Comparative Study on Postoperative Immobilization in Reverse Total Shoulder Arthroplasty: 4 Weeks vs. 6 Weeks of Immobilization Yields Similar Clinical and Functional Outcomes
Abstract
1. Introduction
2. Materials and Methods
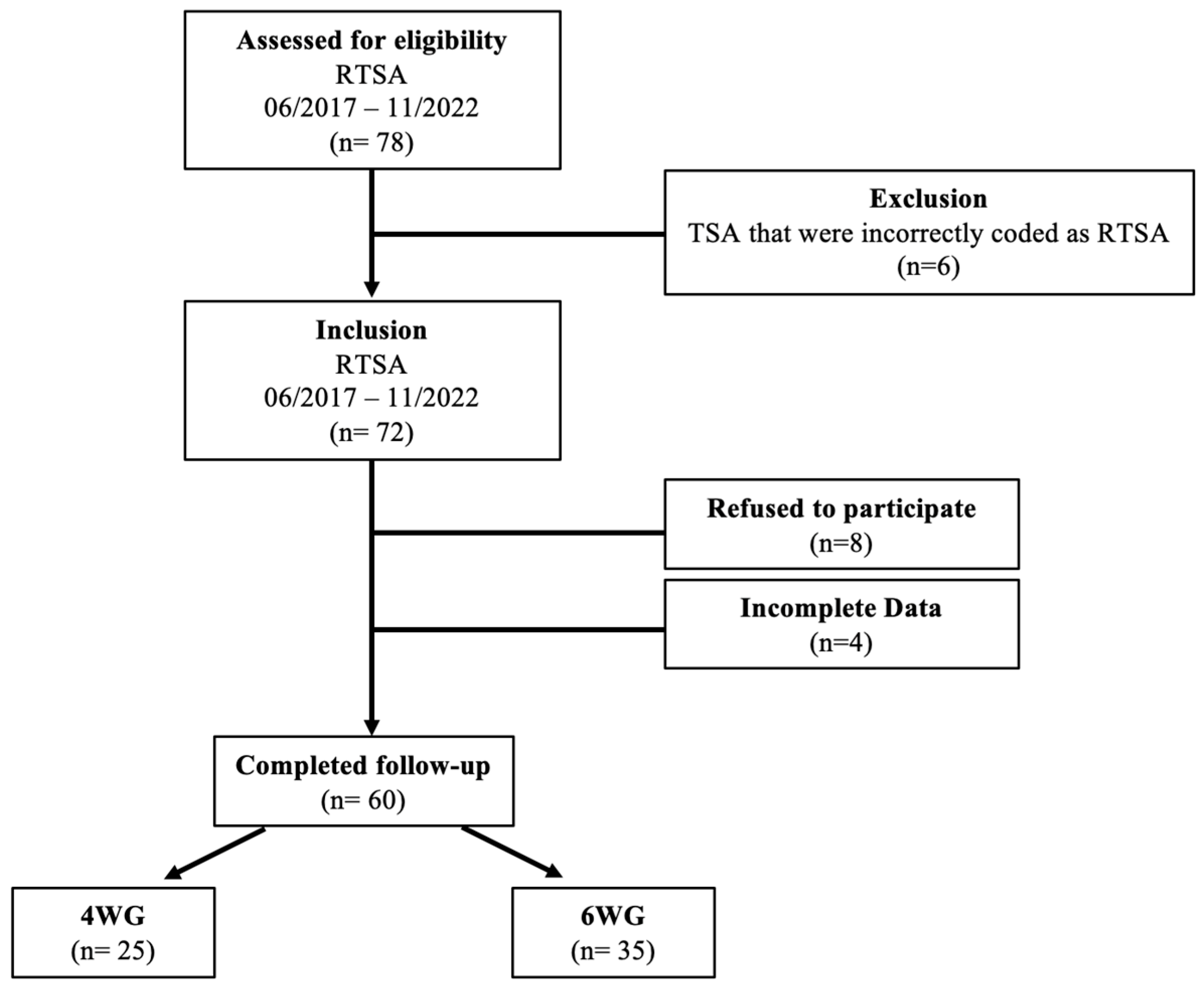
2.1. Patient Selection
2.2. Surgical Management
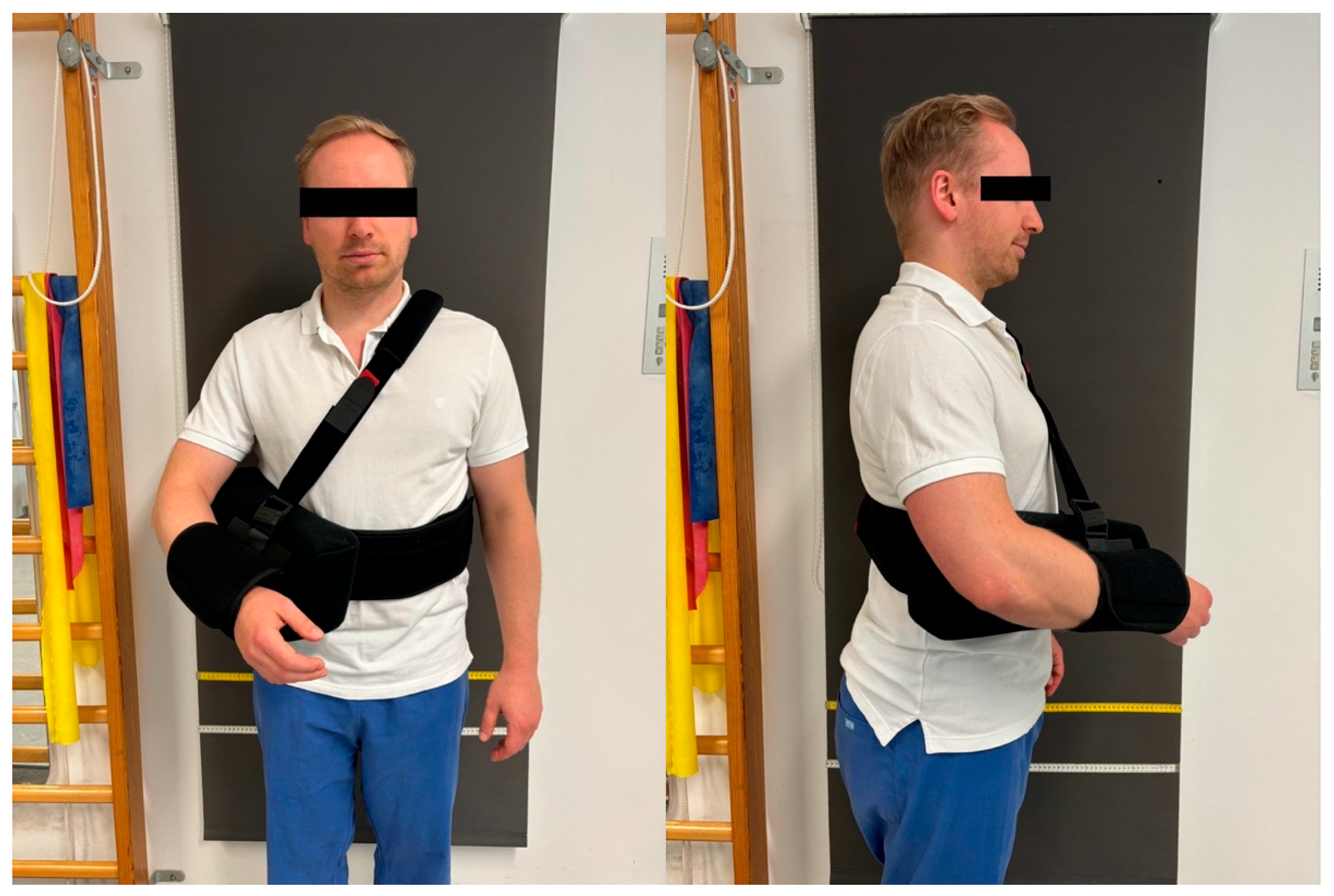
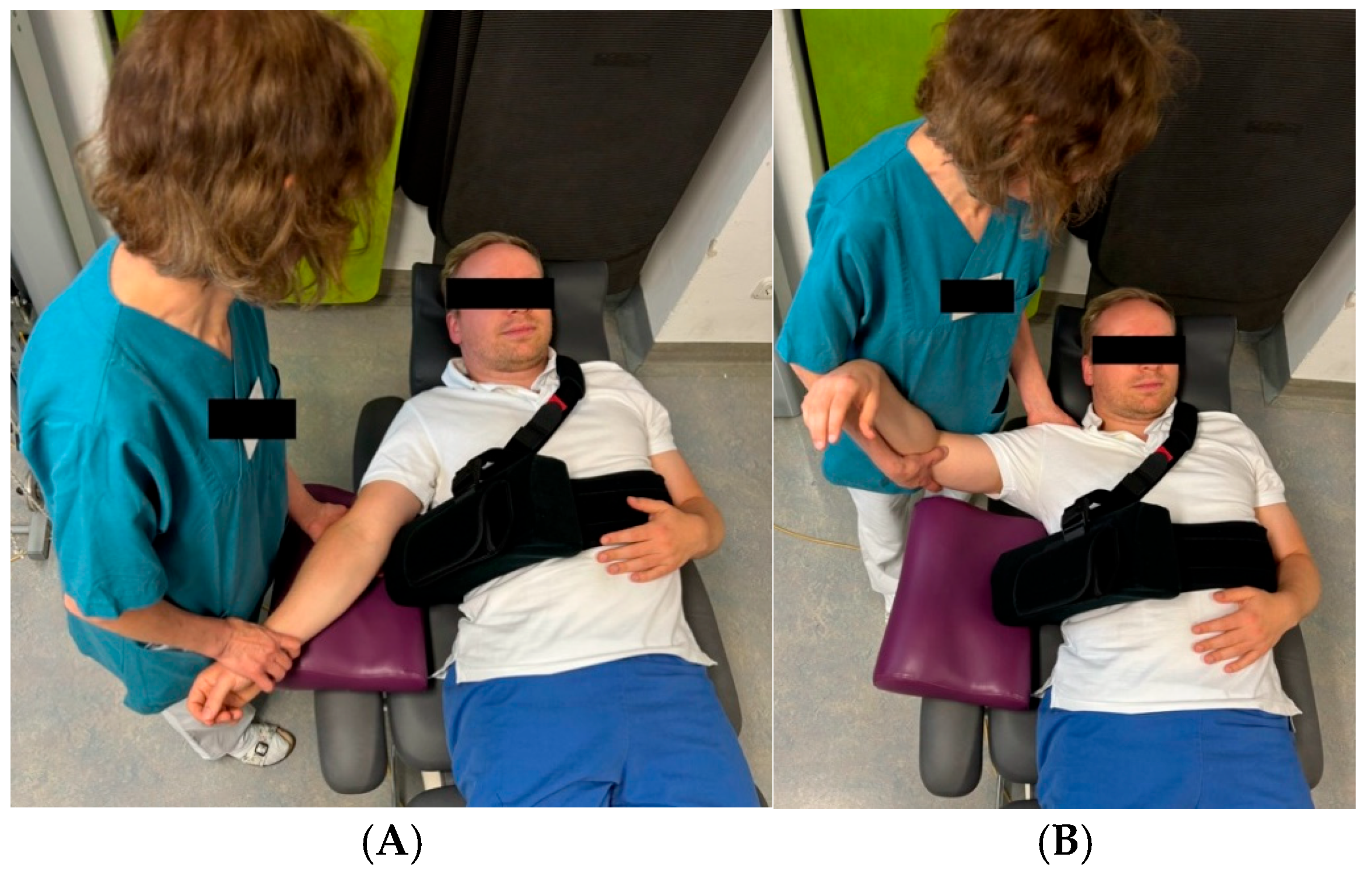
2.3. Postoperative Rehabilitation
2.4. Outcome Evaluation
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
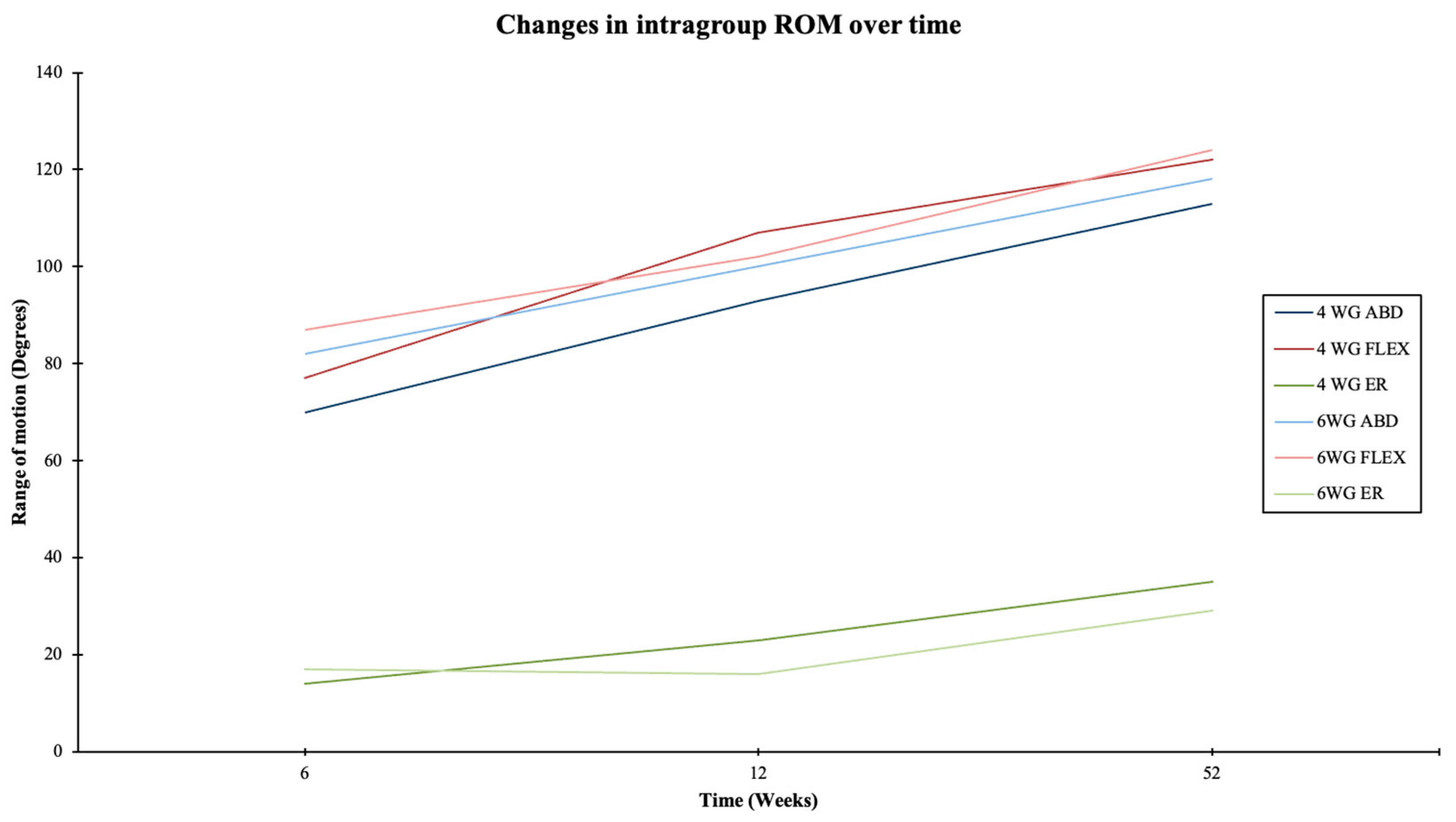
3.2. Clinical Results and Functional Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franceschi, F.; Giovannetti de Sanctis, E.; Gupta, A.; Athwal, G.S.; Di Giacomo, G. Reverse shoulder arthroplasty: State-of-the-art. J. ISAKOS 2023, 8, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Watkinson, D.; Hatzidakis, A.M.; Hovorka, I. Neer Award 2005: The Grammont reverse shoulder prosthesis: Results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J. Shoulder Elb. Surg. 2006, 15, 527–540. [Google Scholar] [CrossRef]
- Flatow, E.L.; Harrison, A.K. A history of reverse total shoulder arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 2432–2439. [Google Scholar] [CrossRef]
- Boudreau, S.; Boudreau, E.D.; Higgins, L.D.; Wilcox, R.B. 3rd. Rehabilitation following reverse total shoulder arthroplasty. J. Orthop. Sports Phys. Ther. 2007, 37, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Kwaees, T.A.; Charalambous, C.P. Reverse shoulder arthroplasty--minimum age for surgery, postoperative rehabilitation and long term restrictions. A delphi consensus study. Ortop. Traumatol. Rehabil. 2014, 16, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.M.; Oliva, F.; Nastrucci, G.; Casillo, P.; Di Giunta, A.; Susanna, M.; Ascione, F. Reverse shoulder arthroplasty patient personalized rehabilitation protocol. Preliminary results according to prognostic groups. Muscles Ligaments Tendons J. 2017, 7, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.L.; Rosenzweig, L. Anatomical and biomechanical framework for shoulder arthroplasty rehabilitation. J. Hand Ther. 2017, 30, 167–174. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Clark, N.J.; Assenmacher, A.T.; Samuelsen, B.T.; Sanchez-Sotelo, J.; Cofield, R.H.; Sperling, J.W. What Are the Complications, Survival, and Outcomes After Revision to Reverse Shoulder Arthroplasty in Patients Older Than 80 Years? Clin. Orthop. Relat. Res. 2017, 475, 2744–2751. [Google Scholar] [CrossRef]
- Clark, J.C.; Ritchie, J.; Song, F.S.; Kissenberth, M.J.; Tolan, S.J.; Hart, N.D.; Hawkins, R.J. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J. Shoulder Elb. Surg. 2012, 21, 36–41. [Google Scholar] [CrossRef]
- Farshad, M.; Gerber, C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int. Orthop. 2010, 34, 1075–1082. [Google Scholar] [CrossRef]
- Hagen, M.S.; Allahabadi, S.; Zhang, A.L.; Feeley, B.T.; Grace, T.; Ma, C.B. A randomized single-blinded trial of early rehabilitation versus immobilization after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Bullock, G.S.; Garrigues, G.E.; Ledbetter, L.; Kennedy, J. A Systematic Review of Proposed Rehabilitation Guidelines Following Anatomic and Reverse Shoulder Arthroplasty. J. Orthop. Sports Phys. Ther. 2019, 49, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hobden, E.; Stiell, I.G.; Wells, G.A. Clinically important change in the visual analog scale after adequate pain control. Acad. Emerg. Med. 2003, 10, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Michener, L.A.; McClure, P.W.; Sennett, B.J. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: Reliability, validity, and responsiveness. J. Shoulder Elb. Surg. 2002, 11, 587–594. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Hung, M.; Keener, J.D.; Bowen, R.C.; McAllister, J.; Chen, W.; Ebersole, G.; Granger, E.K.; Chamberlain, A.M. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 144–148. [Google Scholar] [CrossRef]
- Werner, B.C.; Chang, B.; Nguyen, J.T.; Dines, D.M.; Gulotta, L.V. What Change in American Shoulder and Elbow Surgeons Score Represents a Clinically Important Change After Shoulder Arthroplasty? Clin. Orthop. Relat. Res. 2016, 474, 2672–2681. [Google Scholar] [CrossRef]
- Blacknall, J.; Neumann, L. Rehabilitation following Reverse Total Shoulder Replacement. Shoulder Elb. 2011, 3, 232–240. [Google Scholar] [CrossRef]
- Brander, V.A.; Hinderer, S.R.; Alpiner, N.; Oh, T.H. Rehabilitation in joint and connective tissue diseases. 3. Limb disorders. Arch. Phys. Med. Rehabil. 1995, 76, S47–S56. [Google Scholar] [CrossRef]
- Pierre, P.S.; Frankle, M. Shoulder rehabilitation: Is there a role for home therapy? In Physical Therapy: Theory, Practices and Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 109–126. [Google Scholar]
- Edwards, P.K.; Ebert, J.R.; Joss, B.; Ackland, T.; Wang, A. A randomised trial comparing two rehabilitation approaches following reverse total shoulder arthroplasty. Shoulder Elb. 2021, 13, 557–572. [Google Scholar] [CrossRef]
- Denard, P.J.; Ladermann, A. Immediate versus delayed passive range of motion following total shoulder arthroplasty. J. Shoulder Elb. Surg. 2016, 25, 1918–1924. [Google Scholar] [CrossRef]
- Engel, N.M.; Holschen, M.; Schorn, D.; Witt, K.A.; Steinbeck, J. Results after primary reverse shoulder arthroplasty with and without subscapularis repair: A prospective-randomized trial. Arch. Orthop. Trauma. Surg. 2023, 143, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Alta, T.D.; Veeger, H.E.; Janssen, T.W.; Willems, W.J. Are shoulders with a reverse shoulder prosthesis strong enough? A pilot study. Clin. Orthop. Relat. Res. 2012, 470, 2185–2192. [Google Scholar] [CrossRef]
- Collin, P.; Matsukawa, T.; Denard, P.J.; Gain, S.; Ladermann, A. Pre-operative factors influence the recovery of range of motion following reverse shoulder arthroplasty. Int. Orthop. 2017, 41, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Ackland, D.C.; Roshan-Zamir, S.; Richardson, M.; Pandy, M.G. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J. Bone Jt. Surg. Am. 2010, 92, 1221–1230. [Google Scholar] [CrossRef]
- Cheung, E.; Willis, M.; Walker, M.; Clark, R.; Frankle, M.A. Complications in reverse total shoulder arthroplasty. J. Am. Acad. Orthop. Surg. 2011, 19, 439–449. [Google Scholar] [CrossRef]
- Chalmers, P.N.; Rahman, Z.; Romeo, A.A.; Nicholson, G.P. Early dislocation after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2014, 23, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.V.; Sarkissian, E.J.; Sox-Harris, A.; Comer, G.C.; Saleh, J.R.; Diaz, R.; Costouros, J.G. Instability after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 1946–1952. [Google Scholar] [CrossRef]
- Terrier, A.; Scheuber, P.; Pioletti, D.P.; Farron, A. Activities of daily living with reverse prostheses: Importance of scapular compensation for functional mobility of the shoulder. J. Shoulder Elb. Surg. 2013, 22, 948–953. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Samitier, G.; Torrens, C.; Wright, T.W. Reverse shoulder arthroplasty. Part 2: Systematic review of reoperations, revisions, problems, and complications. Int. J. Shoulder Surg. 2015, 9, 60–67. [Google Scholar] [CrossRef]
- Howard, M.C.; Trasolini, N.A.; Waterman, B.R. Optimizing Outcomes After Reverse Total Shoulder Arthroplasty: Rehabilitation, Expected Outcomes, and Maximizing Return to Activities. Curr. Rev. Musculoskelet. Med. 2023, 16, 145–153. [Google Scholar] [CrossRef]
- Rooney, S.I.; Loro, E.; Sarver, J.J.; Peltz, C.D.; Hast, M.W.; Tseng, W.J.; Kuntz, A.F.; Liu, X.S.; Khurana, T.S.; Soslowsky, L.J. Exercise protocol induces muscle, tendon, and bone adaptations in the rat shoulder. Muscles Ligaments Tendons J. 2014, 4, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Vourazeris, J.D.; Wright, T.W.; Struk, A.M.; King, J.J.; Farmer, K.W. Primary reverse total shoulder arthroplasty outcomes in patients with subscapularis repair versus tenotomy. J. Shoulder Elb. Surg. 2017, 26, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Dines, D.M.; Warren, R.F.; Altchek, D.W.; Moeckel, B. Posttraumatic changes of the proximal humerus: Malunion, nonunion, and osteonecrosis. Treatment with modular hemiarthroplasty or total shoulder arthroplasty. J. Shoulder Elb. Surg. 1993, 2, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bindi, V.E.; Buchanan, T.R.; Cueto, R.J.; Hones, K.M.; Wright, T.W.; Schoch, B.S.; King, J.J.; Hao, K.A. Mitigating the Risk of Instability After Reverse Shoulder Arthroplasty: A Critical Analysis Review of Patient and Surgical Factors. JBJS Rev. 2024, 12, e24.00095. [Google Scholar] [CrossRef]
- Mahendraraj, K.A.; Abboud, J.; Armstrong, A.; Austin, L.; Brolin, T.; Entezari, V.; Friedman, L.; Garrigues, G.E.; Grawe, B.; Gulotta, L. Predictors of acromial and scapular stress fracture after reverse shoulder arthroplasty: A study by the ASES Complications of RSA Multicenter Research Group. J. Shoulder Elb. Surg. 2021, 30, 2296–2305. [Google Scholar] [CrossRef]
- Padegimas, E.M.; Zmistowski, B.M.; Restrepo, C.; Abboud, J.A.; Lazarus, M.D.; Ramsey, M.L.; Williams, G.R.; Namdari, S. Instability After Reverse Total Shoulder Arthroplasty: Which Patients Dislocate? Am. J. Orthop. 2016, 45, E444–E450. [Google Scholar] [PubMed]
- Parsons, M.; Elwell, J.; Muh, S.; Wright, T.; Flurin, P.; Zuckerman, J.; Roche, C. Impact of accumulating risk factors on the incidence of dislocation after primary reverse total shoulder arthroplasty using a medial glenoid-lateral humerus onlay prosthesis. J. Shoulder Elb. Surg. 2024, 33, 1781–1788. [Google Scholar] [CrossRef]
- Shannon, S.F.; Wagner, E.R.; Houdek, M.T.; Cross, W.W., 3rd; Sanchez-Sotelo, J. Reverse shoulder arthroplasty for proximal humeral fractures: Outcomes comparing primary reverse arthroplasty for fracture versus reverse arthroplasty after failed osteosynthesis. J. Shoulder Elb. Surg. 2016, 25, 1655–1660. [Google Scholar] [CrossRef]
- Garofalo, R.; Fontanarosa, A.; Lassandro, N.; De Crescenzo, A. Reverse Total Shoulder Arthroplasty with a Cementless and Metaphyseal Stem Fixation Is a Viable Option for the Treatment of Proximal Humeral Fractures with Calcar Involvement. J. Clin. Med. 2023, 12, 1443. [Google Scholar] [CrossRef]
- King, J.J.; Farmer, K.W.; Struk, A.M.; Wright, T.W. Uncemented versus cemented humeral stem fixation in reverse shoulder arthroplasty. Int. Orthop. 2015, 39, 291–298. [Google Scholar] [CrossRef]
- Mazaleyrat, M.; Favard, L.; Garaud, P.; Boileau, P.; Berhouet, J. Press-fit vs. cemented humeral stem fixation for reverse shoulder arthroplasty: Functional outcomes at a mean follow-up of 9.5 years. J. Shoulder Elb. Surg. 2021, 30, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Salesky, M.A.; Grace, T.R.; Feeley, B.T.; Ma, C.B.; Zhang, A.L. Effects of cemented versus press-fit primary humeral stem fixation in the setting of revision shoulder arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Matsen, F.A., 3rd; Iannotti, J.P.; Rockwood, C.A., Jr. Humeral fixation by press-fitting of a tapered metaphyseal stem: A prospective radiographic study. J. Bone Jt. Surg. Am. 2003, 85, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Guarrella, V.; Chelli, M.; Domos, P.; Ascione, F.; Boileau, P.; Walch, G. Risk factors for instability after reverse shoulder arthroplasty. Shoulder Elb. 2021, 13, 51–57. [Google Scholar] [CrossRef]
- Pena, L.; Pena, J.; Lopez-Anglada, E.; Brana, A.F. Instability after reverse total shoulder arthroplasty: Risk factors and how to avoid them. Acta Orthop. Belg. 2022, 88, 372–379. [Google Scholar] [CrossRef]
- Choi, S.; Bae, J.-H.; Kwon, Y.S.; Kang, H. Clinical outcomes and complications of cementless reverse total shoulder arthroplasty during the early learning curve period. J. Orthop. Surg. Res. 2019, 14, 53. [Google Scholar] [CrossRef]
- Doyle, T.R.; Downey, S.; Hurley, E.T.; Klifto, C.; Mullett, H.; Denard, P.J.; Garrigues, G.E.; Menendez, M.E. Midterm outcomes of primary reverse shoulder arthroplasty: A systematic review of studies with minimum 5-year follow-up. JSES Rev. Rep. Tech. 2024, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simovitch, R.W.; Friedman, R.J.; Cheung, E.V.; Flurin, P.H.; Wright, T.; Zuckerman, J.D.; Roche, C. Rate of Improvement in Clinical Outcomes with Anatomic and Reverse Total Shoulder Arthroplasty. J. Bone Jt. Surg. Am. 2017, 99, 1801–1811. [Google Scholar] [CrossRef]
- Mulieri, P.; Dunning, P.; Klein, S.; Pupello, D.; Frankle, M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J. Bone Jt. Surg. Am. 2010, 92, 2544–2556. [Google Scholar] [CrossRef]
- Steen, B.M.; Cabezas, A.F.; Santoni, B.G.; Hussey, M.M.; Cusick, M.C.; Kumar, A.G.; Frankle, M.A. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: A matched cohort. J. Shoulder Elb. Surg. 2015, 24, 1433–1441. [Google Scholar] [CrossRef]
- Uschok, S.; Herrmann, S.; Pauly, S.; Perka, C.; Greiner, S. Reverse shoulder arthroplasty: The role of physical therapy on the clinical outcome in the mid-term to long-term follow-up. Arch. Orthop. Trauma. Surg. 2018, 138, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Cuff, D.; Pupello, D.; Virani, N.; Levy, J.; Frankle, M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J. Bone Jt. Surg. Am. 2008, 90, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Mollon, B.; Mahure, S.A.; Roche, C.P.; Zuckerman, J.D. Impact of glenosphere size on clinical outcomes after reverse total shoulder arthroplasty: An analysis of 297 shoulders. J. Shoulder Elb. Surg. 2016, 25, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Adomaviciene, A.; Daunoraviciene, K.; Sidlauskaite, R.; Griskevicius, J.; Kubilius, R.; Varzaityte, L.; Raistenskis, J. Analysis of Functional Recovery and Subjective Well-Being after Arthroscopic Rotator Cuff Repair. Medicina 2021, 57, 715. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Sierra, J.; Santaren-Rosell, M.; Albeniz, A.P.; Fonseca-Pedrero, E. Dimensional structure of the Spanish version of the Positive and Negative Affect Schedule (PANAS) in adolescents and young adults. Psychol. Assess. 2015, 27, e1–e9. [Google Scholar] [CrossRef]
- Bolcato, V.; Franzetti, C.; Fassina, G.; Basile, G.; Martinez, R.M.; Tronconi, L.P. Comparative study on informed consent regulation in health care among Italy, France, United Kingdom, Nordic Countries, Germany, and Spain. J. Forensic Leg. Med. 2024, 103, 102674. [Google Scholar] [CrossRef]

| Parameter | 4 WG | 6 WG | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean/ Median | ±SD/ IQR | n (%) | Mean/ Median | ±SD/ IQR | |||
| Age (years) | 59.7 | 7.0 | 75.5 | 5.7 | 0.198 | |||
| Sex | 0.777 | |||||||
| Male | 7 (28.0) | 11 (31.4) | ||||||
| Female | 18 (72.0) | 24 (68.6) | ||||||
| BMI (kg/m2) | 28.5 | 4.4 | 27.4 | 7.8 | 0.828 | |||
| Diagnosis | 0.535 | |||||||
| CTA | 10 (40.0) | 19 (54.3) | ||||||
| OA | 13 (52.0) | 11 (31.4) | ||||||
| Pathological fracture | 1 (4.0) | 1 (2.9) | ||||||
| Chronic instability | 0 (0.0) | 2 (5.7) | ||||||
| Humeral head necrosis | 0 (0.0) | 2 (5.7) | ||||||
| Infection | 1 (4.0) | 0 (0.0) | ||||||
| Implant type | 0.103 | |||||||
| RTSA | 5 (20.0) | 14 (40.0) | ||||||
| BioRTSA | 20 (80.0) | 21 (60.0) | ||||||
| Side | 0.862 | |||||||
| Left | 12 (48.0) | 16 (45.7) | ||||||
| Right | 13 (52.0) | 19 (54.3) | ||||||
| Fixation | 0.454 | |||||||
| Cemented | 3 (12.0) | 7 (20.0) | ||||||
| Press-fit | 22 (88.0) | 28 (80.0) | ||||||
| 4 WG | 6 WG | p-Value | |||
|---|---|---|---|---|---|
| 6 Weeks Postop | Mean/Median | ±SD/IQR | Mean/Median | ±SD/IQR | |
| ABD | 80 | 30 | 90 | 20 | 0.061 |
| FLEX | 80 | 30 | 90 | 20 | 0.229 |
| ER | 10 | 15 | 20 | 20 | 0.295 |
| IR | 80 | 43 | 80 | 0 | 0.858 |
| ASES | 54 | 10 | 57 | 11 | 0.295 |
| SST | 2 | 1 | 2 | 2 | 0.067 |
| VAS | 0 | 2 | 2 | 2 | 0.001 * |
| SWB | 0 | 1 | 1 | 1 | 0.007 * |
| 3 months postop | |||||
| ABD | 90 | 20 | 90 | 20 | 0.301 |
| FLEX | 110 | 35 | 95 | 20 | 0.311 |
| ER | 20 | 28 | 20 | 10 | 0.157 |
| IR | 80 | 30 | 85 | 10 | 0.264 |
| ASES | 65 | 13 | 57 | 12 | 0.023 * |
| SST | 4 | 5 | 3 | 2 | 0.698 |
| VAS | 0 | 2 | 2 | 1 | 0.012 * |
| SWB | 0 | 1 | 1 | 0 | 0.007 * |
| 1 year postop | |||||
| ABD | 113 | 37 | 118 | 30 | 0.327 |
| FLEX | 122 | 31 | 124 | 29 | 0.390 |
| ER | 30 | 13 | 30 | 20 | 0.054 |
| IR | 90 | 0 | 90 | 20 | 0.337 |
| ASES | 90 | 19 | 83 | 12 | 0.071 |
| SST | 9 | 6 | 7 | 2 | 0.093 |
| VAS | 0 | 0 | 0 | 1 | 0.335 |
| SWB | 0 | 1 | 0 | 1 | 0.831 |
| 6 WG | Mean | SD | Mauchly | p-Value | Epsilon | Greenhouse–Geisser p-Value | |
|---|---|---|---|---|---|---|---|
| Direction | FU | ||||||
| ABD | 0.034 | 0.883 | <0.001 | ||||
| 6 weeks | 82 | 20 | |||||
| 3 months | 100 | 26 | |||||
| 1 year | 118 | 30 | |||||
| FLEX | 0.011 | 0.843 | <0.001 | ||||
| 6 weeks | 87 | 18 | |||||
| 3 months | 102 | 19 | |||||
| 1 year | 124 | 29 | |||||
| ER | |||||||
| 6 weeks | 17 | 12 | 0.248 | <0.001 | |||
| 3 months | 16 | 9 | |||||
| 1 year | 29 | 14 | |||||
| IR | |||||||
| 6 weeks | 78 | 13 | 0.154 | <0.001 | |||
| 3 months | 80 | 15 | |||||
| 1 year | 91 | 14 |
| 4 WG | Mean | SD | Mauchly | p-Value | |
|---|---|---|---|---|---|
| Direction | FU | ||||
| ABD | 0.543 | <0.001 | |||
| 6 weeks | 70 | 23 | |||
| 3 months | 93 | 27 | |||
| 1 year | 113 | 37 | |||
| FLEX | 0.525 | <0.001 | |||
| 6 weeks | 77 | 27 | |||
| 3 months | 107 | 28 | |||
| 1 year | 122 | 31 | |||
| ER | 0.449 | <0.001 | |||
| 6 weeks | 14 | 11 | |||
| 3 months | 23 | 17 | |||
| 1 year | 35 | 9 | |||
| IR | 0.442 | 0.008 | |||
| 6 weeks | 71 | 22 | |||
| 3 months | 76 | 15 | |||
| 1 year | 85 | 16 |
| 4 WG | Mean | SD | Mauchly | p-Value | |
|---|---|---|---|---|---|
| Score | FU | ||||
| ASES | 0.088 | <0.001 | |||
| 6 weeks | 53.7 | 9.8 | |||
| 3 months | 64.6 | 13.3 | |||
| 1 year | 83.5 | 16.1 | |||
| SST | 0.054 | <0.001 | |||
| 6 weeks | 2.6 | 1.3 | |||
| 3 months | 4.0 | 2.3 | |||
| 1 year | 8.0 | 3.2 | |||
| VAS | 0.133 | 0.291 | |||
| 6 weeks | 0.8 | 1.3 | |||
| 3 months | 0.8 | 1.2 | |||
| 1 year | 0.4 | 1.0 | |||
| SWB | Friedman | 0.107 | |||
| 6 weeks | 0.3 | 0.5 | |||
| 3 months | 0.6 | 0.9 | |||
| 1 year | 0.4 | 0.8 |
| 6 WG | Mean | SD | Mauchly | p-Value | |
|---|---|---|---|---|---|
| Score | FU | ||||
| ASES | 0.530 | <0.001 | |||
| 6 weeks | 56.6 | 11.2 | |||
| 3 months | 56.9 | 12.1 | |||
| 1 year | 80.1 | 12.1 | |||
| SST | 0.956 | <0.001 | |||
| 6 weeks | 2.1 | 1.2 | |||
| 3 months | 3.8 | 1.9 | |||
| 1 year | 7.1 | 2.1 | |||
| VAS | 0.691 | <0.001 | |||
| 6 weeks | 2.1 | 1.4 | |||
| 3 months | 1.5 | 1.1 | |||
| 1 year | 0.6 | 1.2 | |||
| SWB | Friedman | <0.001 | |||
| 6 weeks | 0.7 | 0.5 | |||
| 3 months | 1.1 | 0.6 | |||
| 1 year | 0.4 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochberger, F.; Wild, M.F.; Heinz, T.; Rudert, M.; List, K. Comparative Study on Postoperative Immobilization in Reverse Total Shoulder Arthroplasty: 4 Weeks vs. 6 Weeks of Immobilization Yields Similar Clinical and Functional Outcomes. J. Clin. Med. 2024, 13, 6363. https://doi.org/10.3390/jcm13216363
Hochberger F, Wild MF, Heinz T, Rudert M, List K. Comparative Study on Postoperative Immobilization in Reverse Total Shoulder Arthroplasty: 4 Weeks vs. 6 Weeks of Immobilization Yields Similar Clinical and Functional Outcomes. Journal of Clinical Medicine. 2024; 13(21):6363. https://doi.org/10.3390/jcm13216363
Chicago/Turabian StyleHochberger, Felix, Moritz Friedrich Wild, Tizian Heinz, Maximilian Rudert, and Kilian List. 2024. "Comparative Study on Postoperative Immobilization in Reverse Total Shoulder Arthroplasty: 4 Weeks vs. 6 Weeks of Immobilization Yields Similar Clinical and Functional Outcomes" Journal of Clinical Medicine 13, no. 21: 6363. https://doi.org/10.3390/jcm13216363
APA StyleHochberger, F., Wild, M. F., Heinz, T., Rudert, M., & List, K. (2024). Comparative Study on Postoperative Immobilization in Reverse Total Shoulder Arthroplasty: 4 Weeks vs. 6 Weeks of Immobilization Yields Similar Clinical and Functional Outcomes. Journal of Clinical Medicine, 13(21), 6363. https://doi.org/10.3390/jcm13216363






