Association of High-Risk Obstructive Sleep Apnea with Artificial Intelligence-Guided, CT-Based Severity Scores in Patients with COVID-19 Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Chest CT Scan Protocol and Assessment
2.2.1. Sample Size
2.2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19 Pandemic). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 26 May 2024).
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Fadel, R.A.; Malette, K.M.; Hammond, C.; Abdulla, H.; Entz, A.; Demertzis, Z.; Hanna, Z.; Failla, A.; Dagher, C.; et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw. Open 2020, 3, e2012270. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- McSharry, D.; Malhotra, A. Potential influences of obstructive sleep apnea and obesity on COVID-19 severity. J. Clin. Sleep. Med. 2020, 16, 1645. [Google Scholar] [CrossRef]
- Tufik, S.; Gozal, D.; Ishikura, I.A.; Pires, G.N.; Andersen, M.L. Does obstructive sleep apnea lead to increased risk of COVID-19 infection and severity? J. Clin. Sleep. Med. 2020, 16, 1425–1426. [Google Scholar] [CrossRef]
- Maas, M.B.; Kim, M.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Obstructive Sleep Apnea and Risk of COVID-19 Infection, Hospitalization and Respiratory Failure. Sleep. Breath. 2020, 25, 2105–2106. [Google Scholar] [CrossRef]
- Cade, B.E.; Dashti, H.S.; Hassan, S.M.; Redline, S.; Karlson, E.W. Sleep Apnea and COVID-19 Mortality and Hospitalization. Am. J. Respir. Crit. Care Med. 2020, 202, 1462–1464. [Google Scholar] [CrossRef]
- Peker, Y.; Celik, Y.; Arbatli, S.; Isik, S.R.; Balcan, B.; Karataş, F.; Uzel, F.I.; Tabak, L.; Çetin, B.; Baygül, A.; et al. Effect of High-Risk Obstructive Sleep Apnea on Clinical Outcomes in Adults with Coronavirus Disease 2019: A Multicenter, Prospective, Observational Clinical Trial. Ann. Am. Thorac Soc. 2021, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Baygül, A.; Peker, Y. Validation of the Modified Berlin Questionnaire for the Diagnosis of Obstructive Sleep Apnea in Patients with a History of COVID-19 Infection. J. Clin. Med. 2023, 12, 3047. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, K.; Min, J.K. Artificial Intelligence and Machine Learning in Cardiovascular Imaging. Methodist. Debakey Cardiovasc. J. 2020, 16, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef]
- Atceken, Z.; Celik, Y.; Atasoy, C.; Peker, Y. The Diagnostic Utility of Artificial Intelligence-Guided Computed Tomography-Based Severity Scores for Predicting Short-Term Clinical Outcomes in Adults with COVID-19 Pneumonia. J. Clin. Med. 2023, 12, 7039. [Google Scholar] [CrossRef]
- Demir, A.; Ardic, S.; Firat, H.; Karadeniz, D.; Aksu, M.; Ucar, Z.; Sevim, S.; Ozgen, F.; Yilmaz, H.; Itil, O.; et al. Prevalence of sleep disorders in the Turkish adult population epidemiology of sleep study. Sleep. Biol. Rhythm. 2015, 13, 298–308. [Google Scholar] [CrossRef]
- van Oosten, E.M.; Hamilton, A.; Petsikas, D.; Payne, D.; Redfearn, D.P.; Zhang, S.; Hopman, W.M.; Baranchuk, A. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 919–923. [Google Scholar] [CrossRef]
- Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253.
- Chamberlin, J.; Kocher, M.R.; Waltz, J.; Snoddy, M.; Stringer, N.F.C.; Stephenson, J.; Sahbaee, P.; Sharma, P.; Rapaka, S.; Schoepf, U.J.; et al. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for lung cancer screening: Accuracy and prognostic value. BMC Med. 2021, 19, 55. [Google Scholar] [CrossRef]
- Abadia, A.F.; Yacoub, B.; Stringer, N.; Snoddy, M.; Kocher, M.; Schoepf, U.J.; Aquino, G.J.; Kabakus, I.; Dargis, D.; Hoelzer, P.; et al. Diagnostic Accuracy and Performance of Artificial Intelligence in Detecting Lung Nodules in Patients With Complex Lung Disease: A Noninferiority Study. J. Thorac. Imaging 2022, 37, 154–161. [Google Scholar] [CrossRef]
- Arish, N.; Izbicki, G.; Rokach, A.; Jarjou’i, A.; Kalak, G.; Goldberg, S. Association of the risk of obstructive sleep apnoea with the severity of COVID-19. PLoS ONE 2023, 18, e0284063. [Google Scholar] [CrossRef] [PubMed]
- Breville, G.; Herrmann, F.; Adler, D.; Deffert, C.; Bommarito, G.; Stancu, P.; Accorroni, A.; Uginet, M.; Assal, F.; Tamisier, R.; et al. Obstructive sleep apnea: A major risk factor for COVID-19 encephalopathy? BMC Neurol. 2023, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Vicini, C.; Lechien, J.R.; Ravaglia, C.; Poletti, V.; di Cesare, S.; Amicarelli, E.; Gardelli, L.; Grosso, C.; Patacca, A.; et al. Association Between Severity of COVID-19 Respiratory Disease and Risk of Obstructive Sleep Apnea. Ear Nose Throat J. 2024, 103, Np10–NP15. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Harmon, S.A.; Sanford, T.H.; Xu, S.; Turkbey, E.B.; Roth, H.; Xu, Z.; Yang, D.; Myronenko, A.; Anderson, V.; Amalou, A.; et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020, 11, 4080. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.C.; Diao, K.Y.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat. Med. 2020, 26, 1224–1228. [Google Scholar] [CrossRef]
- Bernal-Ramirez, J.M.; Chavez-Barba, O.A.; Cobian-Machuca, H.; Delgado-Figueroa, N.; Martinez-Solano, L.F.; Aguirre-Diaz, S.A.; Gonzalez-Diaz, E.; Figueroa-Sanchez, M.; Alanis-Salazar10, R.M. Comparing the diagnostic performance of an artificial intelligence system with human readers in the tomographic evaluation of SARS-CoV-2 pneumonia.
- Kardos, A.S.; Simon, J.; Nardocci, C.; Szabó, I.V.; Nagy, N.; Abdelrahman, R.H.; Zsarnóczay, E.; Fejér, B.; Futácsi, B.; Müller, V.; et al. The diagnostic performance of deep-learning-based CT severity score to identify COVID-19 pneumonia. Br. J. Radiol. 2022, 95, 20210759. [Google Scholar] [CrossRef]
- Sezer, R.; Esendagli, D.; Erol, C.; Hekimoglu, K. New challenges for management of COVID-19 patients: Analysis of MDCT based “Automated pneumonia analysis program”. Eur. J. Radiol. Open 2021, 8, 100370. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Prosch, H.; Schaefer-Prokop, C.; Bohn, K.P.; Alberts, I.; Mingels, C.; Thurnher, M.; Cumming, P.; Shi, K.; Peters, A.; et al. A comprehensive review of imaging findings in COVID-19—Status in early 2021. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2500–2524. [Google Scholar] [CrossRef] [PubMed]
- Tasmi, S.T.; Raihan, M.M.S.; Shams, A.B. Obstructive sleep apnea (OSA) and COVID-19: Mortality prediction of COVID-19-infected patients with OSA using machine learning approaches. COVID 2022, 2, 877–894. [Google Scholar] [CrossRef]
- Kimura-Sandoval, Y.; Arevalo-Molina, M.E.; Cristancho-Rojas, C.N.; Kimura-Sandoval, Y.; Rebollo-Hurtado, V.; Licano-Zubiate, M.; Chapa-Ibargüengoitia, M.; Muüoz-López, G. Validation of chest computed tomography artificial intelligence to determine the requirement for mechanical ventilation and risk of mortality in hospitalized coronavirus disease-19 patients in a tertiary care center in Mexico City. Rev. Investig. Clínica 2021, 73, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, D. Artificial intelligence in health care. J. Paediatr. Child. Health 2022, 10, 1493–1495. [Google Scholar] [CrossRef]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]

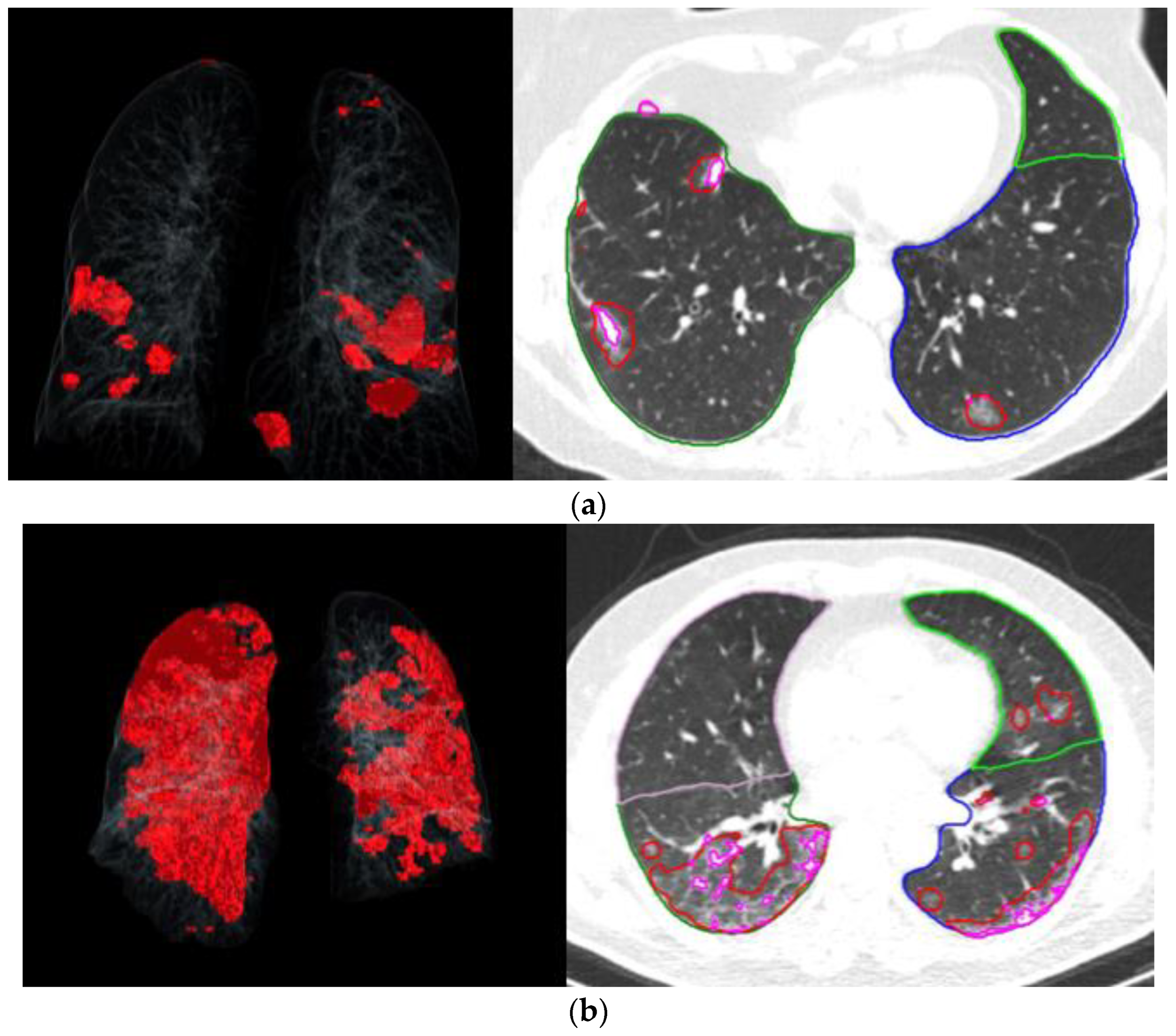
| Variables | <5 No or Minimal TOR N = 136 | ≥5, <15 Mild to Moderate TOR N = 54 | ≥15 Severe TOR N = 31 | p-Value |
|---|---|---|---|---|
| HR-OSA | 15 (11.0) | 12 (22.2) | 12 (38.7) | <0.001 |
| Demographic Characteristics Age ≥ 65 yrs | 15 (11.0) | 20 (37.0) | 8 (25.8) | <0.001 |
| Age, yrs | 48.1 (37.4–58.4) | 58.7 (51.5–72.0) | 56.4 (49.4–66.7) | <0.001 |
| Sex | 79 (58.1) | 30 (55.6) | 21 (67.7) | 0.526 |
| BMI, kg/m2 | 25.9 (24.0–29.9) | 29.0 (25.8–32.9) | 28.3 (25.8–31.2) | 0.001 |
| Comorbidities Current Smoking | 15 (11.9) | 4 (7.7) | 0 (0.0) | 0.123 |
| Obesity | 34 (25.0) | 24 (44.4) | 10 (32.3) | 0.032 |
| Hypertension | 32 (23.5) | 24 (44.4) | 19 (61.3) | <0.001 |
| Diabetes | 15 (11.0) | 14 (25.9) | 3 (9.7) | 0.022 |
| CAD | 5 (3.7) | 8 (14.8) | 4 (12.9) | 0.017 |
| CHF | 1 (0.7) | 0 (0.0) | 2 (6.5) | 0.028 |
| Atrial Fibrillation | 1 (0.7) | 1 (1.9) | 1 (3.2) | 0.522 |
| Cardiac Disease | 6 (4.4) | 9 (16.7) | 7 (22.6) | 0.002 |
| COPD | 3 (2.2) | 1 (1.9) | 1 (3.2) | 0.917 |
| Asthma | 3 (2.2) | 1 (1.9) | 1 (3.2) | 0.917 |
| Lung Disease | 6 (4.4) | 2 (3.7) | 2 (6.5) | 0.837 |
| Cerebrovascular Disease | 0 (0.0) | 0 (0.0) | 3 (9.7) | <0.001 |
| Dementia | 1 (0.7) | 2 (3.7) | 0 (0.0) | 0.219 |
| Psychiatric Disease | 2 (1.5) | 1 (1.9) | 0 (0.0) | 0.764 |
| Known OSA | 0 (0.0) | 1 (1.9) | 1 (3.2) | 0.162 |
| Malignancy | 6 (4.4) | 3 (5.6) | 0 (0.0) | 0.436 |
| Chronic Kidney Disease | 2 (1.5) | 3 (5.6) | 2 (6.5) | 0.185 |
| Hyperlipidemia | 2 (1.5) | 6 (11.1) | 2 (6.5) | 0.013 |
| Hypothyroidism | 7 (5.1) | 5 (9.3) | 3 (9.7) | 0.470 |
| Hospitalization | 80 (58.8) | 43 (79.6) | 29 (93.5) | <0.001 |
| Supplemental Oxygen | 17 (12.5) | 17 (31.5) | 24 (77.4) | <0.001 |
| ICU | 4 (2.9) | 4 (7.4) | 14 (6.3) | 0.003 |
| Drug Treatment Drug treatment for COVID | 91 (91.2) | 53 (98.1) | 30 (96.8) | 0.153 |
| Statins | 3 (2.2) | 9 (16.7) | 2 (6.5) | 0.001 |
| Immune Suppressive | 3 (2.2) | 0 (0.0) | 0 (0.0) | 0.387 |
| Chloroquine | 109 (80.1) | 50 (92.6) | 29 (93.5) | 0.034 |
| Azithromycin | 57 (41.9) | 36 (66.7) | 17 (54.8) | 0.007 |
| Favipiravir | 21 (15.4) | 12 (22.2) | 15 (48.4) | <0.001 |
| Oseltamivir | 30 (22.1) | 12 (22.2) | 15 (48.4) | 0.008 |
| Ritonavir/Lopinavir | 3 (2.2) | 2 (3.7) | 5 (16.1) | 0.003 |
| Tocilizumab | 9 (6.6) | 11 (20.4) | 10 (32.3) | <0.001 |
| Systemic Steroids | 1 (0.7) | 2 (3.7) | 0 (0.0) | 0.219 |
| Anticoagulant | 54 (39.7) | 34 (63.0) | 19 (61.3) | 0.005 |
| Odds Ratio | 95% CI for Odds Ratio | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.034 | 1.008 | 1.060 | 0.01 |
| BMI | 1.032 | 0.959 | 1.110 | 0.40 |
| Male sex | 1.561 | 0.697 | 3.494 | 0.28 |
| Hypertension | 3.789 | 1.724 | 8.324 | <0.001 |
| Diabetes mellitus | 0.595 | 0.170 | 2.086 | 0.41 |
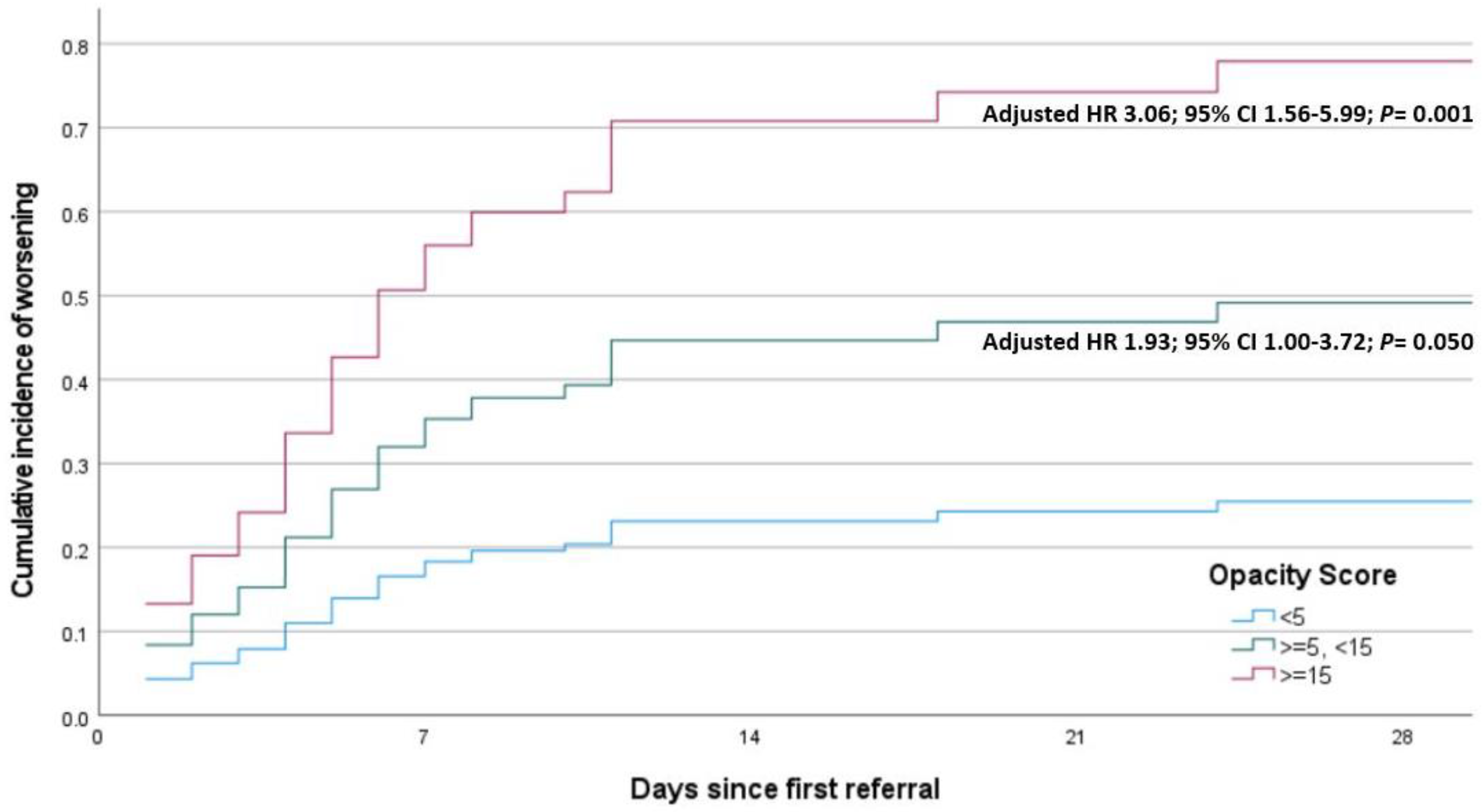
| HR-OSA | 3.813 | 1.663 | 8.740 | 0.002 |
| Odds Ratio | 95% CI for Odds Ratio | p-Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.016 | 0.986 | 1.047 | 0.30 |
| BMI | 0.979 | 0.899 | 1.066 | 0.62 |
| Male sex | 1.464 | 0.626 | 3.428 | 0.38 |
| Hypertension | 2.926 | 1.216 | 7.043 | 0.017 |
| HR-OSA | 3.068 | 1.265 | 7.440 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atceken, Z.; Celik, Y.; Atasoy, C.; Peker, Y. Association of High-Risk Obstructive Sleep Apnea with Artificial Intelligence-Guided, CT-Based Severity Scores in Patients with COVID-19 Pneumonia. J. Clin. Med. 2024, 13, 6415. https://doi.org/10.3390/jcm13216415
Atceken Z, Celik Y, Atasoy C, Peker Y. Association of High-Risk Obstructive Sleep Apnea with Artificial Intelligence-Guided, CT-Based Severity Scores in Patients with COVID-19 Pneumonia. Journal of Clinical Medicine. 2024; 13(21):6415. https://doi.org/10.3390/jcm13216415
Chicago/Turabian StyleAtceken, Zeynep, Yeliz Celik, Cetin Atasoy, and Yüksel Peker. 2024. "Association of High-Risk Obstructive Sleep Apnea with Artificial Intelligence-Guided, CT-Based Severity Scores in Patients with COVID-19 Pneumonia" Journal of Clinical Medicine 13, no. 21: 6415. https://doi.org/10.3390/jcm13216415
APA StyleAtceken, Z., Celik, Y., Atasoy, C., & Peker, Y. (2024). Association of High-Risk Obstructive Sleep Apnea with Artificial Intelligence-Guided, CT-Based Severity Scores in Patients with COVID-19 Pneumonia. Journal of Clinical Medicine, 13(21), 6415. https://doi.org/10.3390/jcm13216415








