Cosmi, E.; Saccardi, C.; Litta, P.; D’Antona, D.; Nardelli, G.B.; Gizzo, S. Usefulness, methods and rationale of lymph nodes HPV-DNA investigation in estimating risk of early stage cervical cancer recurrence: A systematic literature review. Clin. Exp. Metastasis 2014, 31, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Vintermyr, O.K.; Iversen, O.; Thoresen, S.; Quint, W.; Molijn, A.; de Souza, S.; Rosillon, D.; Holl, K. Recurrent high-grade cervical lesion after primary conization is associated with persistent human papillomavirus infection in Norway. Gynecol. Oncol. 2014, 133, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Liu, J.; Wang, Q.; Feng, Y.; Lou, T.; Wang, S.; Wang, Y.; Jin, M.; Zhang, Z. Oncological and reproductive outcomes of adenocarcinoma in situ of the cervix managed with the loop electrosurgical excision procedure. BMC Cancer 2018, 18, 461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byun, J.M.; Jeong, D.H.; Kim, Y.N.; Jung, E.J.; Lee, K.B.; Sung, M.S.; Kim, K.T. Persistent HPV-16 infection leads to recurrence of high-grade cervical intraepithelial neoplasia. Medicine 2018, 97, e13606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bogani, G.; Pinelli, C.; Chiappa, V.; Martinelli, F.; Lopez, S.; Ditto, A.; Raspagliesi, F. Age-specific predictors of cervical dysplasia recurrence after primary conization: Analysis of 3,212 women. J. Gynecol. Oncol. 2020, 31, e60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bogani, G.; DI Donato, V.; Sopracordevole, F.; Ciavattini, A.; Ghelardi, A.; Lopez, S.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; et al. Recurrence rate after loop electrosurgical excision procedure (LEEP) and laser Conization: A 5-year follow-up study. Gynecol. Oncol. 2020, 159, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Dominoni, M.; Boschi, A.C.; Sosso, C.; Fiandrino, G.; Cesari, S.; Gardella, B. Clinical Significance of the Interaction between Human Papillomavirus (HPV) Type 16 and Other High-Risk Human Papillomaviruses in Women with Cervical Intraepithelial Neoplasia (CIN) and Invasive Cervical Cancer. J. Oncol. 2020, 2020, 6508180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinillo, A.; Dominoni, M.; Boschi, A.C.; Cesari, S.; Fiandrino, G.; Gardella, B. The relationship of human papillomavirus infection with endocervical glandular involvement on cone specimens in women with cervical intraepithelial neoplasia. Gynecol. Oncol. 2020, 159, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Covens, A.; Durand, N.; Ghorab, Z.; Gien, L.T.; Osborne, R.; Vicus, D.; Kupets, R. Role of HPV in the Prediction of Persistence/Recurrence After Treatment for Cervical Precancer. J. Obstet. Gynaecol. Can. 2023, 45, 102171. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Hu, Y. Risk factors associated with HPV persistence after conization in high-grade squamous intraepithelial lesion. Arch. Gynecol. Obstet. 2021, 304, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, B. Can prophylactic HPV vaccination reduce the recurrence of cervical lesions after surgery? Review and prospect. Infect. Agent. Cancer 2023, 18, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jentschke, M.; Kampers, J.; Becker, J.; Sibbertsen, P.; Hillemanns, P. Prophylactic HPV vaccination after conization: A systematic review and meta-analysis. Vaccine 2020, 38, 6402–6409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimagambetova, G.; Bapayeva, G.; Ukybassova, T.; Kamzayeva, N.; Sakhipova, G.; Shanazarov, N.; Terzic, M. Risks of Cervical Cancer Recurrence After Fertility-Sparing Surgery and the Role of Human Papillomavirus Infection Types. J. Clin. Med. 2024, 13, 6318. https://doi.org/10.3390/jcm13216318
Aimagambetova G, Bapayeva G, Ukybassova T, Kamzayeva N, Sakhipova G, Shanazarov N, Terzic M. Risks of Cervical Cancer Recurrence After Fertility-Sparing Surgery and the Role of Human Papillomavirus Infection Types. Journal of Clinical Medicine. 2024; 13(21):6318. https://doi.org/10.3390/jcm13216318
Chicago/Turabian StyleAimagambetova, Gulzhanat, Gauri Bapayeva, Talshyn Ukybassova, Nazira Kamzayeva, Gulnara Sakhipova, Nasrulla Shanazarov, and Milan Terzic. 2024. "Risks of Cervical Cancer Recurrence After Fertility-Sparing Surgery and the Role of Human Papillomavirus Infection Types" Journal of Clinical Medicine 13, no. 21: 6318. https://doi.org/10.3390/jcm13216318
APA StyleAimagambetova, G., Bapayeva, G., Ukybassova, T., Kamzayeva, N., Sakhipova, G., Shanazarov, N., & Terzic, M. (2024). Risks of Cervical Cancer Recurrence After Fertility-Sparing Surgery and the Role of Human Papillomavirus Infection Types. Journal of Clinical Medicine, 13(21), 6318. https://doi.org/10.3390/jcm13216318







