The Frozen Elephant Trunk Procedure—8 Years of Experience from Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics and Definitions
- Acute aortic dissection (AAD)—patients with the onset of symptoms < 14 days from surgery;
- Chronic aortic dissection (non-AAD)—patients with the onset of symptoms > 14 days from surgery;
- Redo surgery—patients with previous repair/surgery in any part of the thoracic aorta.
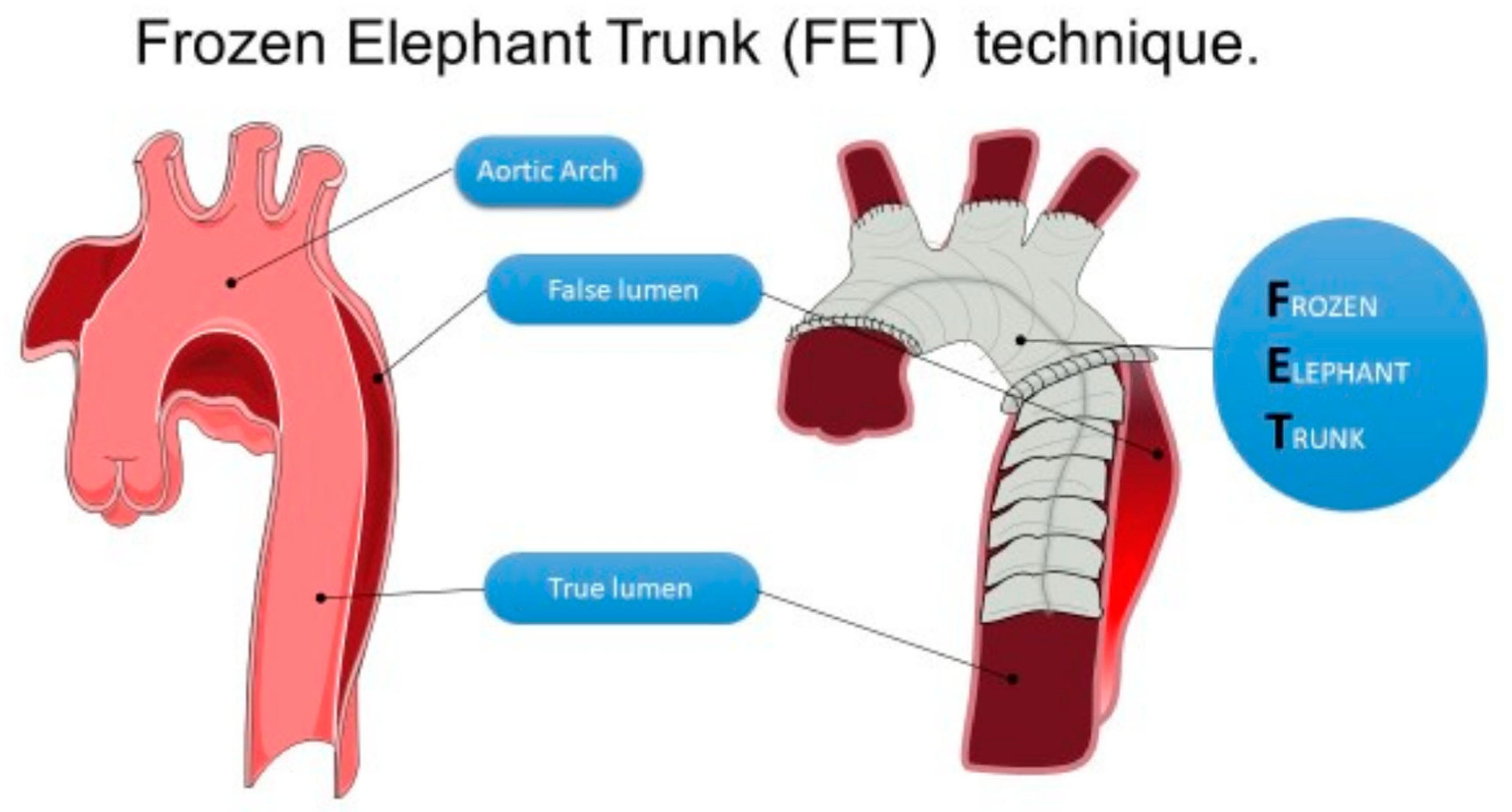
2.2. Surgical Management
2.3. Data Management and Endpoints
2.4. Statistical Analysis
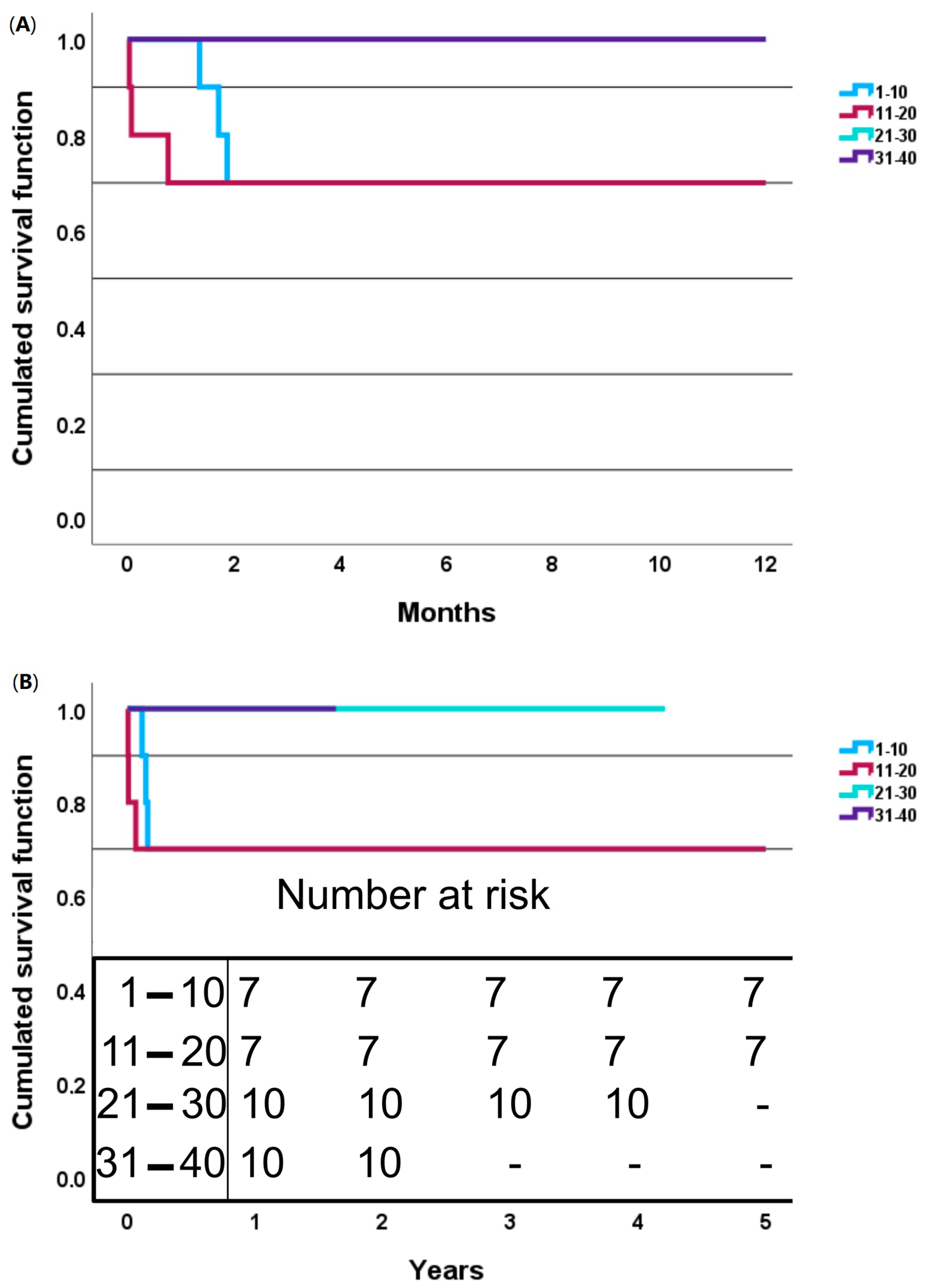
3. Results
3.1. Characteristics of the Patients
3.2. Intraoperative and Postoperative Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christodoulou, K.C.; Karangelis, D.; Efenti, G.M.; Sdrevanos, P.; Browning, J.R.; Konstantinou, F.; Georgakarakos, E.; Mitropoulos, F.A.; Mikroulis, D. Current knowledge and contemporary management of non-A non-B aortic dissections. World J. Cardiol. 2023, 15, 244–252. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, E334–E482. [Google Scholar] [CrossRef]
- Karck, M.; Chavan, A.; Hagl, C.; Friedrich, H.; Galanski, M.; Haverich, A. The frozen elephant trunk technique: A new treatment for thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2003, 125, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Sherzad, H.; Bashir, M.; Mariscalco, G. The frozen elephant trunk procedure: Indications, outcomes and future directions. Cardiovasc. Diagn. Ther. 2022, 12, 708–721. [Google Scholar] [CrossRef]
- Karck, M.; Kamiya, H. Progress of the treatment for extended aortic aneurysms; is the frozen elephant trunk technique the next standard in the treatment of complex aortic disease including the arch? Eur. J. Cardio-Thorac. Surg. 2008, 33, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Tan, L.; Tang, H.; Zhou, X.; Xiao, J.; Xie, D.; Li, J.; Chen, Y. Total Arch Replacement With Frozen Elephant Trunk Using a NEW “Brain-Heart-First” Strategy for Acute DeBakey Type I Aortic Dissection Can Be Performed Under Mild Hypothermia (≥30°C) With Satisfactory Outcomes. Front. Cardiovasc. Med. 2022, 9, 806822. [Google Scholar] [CrossRef] [PubMed]
- Zembala, M.; Krasoń, M.; Hrapkowicz, T.; Przybylski, R.; Filipiak, K.; Borowicz, M.; Niklewski, T.; Głowacki, J.; Wolny, T.; Nadziakiewicz, P.; et al. CARDIAC SURGERY The use of a new hybrid stent graft for the repair of extensive thoracic aortic aneurysms with the frozen elephant trunk method—First Polish experiences. Pol. J. Cardio-Thorac. Surg. 2014, 3, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kosiorowska, M.; Berezowski, M.; Widenka, K.; Kreibich, M.; Beyersdorf, F.; Czerny, M.; Rylski, B. Non-A non-B acute aortic dissection with entry tear in the aortic arch. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.Y.; Basharat, K.; Zahra, S.A.; Tran, T.; Rimmer, L.; Harky, A.; Idhrees, M.; Bashir, M. “Proximalization is Advancement”—Zone 3 Frozen Elephant Trunk vs Zone 2 Frozen Elephant Trunk: A Literature Review. Vasc. Endovasc. Surg. 2021, 55, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Arnold, Z.; Geisler, D.; Aschacher, T.; Winkler, B.; Lenz, V.; Crailsheim, I.; Folkmann, S.; Harrer, M.; Moidl, R.; Grabenwöger, M.; et al. Long-Term Results with 187 Frozen Elephant Trunk Procedures. J. Clin. Med. 2023, 12, 4143. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, K.; Motekallemi, A.; Dell’Aquila, A.M.; Oberhuber, A.; Schaefers, J.F.; Ibrahim, A.; Martens, S.; Rukosujew, A. Single-Center Experience with the ThoraflexTM Hybrid Prosthesis: Indications, Implantation Technique and Results. Front. Cardiovasc. Med. 2022, 9, 924838. [Google Scholar] [CrossRef] [PubMed]
- Murana, G.; Campanini, F.; Fiaschini, C.; Barberio, G.; Folesani, G.; Pacini, D. Spinal cord injury after frozen elephant trunk procedures—Prevention and management. Ann. Cardiothorac. Surg. 2023, 12, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Porterie, J.; Hostalrich, A.; Dagenais, F.; Marcheix, B.; Chaufour, X.; Ricco, J.-B. Hybrid Treatment of Complex Diseases of the Aortic Arch and Descending Thoracic Aorta by Frozen Elephant Trunk Technique. J. Clin. Med. 2023, 12, 5693. [Google Scholar] [CrossRef] [PubMed]
- Kayali, F.; Qutaishat, S.; Jubouri, M.; Chikhal, R.; Tan, S.Z.C.P.; Bashir, M. Kinking of Frozen Elephant Trunk Hybrid Prostheses: Incidence, Mechanism, and Management. Front. Cardiovasc. Med. 2022, 9, 912071. [Google Scholar] [CrossRef] [PubMed]
- Kayali, F.; Chikhal, R.; Agbobu, T.; Jubouri, M.; Patel, R.; Chen, E.P.; Mohammed, I.; Bashir, M. Evidence-based frozen elephant trunk practice: A narrative review. Cardiovasc. Diagn. Ther. 2023, 13, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; Alfonsi, J.; Berretta, P.; Cefarelli, M.; Gatta, E.; Di Eusanio, M. Normothermic frozen elephant trunk: Our experience and literature review. Cardiovasc. Diagn. Ther. 2022, 12, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Burysz, M.; Batko, J.; Bartuś, K.; Ogorzeja, W.; Litwinowicz, R.A. Hybrid treatment of penetrating aortic trauma. Pol. J. Cardio-Thorac. Surg. 2024, 21, 65–66. [Google Scholar] [CrossRef] [PubMed]

| AAD (n = 28) | Non-AAD (n = 6) | Redo FET (n = 6) | Total | p | ||
|---|---|---|---|---|---|---|
| Age (years) | 60 (53–67) | 64 (60–65) | 51 (42–64) | 60 (53–66) | 0.34 | |
| Male | 18 (64.3%) | 4 (66.7%) | 5 (83.3%) | 27 (67.5%) | 0.66 | |
| Body mass index (kg/m2) | 28.7 (24.1–30.8) | 24.1 (23.4–27.1) | 27.2 (21–30.7) | 27.8 (23.4–30.7) | 0.59 | |
| DM2 | 3 (10.7%) | 0 (0%) | 0 (0%) | 3 (7.5%) | 0.50 | |
| Hypertension | 26 (92.9%) | 6 (100%) | 5 (83.3%) | 37 (92.5%) | 0.54 | |
| Atrial fibrillation | 0 (0%) | 0 (0%) | 2 (33.3%) | 2 (5%) | 0.003 | |
| Chronic heart failure | 1 (3.6%) | 0 (0%) | 0 (0%) | 1 (2.5%) | 0.80 | |
| Pulmonary disease | 7 (25%) | 1 (16.7%) | 3 (50%) | 11 (27.5%) | 0.37 | |
| Ever smoker | Active | 20 (71.4%) | 0 (0%) | 2 (33.3%) | 22 (55%) | 0.02 |
| Previous | 7 (25%) | 5 (83.3%) | 3 (50%) | 15 (37.5%) | ||
| Vascular disease | Peripheral | 8 (28.6%) | 2 (33.3%) | 3 (50%) | 13 (32.5%) | 0.25 |
| Cerebral | 0 (0%) | 1 (16.7%) | 0 (0%) | 1 (2.5%) | ||
| Both | 3 (10.7%) | 0 (0%) | 0 (0%) | 3 (7.5%) | ||
| Non-elective surgery | 27 (96.4%) | 3 (50%) | 1 (16.7%) | 31 (77.5%) | <0.001 | |
| Tamponade | 5 (17.9%) | 1 (16.7%) | 1 (16.7%) | 7 (17.5%) | 0.99 | |
| Ejection fraction (%) | 40 (40–48) | 60 (40–65) | 45 (40–50) | 40 (40–53) | 0.18 | |
| Euroscore II | 29.5 (22.8–48.1) | 23.6 (14.3–28.6) | 8 (5.5–11.6) | 25.9 (11.7–38.7) | 0.05 | |
| Parts of aorta involved | Type A | 8 (28.6%) | 0 (0%) | 0 (0%) | 8 (20%) | 0.007 |
| Ascending + arch | 12 (42.9%) | 2 (33.3%) | 3 (50%) | 17 (42.5%) | ||
| Ascending + arch + descending | 3 (10.7%) | 2 (33.3%) | 0 (0%) | 5 (12.5%) | ||
| Arch + descending | 0 (0%) | 2 (33.3%) | 3 (50%) | 5 (12.5%) | ||
| Thoracic + abdominal | 5 (17.9%) | 0 (0%) | 0 (0%) | 5 (12.5%) | ||
| Preoperative new-onset neurological symptoms | TIA | 9 (32.1%) | 1 (16.7%) | 2 (33.3%) | 12 (30%) | 0.51 |
| Peripheral paresis | 3 (10.7%) | 0 (0%) | 0 (0%) | 3 (7.5%) | ||
| Cerebral ischemia | 4 (14.3%) | 0 (0%) | 0 (0%) | 4 (10%) | ||
| Preoperative ischemic non-neurological symptoms | Lower-limb ischemia | 10 (35.7%) | 1 (16.7%) | 2 (33.3%) | 13 (32.5%) | 0.39 |
| Cardiac arrest | 4 (14.3%) | 2 (33.3%) | 0 (0%) | 6 (15%) | ||
| Lower-limb ischemia and cardiac arrest | 3 (10.7%) | 0 (0%) | 0 (0%) | 3 (7.5%) | ||
| Mesenteric ischemia | 1 (3.6%) | 0 (0%) | 0 (0%) | 1 (2.5%) | ||
| Mesenteric and lower-limb ischemia | 0 (0%) | 0 (0%) | 1 (16.7%) | 1 (2.5%) | ||
| Previous aortic procedures | AVR | 0 (0%) | 0 (0%) | 2 (33.3%) | 2 (5%) | <0.001 |
| Abdominal stent graft | 2 (7.1%) | 2 (33.3%) | 0 (0%) | 4 (10%) | ||
| AAD surgery | 0 (0%) | 1 (16.7%) | 2 (33.3%) | 3 (7.5%) | ||
| Bentall procedure | 0 (0%) | 0 (0%) | 2 (33.3%) | 2 (5%) | ||
| AAD (n = 28) | Non-AAD (n = 6) | Redo FET (n = 6) | Total | p | ||
|---|---|---|---|---|---|---|
| Hypothermia temperature (°C) | 28 (28–32) | 29 (28–32) | 28 (28–28) | 28 (28–32) | 0.80 | |
| Surgery time (min) | 320 (280–488) | 465 (380–560) | 350 (270–385) | 355 (280–488) | 0.25 | |
| Cardiopulmonary bypass time (min) | 195 (158–293) | 323 (202–420) | 206 (157–210) | 206 (160–301) | 0.07 | |
| Aortic cross-clamp time (min) | 120 (110–162) | 126 (115–140) | 121 (90–135) | 120 (111–155) | 0.71 | |
| Distal hypothermic circulatory arrest time (min) | 35 (30–43) | 35 (25–40) | 30 (25–34) | 35 (30–43) | 0.40 | |
| Type of surgery | FET | 21 (75%) | 4 (66.7%) | 6 (100%) | 31 (77.5%) | 0.53 |
| FET + Bentall | 2 (7.1%) | 0 (0%) | 0 (0%) | 2 (5%) | ||
| FET + Florida sleeve | 3 (10.7%) | 2 (33.3%) | 0 (0%) | 5 (12.5%) | ||
| Complex procedure | 2 (7.1%) | 0 (0%) | 0 (0%) | 2 (5%) | ||
| Prosthesis length | 100 | 12 (42.9%) | 1 (16.7%) | 5 (83.3%) | 18 (45%) | 0.16 |
| 120 | 2 (7.1%) | 0 (0%) | 0 (0%) | 2 (5%) | ||
| 150 | 14 (50%) | 5 (83.3%) | 1 (16.7%) | 20 (50%) | ||
| Arterial cannulation site | Femoral | 26 (92.9%) | 5 (83.3%) | 6 (100%) | 37 (92.5%) | 0.63 |
| Brachiocephalic | 1 (3.6%) | 1 (16.7%) | 0 (0%) | 2 (5%) | ||
| Subclavian | 1 (3.6%) | 0 (0%) | 0 (0%) | 1 (2.5%) | ||
| Anastomosis zone | 1 | 2 (7.1%) | 0 (0%) | 0 (0%) | 2 (5%) | 0.03 |
| 2 | 22 (78.6%) | 2 (33.3%) | 6 (100%) | 30 (75%) | ||
| 4 | 4 (14.3%) | 4 (66.7%) | 0 (0%) | 8 (20%) | ||
| Cell server | 17 (60.7%) | 2 (33.3%) | 4 (66.7%) | 23 (57.5%) | 0.42 | |
| Delayed chest closure | 25 (89.3%) | 6 (100%) | 5 (83.3%) | 36 (90%) | 0.61 | |
| AAD (n = 28) | Non-AAD (n = 6) | Redo FET (n = 6) | Total | p | ||
|---|---|---|---|---|---|---|
| 30-day mortality | 2 (7.1%) | 0 (0%) | 1 (16.7%) | 3 (7.5%) | 0.54 | |
| Complications | Peripheral paresis | 3 (10.7%) | 1 (16.7%) | 0 (0%) | 4 (10%) | 0.10 |
| Bleeding | 0 (0%) | 1 (16.7%) | 0 (0%) | 1 (2.5%) | ||
| Paraparesis | 0 (0%) | 1 (16.7%) | 0 (0%) | 1 (2.5%) | ||
| Multiorgan failure | 1 (3.6%) | 0 (0%) | 0 (0%) | 1 (2.5%) | ||
| Cerebral ischemia | 5 (17.9%) | 0 (0%) | 0 (0%) | 5 (12.5%) | ||
| Hospitalization time (days) | 20 (15–28) | 22 (11–30) | 21 (19–23) | 21 (15–29) | 0.91 | |
| Next-stage stent graft implantation | 4 (14.3%) | 2 (33.3%) | 0 (0%) | 6 (15%) | 0.27 | |
| Consecutive Number of Patients | 1–10 | 11–20 | 21–30 | 31–40 | Total | p |
|---|---|---|---|---|---|---|
| Hypothermia temperature (°C) | 28 (25–28) | 29 (28–32) | 30 (28–32) | 28 (28–32) | 28 (28–32) | 0.12 |
| Surgery time (min) | 533 (500–560) | 405 (280–470) | 320 (300–385) | 256 (235–285) | 355 (280–488) | <0.001 +†• |
| Cardiopulmonary bypass time (min) | 327 (299–360) | 206 (150–243) | 199 (172–231) | 160 (157–172) | 206 (160–301) | <0.001 *+† |
| Aortic cross-clamp time (min) | 165 (115–208) | 125 (90–140) | 122 (118–135) | 110 (101–115) | 120 (111–155) | 0.03 † |
| Distal hypothermic circulatory arrest time (min) | 40 (40–50) | 30 (10–35) | 43 (30–43) | 30 (25–30) | 35 (30–43) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burysz, M.; Horosin, G.; Olejek, W.; Kowalewski, M.; Bartuś, K.; Słomka, A.; Litwinowicz, R.; Batko, J. The Frozen Elephant Trunk Procedure—8 Years of Experience from Poland. J. Clin. Med. 2024, 13, 6544. https://doi.org/10.3390/jcm13216544
Burysz M, Horosin G, Olejek W, Kowalewski M, Bartuś K, Słomka A, Litwinowicz R, Batko J. The Frozen Elephant Trunk Procedure—8 Years of Experience from Poland. Journal of Clinical Medicine. 2024; 13(21):6544. https://doi.org/10.3390/jcm13216544
Chicago/Turabian StyleBurysz, Marian, Grzegorz Horosin, Wojciech Olejek, Mariusz Kowalewski, Krzysztof Bartuś, Artur Słomka, Radosław Litwinowicz, and Jakub Batko. 2024. "The Frozen Elephant Trunk Procedure—8 Years of Experience from Poland" Journal of Clinical Medicine 13, no. 21: 6544. https://doi.org/10.3390/jcm13216544
APA StyleBurysz, M., Horosin, G., Olejek, W., Kowalewski, M., Bartuś, K., Słomka, A., Litwinowicz, R., & Batko, J. (2024). The Frozen Elephant Trunk Procedure—8 Years of Experience from Poland. Journal of Clinical Medicine, 13(21), 6544. https://doi.org/10.3390/jcm13216544








