Standardized 3D Transoesophageal Echocardiography Manoeuvre for Enhanced Tenting Height Evaluation During Transcatheter Mitral Valve Edge-to-Edge Repair
Abstract
1. Introduction
2. Materials and Methods
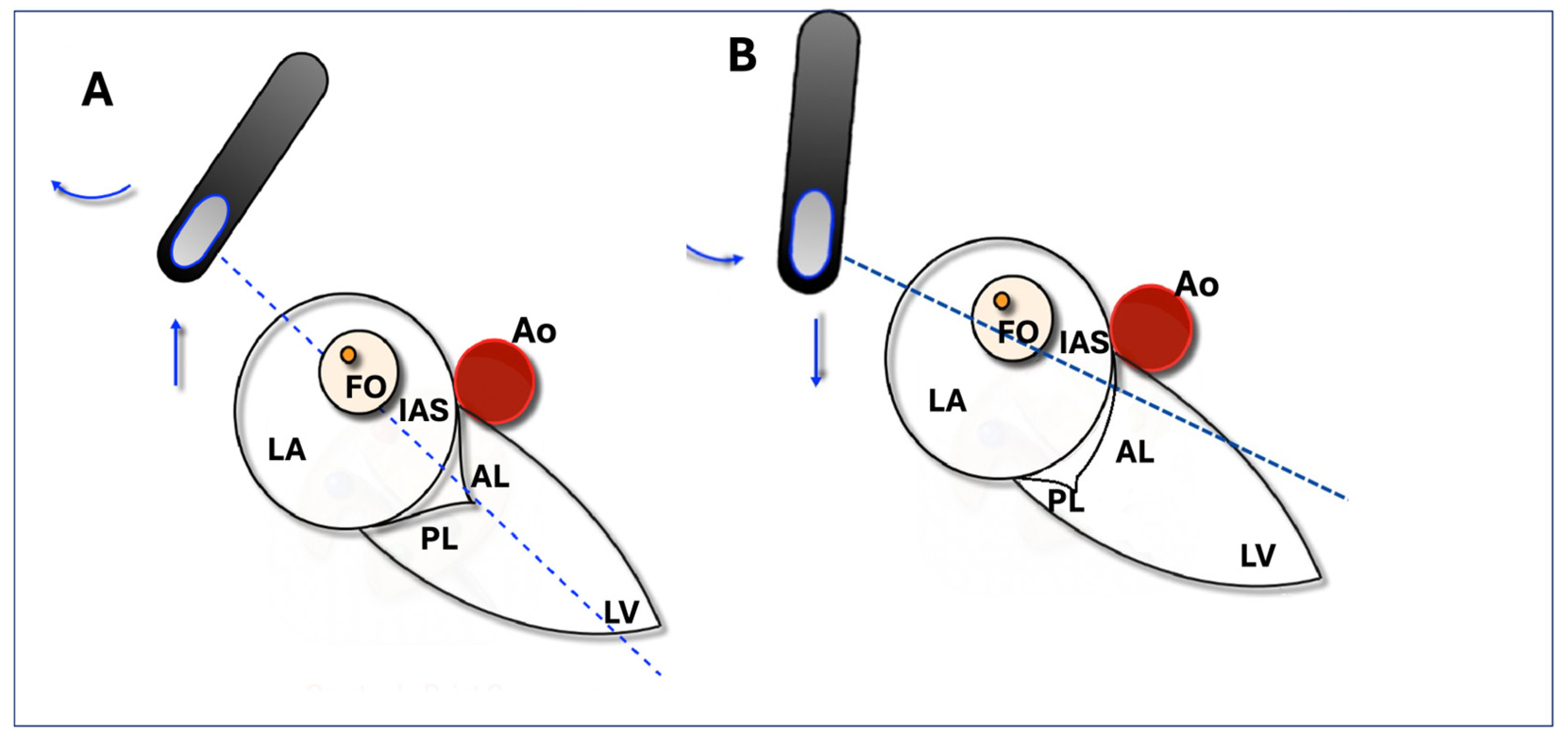
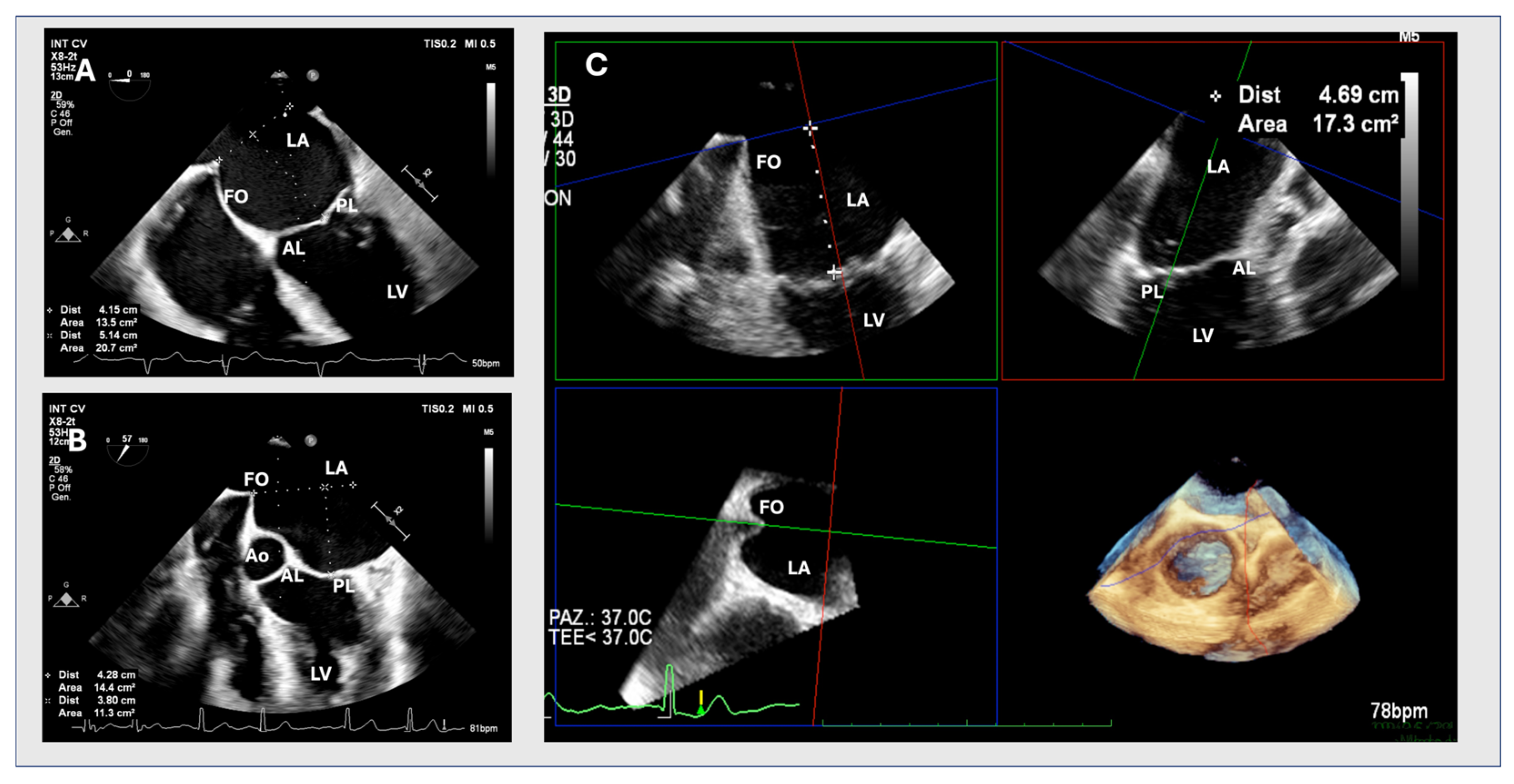
2.1. Two-Dimensional Method
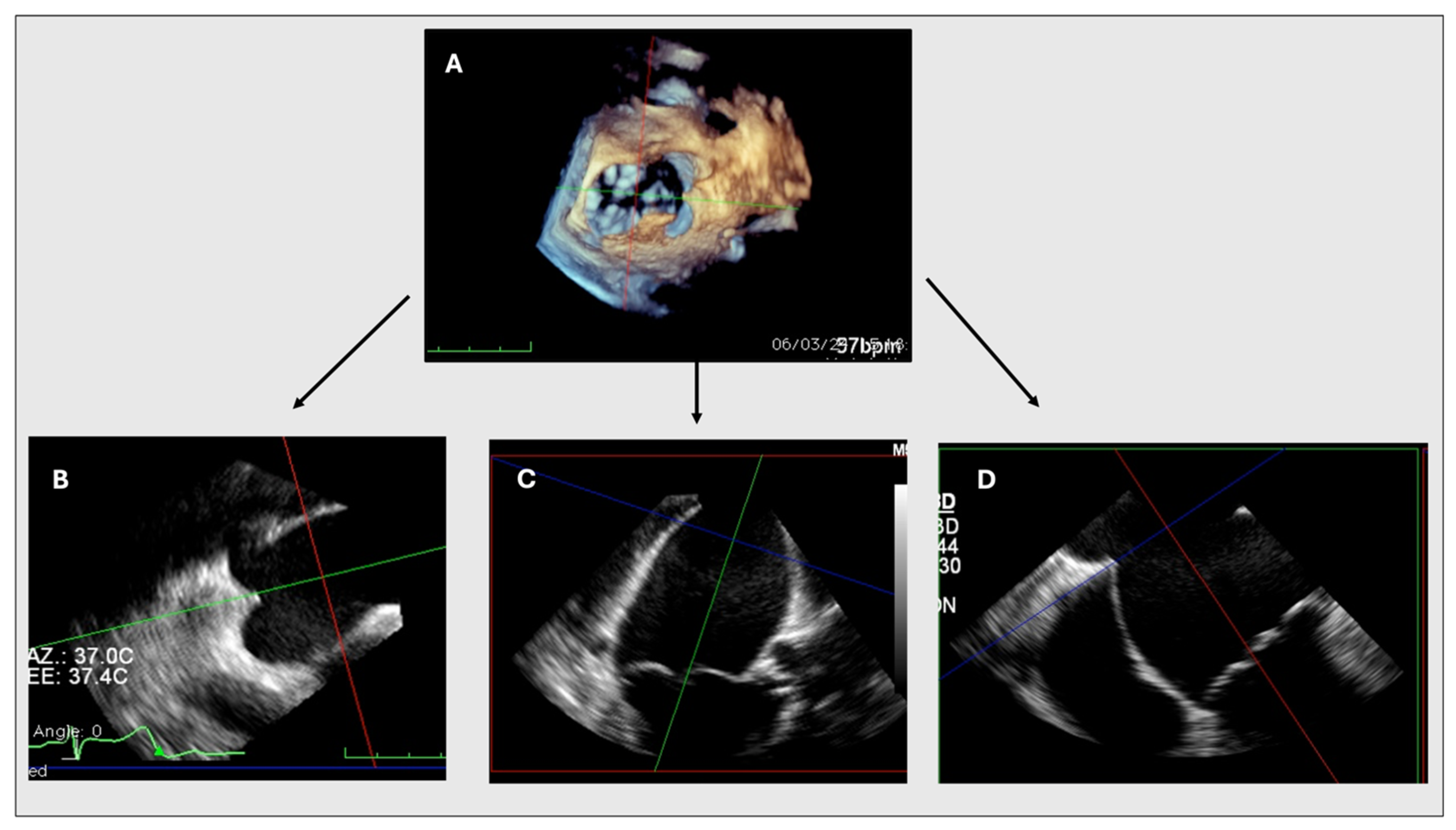
2.2. MPR Method
2.3. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Baseline MR Echocardiographic Parameters
3.3. Procedural Characteristics and Echocardiographic Results
3.4. Correlations Between 2D and MPR Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hausleiter, J.; Stocker, T.J.; Adamo, M.; Karam, N.; Swaans, M.J.; Praz, F. Mitral Valve Transcatheter Edge-to-Edge Repair. EuroIntervention 2023, 18, 957–976. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Saric, M.; Faletra, F.F.; Garg, R.; Gillam, L.D.; Horton, K.; Khalique, O.K.; Little, S.H.; Mackensen, G.B.; Oh, J.; et al. Recommended Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2022, 35, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Ancona, F.; Bartel, T.; Brochet, E.; Dweck, M.; Faletra, F.; Lancellotti, P.; Mahmoud-Elsayed, H.; Marsan, N.A.; Maurovich-Hovart, P.; et al. Multimodality Imaging for Patient Selection, Procedural Guidance, and Follow-up of Transcatheter Interventions for Structural Heart Disease: A Consensus Document of the EACVI Task Force on Interventional Cardiovascular Imaging: Part 1: Access Routes, Transcatheter Aortic Valve Implantation, and Transcatheter Mitral Valve Interventions. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e209–e268. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; El Sabbagh, A.; Lane, C.; Killu, A.M.; Alkhouli, M.; Pollak, P.M.; Thaden, J.J.; Eleid, M.F.; Friedman, P.A.; Rihal, C.S. Anatomic Approach to Transseptal Puncture for Structural Heart Interventions. JACC Cardiovasc. Interv. 2021, 14, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Ho, S.Y. The Interatrial Septum, Septal Atrio-Ventricular Junction, and Membranous Septum. In Atlas of Non-Invasive Imaging in Cardiac Anatomy; Faletra, F.F., Narula, J., Ho, S.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–61. ISBN 978-3-030-35506-7. [Google Scholar]
- Russo, G.; d’Aiello, A.; Pedicino, D.; Kuwata, S.; Sangiorgi, G.M.; Taramasso, M.; Maisano, F. Understanding Transcatheter Edge-to-Edge Repair “Knobology”: Advanced Catheter Steering for Different Scenarios of Transseptal Puncture. Catheter. Cardiovasc. Interv. 2024, 103, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Maisano, F.; Massaro, G.; Terlizzese, G.; Mariano, E.; Bonanni, M.; Matteucci, A.; Bezzeccheri, A.; Benedetto, D.; Chiricolo, G.; et al. Challenges and Open Issues in Transcatheter Mitral Valve Implantation: Smooth Seas Do Not Make Skillful Sailors. Front. Cardiovasc. Med. 2021, 8, 738756. [Google Scholar] [CrossRef] [PubMed]
- Harmel, E.; Pausch, J.; Gross, T.; Petersen, J.; Sinning, C.; Kubitz, J.; Reichenspurner, H.; Girdauskas, E. Standardized Subannular Repair Improves Outcomes in Type IIIb Functional Mitral Regurgitation. Ann. Thorac. Surg. 2019, 108, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, J.; Harb, S.C.; Krishnaswamy, A.; Kapadia, S.R.; Jaber, W.A.; Miyasaka, R. Echocardiographic Guidance of Transcatheter Mitral Valve Edge-To-Edge Repair. Struct. Heart 2020, 4, 397–412. [Google Scholar] [CrossRef]
- Pino, P.G.; Madeo, A.; Lucà, F.; Ceravolo, R.; di Fusco, S.A.; Benedetto, F.A.; Bisignani, G.; Oliva, F.; Colivicchi, F.; Gulizia, M.M.; et al. Clinical Utility of Three-Dimensional Echocardiography in the Evaluation of Mitral Valve Disease: Tips and Tricks. J. Clin. Med. 2023, 12, 2522. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Pagnesi, M.; Ghizzoni, G.; Estévez-Loureiro, R.; Raposeiras-Roubin, S.; Tomasoni, D.; Stolfo, D.; Sinagra, G.; Rubbio, A.P.; Bedogni, F.; et al. Evolution of tricuspid regurgitation after transcatheter edge-to-edge mitral valve repair for secondary mitral regurgitation and its impact on mortality. Eur. J. Heart Fail. 2022, 24, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406, Erratum in: N. Engl. J. Med. 2011, 365, 189. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Meucci, F.; Ancona, F.; Pardo Sanz, A.; Zamorano, J.L. Echocardiographic Guidance in Transcatheter Structural Cardiac Interventions. EuroIntervention 2022, 17, 1205–1226. [Google Scholar] [CrossRef] [PubMed]
- Katz, W.E.; Conrad Smith, A.J.; Crock, F.W.; Cavalcante, J.L. Echocardiographic Evaluation and Guidance for MitraClip Procedure. Cardiovasc. Diagn. Ther. 2017, 7, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Harb, S.C.; Krishnaswamy, A.; Kapadia, S.R.; Miyasaka, R.L. The Added Value of 3D Real-Time Multiplanar Reconstruction for Intraprocedural Guidance of Challenging MitraClip Cases. JACC Cardiovasc. Imaging 2020, 13, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.M.; Al-Ghamdi, M.A.; Ghabashi, A.E.; Anwar, A.M. A Proposed Maneuver to Guide Transseptal Puncture Using Real-Time Three-Dimensional Transesophageal Echocardiography: Pilot Study. Cardiol. Res. Pract. 2015, 2015, 174051. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H.; Isotani, A.; Shirai, S.; Ando, K. Three-Dimensional Transesophageal Echocardiography-Guided Transseptal Puncture for Percutaneous Mitral Valve Edge-to-Edge Repair Post-Percutaneous Atrial Septal Defect Closure. Clin. Case Rep. 2023, 11, e7794. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (n = 64) | Optimal 2D (n = 52) | Suboptimal 2D (n = 12) | p Value |
|---|---|---|---|---|
| Age, yrs | 79 ± 8 | 79.10 ± 7.9 | 76.25 ± 9.9 | 0.442 |
| Female, n (%) | 25 (39.1) | 22 (42.3) | 3 (25) | 0.338 |
| BMI, kg/m2 | 25.4 ± 4.4 | 25.3 ± 4.8 | 26 ± 3 | 0.349 |
| Hypertension, n (%) | 48 (75) | 41 (78.8) | 7 (58.3) | 0.156 |
| Hyperlipidaemia, n (%) | 37 (57.8) | 29 (55.8) | 8 (66.7) | 0.537 |
| Diabetes, n (%) | 21 (32.8) | 20 (38.5) | 1 (8.3) | 0.084 |
| Previous MI, n (%) | 20(31.3) | 17 (32.7) | 3 (25) | 0.739 |
| Previous PCI, n (%) | 11 (17.2) | 10 (19.2) | 1 (8.3) | 0.673 |
| Previous CABG, | 15 (23.4) | 11 (21.2) | 4 (33.3) | 0.453 |
| Previous TIA/stroke, n (%) | 3 (4.7) | 3 (5.8) | 0 (0) | 0.530 |
| PAD, n (%) | 7 (10.9) | 4 (7.7) | 3 (25) | 0.115 |
| COPD, n (%) | 7 (10.9) | 4 (7.7) | 3 (25) | 0.115 |
| CKD (≥III), n (%) | 21 (32.8) | 18 (34) | 3 (25) | 0.736 |
| Atrial fibrillation/atrial flutter, n (%) | 37 (57.8) | 31 (59.6) | 6 (50) | 0.747 |
| History of cancer, n (%) | 10 (15.6) | 7 (13.5) | 3 (25) | 0.557 |
| CRT-D, n (%) | 6 (9.5) | 3 (5.8) | 3 (25) | 0.060 |
| NYHA ≥ III, n (%) | 38 (58) | 31 (59.6) | 7 (58.3) | 0.465 |
| Previous valvular intervention, n (%) | 5 (7.9) | 3 (5.8) | 2 (16.7) | 0.105 |
| Mechanical aortic valve n (%) | 3 (4.7) | 2 (3.8) | 1 (8.3) | 0.253 |
| Previous TAVI n (%) | 2 (3.1) | 1 (1.9) | 1 (8.3) | 0.126 |
| LVEF, % | 50 (30.5–60) | 50 (35.75–60) | 50 (30.5–60) | 0.809 |
| Variables (n = 64) | |
|---|---|
| PMR, n (%) | 25 (39.1) |
| 16 (25) 9 (14.1) |
| SMR, N (%) | 39 (60.9) |
| 24 (37.5) 15 (23.4) |
| EROA, cm2 | 0.45 (0.40–0.65) |
| VR, mL | 63 (49–85) |
| VC, mm | 7 (6–8) |
| VC3D, cm2 | 0.45 (0.35–0.7) |
| MVA, cm2 | 7 (5–7.75) |
| Mean transvalvular gradient, mmHg | 2 (1–2) |
| Posterior leaflet length, mm | 12 (10–15) |
| LVEF, % | 50 (30.5–60) |
| LVEDD, mm | 52 (47–59) |
| LVESD, mm | 34 (28–42.5) |
| LVEDV, mL | 127 (100.5–147) |
| LVESV, mL | 54 (40–93) |
| PAPs, mmHg | 45 (35–63) |
| TR (≥moderate), n (%) | 40 (62.5) |
| TAPSE, mm | 20 (17.25–23) |
| N. jets (>1), n (%) | 25 (39.1) |
| LAVi, mL/mq | 61 (49.5–73) |
| Variables n = 64 | |
|---|---|
| N. device | |
| 1, n (%) | 44 (68.8) |
| 2, n (%) | 19 (29.7) |
| 3, n (%) | 1 (1.6) |
| Fluoroscopy time, min | 22 (15–28) |
| EROA, cm2 | 0.16 (0.12–0.21) |
| VR, mL | 21 (16.25–27.5) |
| VC, mm | 4 (2–5) |
| VC3D, cm2 | 0.18 (0.12–0.22) |
| MVA, cm2 | 3 (2–3.25) |
| Mean gradient, mmHg | 3 (3–4) |
| LVEF, % | 50 (30.5–60) |
| Pericardial effusion, n (%) | 0 (0) |
| Cardiac perforation, n (%) Procedural success, n (%) | 0 (0) 59 (92.2) |
| Method | Mean (SD) | CI 95% | Paired t-Test | Mean of Differences (CI 95%) | |
|---|---|---|---|---|---|
| TSP height (cm) | MPR | 4.59 (0.63) | 4.46–4.71 | 0.0001 | (0.16–0.30) |
| 2D | 4.36 (0.61) | 4.23–4.48 |
| Method | Median (IQR) | Mann–Whitney | |
|---|---|---|---|
| Mean difference between 2D and 3D measurements of TSP height (cm) | Optimal 2D | 0.2 (0.0–0.4) | 0.0001 |
| Suboptimal 2D | 0.5 (0.22–0.87) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, M.; Trimarchi, G.; Benedetti, G.; D’Agostino, A.; Iuliano, G.; Manzo, R.; Capasso, R.; Cerone, E.; Paradossi, U.; Berti, S.; et al. Standardized 3D Transoesophageal Echocardiography Manoeuvre for Enhanced Tenting Height Evaluation During Transcatheter Mitral Valve Edge-to-Edge Repair. J. Clin. Med. 2024, 13, 6525. https://doi.org/10.3390/jcm13216525
Bonanni M, Trimarchi G, Benedetti G, D’Agostino A, Iuliano G, Manzo R, Capasso R, Cerone E, Paradossi U, Berti S, et al. Standardized 3D Transoesophageal Echocardiography Manoeuvre for Enhanced Tenting Height Evaluation During Transcatheter Mitral Valve Edge-to-Edge Repair. Journal of Clinical Medicine. 2024; 13(21):6525. https://doi.org/10.3390/jcm13216525
Chicago/Turabian StyleBonanni, Michela, Giancarlo Trimarchi, Giovanni Benedetti, Andreina D’Agostino, Giuseppe Iuliano, Rachele Manzo, Rosangela Capasso, Elisa Cerone, Umberto Paradossi, Sergio Berti, and et al. 2024. "Standardized 3D Transoesophageal Echocardiography Manoeuvre for Enhanced Tenting Height Evaluation During Transcatheter Mitral Valve Edge-to-Edge Repair" Journal of Clinical Medicine 13, no. 21: 6525. https://doi.org/10.3390/jcm13216525
APA StyleBonanni, M., Trimarchi, G., Benedetti, G., D’Agostino, A., Iuliano, G., Manzo, R., Capasso, R., Cerone, E., Paradossi, U., Berti, S., & Mariani, M. (2024). Standardized 3D Transoesophageal Echocardiography Manoeuvre for Enhanced Tenting Height Evaluation During Transcatheter Mitral Valve Edge-to-Edge Repair. Journal of Clinical Medicine, 13(21), 6525. https://doi.org/10.3390/jcm13216525








