A Patient Case of Malan Syndrome Involving 19p13.2 Deletion of NFIX with Longitudinal Follow-Up and Future Prospectives
Abstract
1. Introduction
2. Detailed Case Description
2.1. Gross Motor Development
2.2. Therapeutic Intervention
2.3. Oral Sensory Intervention
2.4. Cytogenetic Analysis
2.5. Neuropsychological Testing
3. Discussion
3.1. Current Diagnosis, Management, and Standard of Care
3.2. Toward a Personalized Therapeutic Strategy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baujat, G.; Rio, M.; Rossignol, S.; Sanlaville, D.; Lyonnet, S.; Merrer, M.L.; Munnich, A.; Gicquel, C.; Colleaux, L.; Cormier-Daire, V. Clinical and molecular overlap in overgrowth syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2005, 137, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.Y.; Zeng, H.; Hu, Y.Q.; Xie, L.; Wang, J.; Wang, X.Y.; Yang, Y.F.; Tan, Z.P. 19p13.2 Microdeletion including NFIX associated with overgrowth and intellectual disability suggestive of Malan syndrome. Mol. Cytogenet. 2016, 9, 71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Driller, K.; Pagenstecher, A.; Uhl, M.; Omran, H.; Berlis, A.; Grunder, A.; Sippel, A.E. Nuclear factor I X deficiency causes brain malformation and severe skeletal defects. Mol. Cell Biol. 2007, 27, 3855–3867. [Google Scholar] [CrossRef] [PubMed]
- Gronostajski, R.M. Analysis of nuclear factor I binding to DNA using degenerate oligonucleotides. Nucleic Acids Res. 1986, 14, 9117–9132. [Google Scholar] [CrossRef]
- Gronostajski, R.M.; Adhya, S.; Nagata, K.; Guggenheimer, R.A.; Hurwitz, J. Site-specific DNA binding of nuclear factor I: Analyses of cellular binding sites. Mol. Cell Biol. 1985, 5, 964–971. [Google Scholar]
- Kruse, U.; Sippel, A.E. Transcription factor nuclear factor I proteins form stable homo-and heterodimers. FEBS Lett. 1994, 348, 46–50. [Google Scholar] [CrossRef]
- Piper, M.; Gronostajski, R.; Messina, G. Nuclear Factor One X in Development and Disease. Trends Cell Biol. 2019, 29, 20–30. [Google Scholar] [CrossRef]
- Campbell, C.E.; Piper, M.; Plachez, C.; Yeh, Y.T.; Baizer, J.S.; Osinski, J.M.; Litwack, E.D.; Richards, L.J.; Gronostajski, R.M. The transcription factor Nfix is essential for normal brain development. BMC Dev. Biol. 2008, 8, 52. [Google Scholar] [CrossRef]
- Heng, Y.H.E.; McLeay, R.C.; Harvey, T.J.; Smith, A.G.; Barry, G.; Cato, K.; Plachez, C.; Little, E.; Mason, S.; Dixon, C.; et al. NFIX regulates neural progenitor cell differentiation during hippocampal morphogenesis. Cereb. Cortex 2014, 24, 261–279. [Google Scholar] [CrossRef]
- Riddell, J.; Gazit, R.; Garrison, B.S.; Guo, G.; Saadatpour, A.; Mandal, P.K.; Ebina, W.; Volchkov, P.; Yuan, G.C.; Orkin, S.H.; et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 2014, 157, 549–564. [Google Scholar] [CrossRef]
- Matuzelski, E.; Bunt, J.; Harkins, D.; Lim, J.W.; Gronostajski, R.M.; Richards, L.J.; Harris, L.; Piper, M. Transcriptional regulation of Nfix by NFIB drives astrocytic maturation within the developing spinal cord. Dev. Biol. 2017, 432, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.Z.; Lyons, G.E.; Gronostajski, R.M. Expression patterns of the four nuclear factor I genes during mouse embryogenesis indicate a potential role in development. Dev. Dyn. 1997, 208, 313–325. [Google Scholar] [CrossRef]
- Piper, M.; Harris, L.; Barry, G.; Heng, Y.H.E.; Plachez, C.; Gronostajski, R.M.; Richards, L.J. Nuclear factor one X regulates the development of multiple cellular populations in the postnatal cerebellum. J. Comp. Neurol. 2011, 519, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Biressi, S.; Tagliafico, E.; Lamorte, G.; Monteverde, S.; Tenedini, E.; Roncaglia, E.; Ferrari, S.; Ferrari, S.; Cusella-De Angelis, M.G.; Tajbakhsh, S.; et al. Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells. Dev. Biol. 2007, 304, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Keeshan, K.; O’Connor, C.; Campos, J.; Murphy, B.; Hannon, M.; Michie, A. Nfix expression critically modulates early B lymphopoiesis and myelopoiesis. PLoS ONE 2015, 10, e0120102. [Google Scholar] [CrossRef][Green Version]
- Rossi, G.; Antonini, S.; Bonfanti, C.; Monteverde, S.; Vezzali, C.; Tajbakhsh, S.; Cossu, G.; Messina, G. Nfix Regulates Temporal Progression of Muscle Regeneration through Modulation of Myostatin Expression. Cell Rep. 2016, 14, 2238–2249. [Google Scholar] [CrossRef]
- Stringer, B.W.; Bunt, J.; Day, B.W.; Barry, G.; Jamieson, P.R.; Ensbey, K.S.; Bruce, Z.C.; Goasdoué, K.; Vidal, H.; Charmsaz, S.; et al. Nuclear factor one B (NFIB) encodes a subtype-specific tumour suppressor in glioblastoma. Oncotarget 2016, 7, 29306–29320. [Google Scholar] [CrossRef]
- Macchiaiolo, M.; Panfili, F.M.; Vecchio, D.; Gonfiantini, M.V.; Cortellessa, F.; Caciolo, C.; Zollino, M.; Accadia, M.; Seri, M.; Chinali, M.; et al. A deep phenotyping experience: Up to date in management and diagnosis of Malan syndrome in a single center surveillance report. Orphanet J. Rare Dis. 2022, 17, 235. [Google Scholar] [CrossRef]
- Malan, V.; Rajan, D.; Thomas, S.; Shaw, A.C.; dit Picard, H.L.; Layet, V.; Till, M.; van Haeringen, A.; Mortier, G.; Nampoothiri, S.; et al. Distinct effects of allelic NFIX mutations on nonsense-mediated mRNA decay engender either a Sotos-like or a Marshall-Smith syndrome. Am. J. Hum. Genet. 2010, 87, 189–198. [Google Scholar] [CrossRef]
- Dolan, M.; Mendelsohn, N.J.; Pierpont, M.E.; Schimmenti, L.A.; Berry, S.A.; Hirsch, B. A novel microdeletion/microduplication syndrome of 19p13.13. Genet. Med. 2010, 12, 503–511. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdottir, H.; Turner, D.; Mesirov, J.P. igv.js: An embeddable JavaScript implementation of the Integrative Genomics Viewer (IGV). Bioinformatics 2023, 39, btac830. [Google Scholar] [CrossRef] [PubMed]
- Klaassens, M.; Morrogh, D.; Rosser, E.M.; Jaffer, F.; Vreeburg, M.; Bok, L.A.; Segboer, T.; Van Belzen, M.; Quinlivan, R.M.; Kumar, A.; et al. Malan syndrome: Sotos-like overgrowth with de novo NFIX sequence variants and deletions in six new patients and a review of the literature. Eur. J. Hum. Genet. 2015, 23, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Tatton-Brown, K.; Rahman, N. The NSD1 and EZH2 overgrowth genes, similarities and differences. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, P.; Macchiaiolo, M.; Collotta, M.; Montanaro, F.A.M.; Caciolo, C.; Cumbo, F.; Galassi, P.; Panfili, F.M.; Cortellessa, F.; Zollino, M.; et al. Characterization of Cognitive, Language and Adaptive Profiles of Children and Adolescents with Malan Syndrome. J. Clin. Med. 2022, 11, 4078. [Google Scholar] [CrossRef] [PubMed]
- Bellucco, F.T.; de Mello, C.B.; Meloni, V.A.; Melaragno, M.I. Malan syndrome in a patient with 19p13.2p13.12 deletion encompassing NFIX and CACNA1A genes: Case report and review of the literature. Mol. Genet. Genom. Med. 2019, 7, e997. [Google Scholar] [CrossRef]
- Gurrieri, F.; Cavaliere, M.L.; Wischmeijer, A.; Mammì, C.; Neri, G.; Pisanti, M.A.; Rodella, G.; Laganà, C.; Priolo, M. NFIX mutations affecting the DNA-binding domain cause a peculiar overgrowth syndrome (Malan syndrome): A new patients series. Eur. J. Med. Genet. 2015, 58, 488–491. [Google Scholar] [CrossRef]
- Kuroda, Y.; Mizuno, Y.; Mimaki, M.; Oka, A.; Sato, Y.; Ogawa, S.; Kurosawa, K. Two patients with 19p13.2 deletion (Malan syndrome) involving NFIX and CACNA1A with overgrowth, developmental delay, and epilepsy. Clin. Dysmorphol. 2017, 26, 224–227. [Google Scholar] [CrossRef]
- Mulder, P.A.; van Balkom, I.D.C.; Landlust, A.M.; Priolo, M.; Menke, L.A.; Acero, I.H.; Alkuraya, F.S.; Arias, P.; Bernardini, L.; Bijlsma, E.K.; et al. Development, behaviour and sensory processing in Marshall-Smith syndrome and Malan syndrome: Phenotype comparison in two related syndromes. J. Intellect. Disabil. Res. 2020, 64, 956–969. [Google Scholar] [CrossRef]
- Priolo, M.; Schanze, D.; Tatton-Brown, K.; Mulder, P.A.; Tenorio, J.; Kooblall, K.; Acero, I.H.; Alkuraya, F.S.; Arias, P.; Bernardini, L.; et al. Further delineation of Malan syndrome. Hum. Mutat. 2018, 39, 1226–1237. [Google Scholar] [CrossRef]
- Yoneda, Y.; Saitsu, H.; Touyama, M.; Makita, Y.; Miyamoto, A.; Hamada, K.; Kurotaki, N.; Tomita, H.; Nishiyama, K.; Tsurusaki, Y.; et al. Missense mutations in the DNA-binding/dimerization domain of NFIX cause Sotos-like features. J. Hum. Genet. 2012, 57, 207–211. [Google Scholar] [CrossRef]
- Jezela-Stanek, A.; Kucharczyk, M.; Falana, K.; Jurkiewicz, D.; Mlynek, M.; Wicher, D.; Rydzanicz, M.; Kugaudo, M.; Cieslikowska, A.; Ciara, E.; et al. Malan syndrome (Sotos syndrome 2) in two patients with 19p13.2 deletion encompassing NFIX gene and novel NFIX sequence variant. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2016, 160, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Liang, X.H.; Baker, B.F.; Crooke, R.M. Antisense technology: A review. J. Biol. Chem. 2021, 296, 100416. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Shen, W.; Crooke, S.T. Specific Increase of Protein Levels by Enhancing Translation Using Antisense Oligonucleotides Targeting Upstream Open Frames. Adv. Exp. Med. Biol. 2017, 983, 129–146. [Google Scholar] [PubMed]
- Liang, X.H.; Shen, W.; Sun, H.; Migawa, M.T.; Vickers, T.A.; Crooke, S.T. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat. Biotechnol. 2016, 34, 875–880. [Google Scholar] [CrossRef]
- Colella, P.; Ronzitti, G.; Mingozzi, F. Emerging Issues in AAV-Mediated In Vivo Gene Therapy. Mol. Ther. Methods Clin. Dev. 2018, 8, 87–104. [Google Scholar] [CrossRef]
- Schoch, K.; McConkie-Rosell, A.; Walley, N.; Bhambhani, V.; Feyma, T.; Undiagnosed Diseases Network; Pizoli, C.E.; Smith, E.C.; Tan, Q.K.G.; Shashi, V. Parental perspectives of episodic irritability in an ultra-rare genetic disorder associated with NACC1. Orphanet J. Rare Dis. 2023, 18, 269. [Google Scholar] [CrossRef]


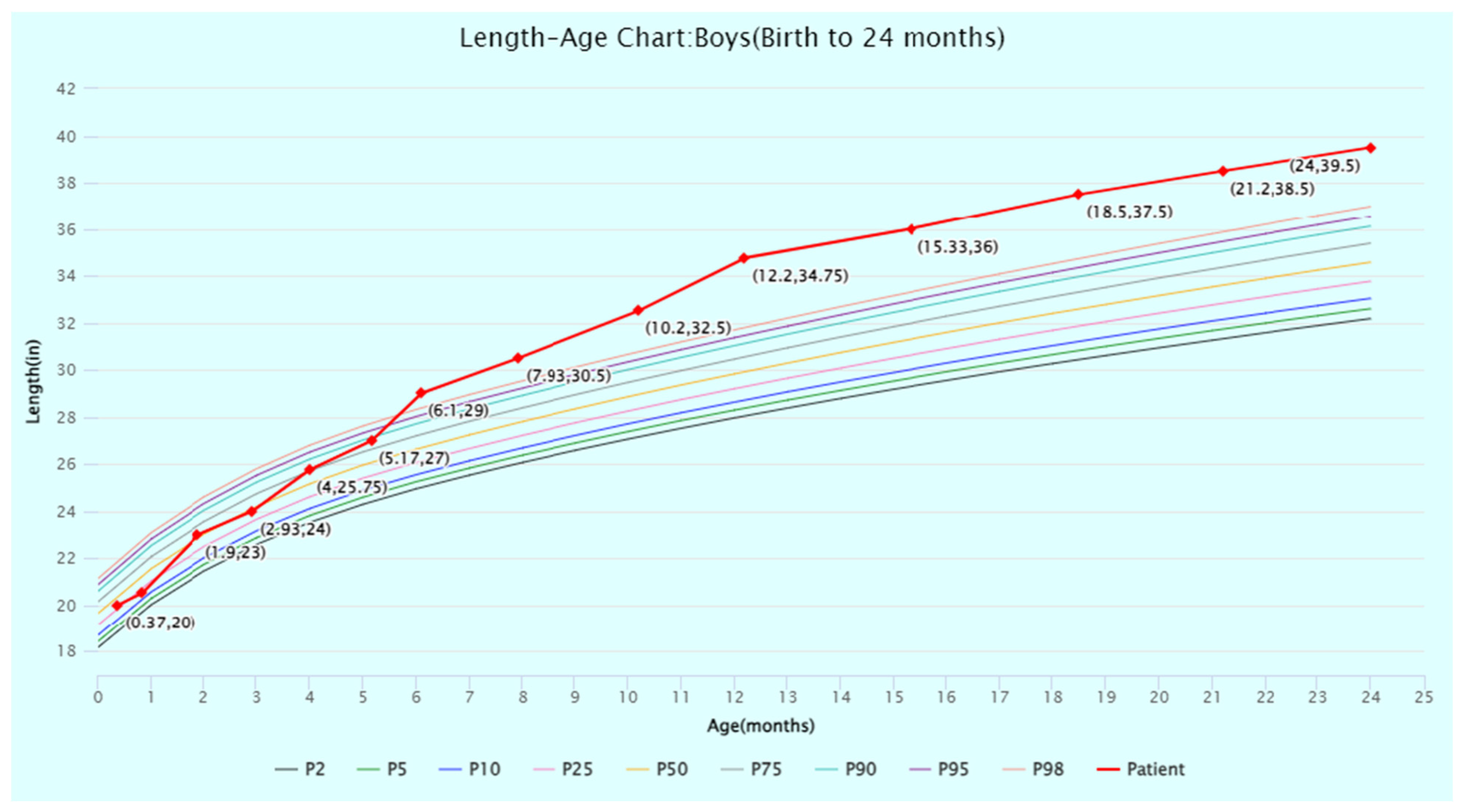
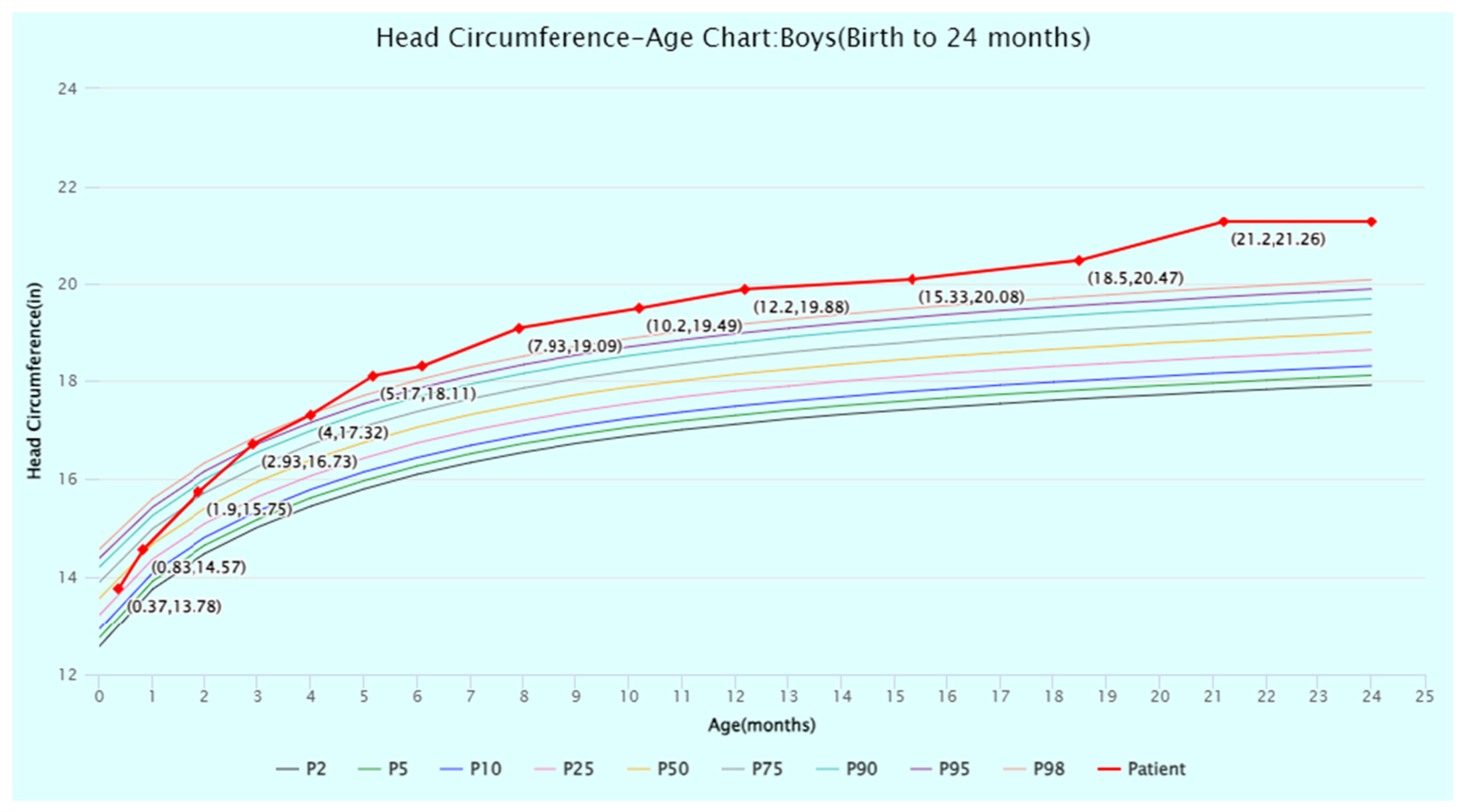


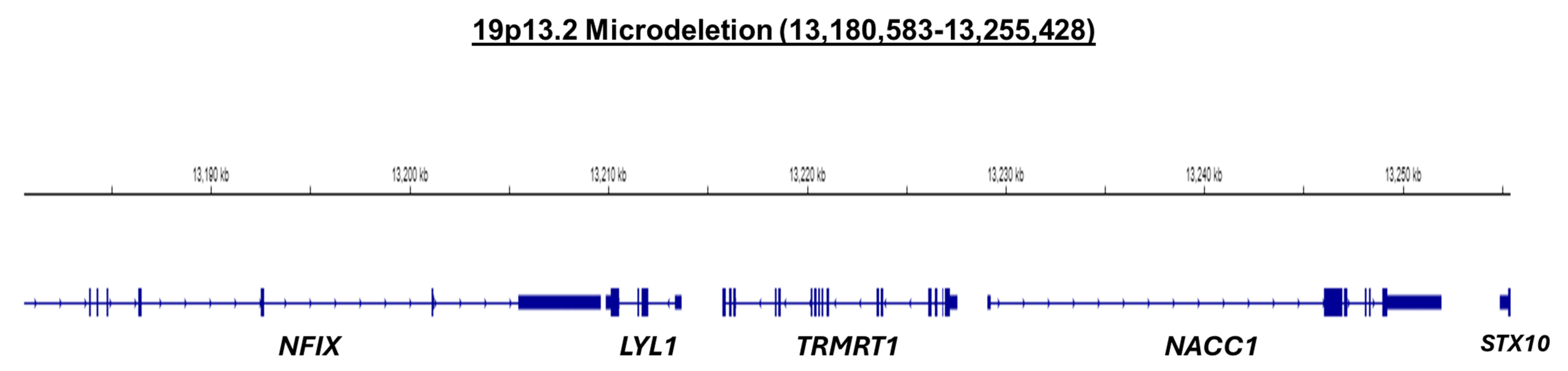
| Domain | Standard Score | Age-Equivalent (Months) |
|---|---|---|
| Cognition | 72 | 8 |
| Communication | 66 | 7 |
| Social–emotional | 75 | 9 |
| Physical development | 58 | 7 |
| Adaptive behavior | 70 | 8 |
| Percentile | |||
|---|---|---|---|
| Skill | 6 Years of Age | 11 Years of Age | 13 Years of Age |
| Verbal | |||
| Similarities a | 2nd | 25th | 37th |
| Information b | 2nd | 10th | 37th |
| Vocabulary c | CNC | CNC | 37th |
| Visual spatial | |||
| Block design d | <1st | 2nd | 9th |
| Matrix reasoning e | <1st | <1st | 5th |
| Picture concepts f | <1st | <1st | 1st |
| Working memory | |||
| Digits (forward) g | N/A | 50th | 50th |
| Digits (backward) | N/A | CNC | 5th |
| Digits (sequence) | N/A | 5th | 2nd |
| Picture span h | N/A | 5th | 9th |
| Processing speed | |||
| Coding i | CNC | CNC | <1st |
| Symbol search j | CNC | CNC | 5th |
| Cancelation k | CNC | CNC | <1st |
| Reading | |||
| Word identification | N/A | 50th | 50th |
| Word comprehension | N/A | 25th | 25th |
| Pseudoword reading | N/A | 10th | 30th |
| Orthographic fluency | N/A | CNC | 27th |
| Comprehension | N/A | 37th | 25th |
| Math | |||
| Numerical operations | CNC | 9th | 25th |
| Problem solving | CNC | 1st | 16th |
| Math fluency | N/A | 7th | 12th |
| Writing | |||
| Spelling | CNC | 20th | 30th |
| Writing fluency | N/A | 6th | 58th |
| Sentence combining | N/A | CNC | 3rd |
| Sentence building | N/A | CNC | 25th |
| Essay composition | N/A | CNC | 4th |
| Characteristic | Percentage of Individuals |
|---|---|
| Macrocephaly | >75% |
| Hypotonia | 50–76% |
| Long and slender body habitus | 59–100% |
| Long hands | 63% |
| Advanced bone age | 80% |
| Scoliosis | 32–75% |
| Pectus carinatum/excavatum | 40–56% |
| Flat occiput, long or triangular face shape | 81% |
| Prominent forehead and frontal bossing | 100% |
| Deeply set eyes | 50% |
| Depressed nasal bridge | 50% |
| Anteverted nares | 69% |
| Down-slanted palpebral fissures | 63% |
| Long philtrum | 56% |
| Thin upper vermillion in a Cupid’s bow shape | 75% |
| Everted lower lip | 81% |
| Small mouth that is often held open | 69% |
| Prominent chin | 88% |
| Ocular abnormalities | 75–100% |
| Strabismus | 32–63% |
| Esotropia | ≤56% |
| Nystagmus | 15–31% |
| Optic nerve hypoplasia | 21–25% |
| Blue sclera | 25–69% |
| Constipation | 50% |
| Malocclusion | 44% |
| Ogival palate/overcrowded teeth | 2–56% |
| Caries | 38% |
| Hypersalivation | 31% |
| Mitral valve regurgitation | 1–31% |
| Ventriculomegaly | 27–50% |
| Corpus callosum and vermis hypoplasia | 22–50% |
| Chiari malformation type I | 9.5–38% |
| Seizures | 13–63% |
| Intellectual disability | 100% |
| Autistic features | 31% |
| Sensitivity to noise | 81% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makker, S.; Gagnon, B.R.; Trew, I.; Mougios, V.; Hanna, A.; Cale, J.M.; McIntosh, C.S. A Patient Case of Malan Syndrome Involving 19p13.2 Deletion of NFIX with Longitudinal Follow-Up and Future Prospectives. J. Clin. Med. 2024, 13, 6575. https://doi.org/10.3390/jcm13216575
Makker S, Gagnon BR, Trew I, Mougios V, Hanna A, Cale JM, McIntosh CS. A Patient Case of Malan Syndrome Involving 19p13.2 Deletion of NFIX with Longitudinal Follow-Up and Future Prospectives. Journal of Clinical Medicine. 2024; 13(21):6575. https://doi.org/10.3390/jcm13216575
Chicago/Turabian StyleMakker, Simran, Bernadine R. Gagnon, Isabella Trew, Vivian Mougios, Anne Hanna, Jessica M. Cale, and Craig S. McIntosh. 2024. "A Patient Case of Malan Syndrome Involving 19p13.2 Deletion of NFIX with Longitudinal Follow-Up and Future Prospectives" Journal of Clinical Medicine 13, no. 21: 6575. https://doi.org/10.3390/jcm13216575
APA StyleMakker, S., Gagnon, B. R., Trew, I., Mougios, V., Hanna, A., Cale, J. M., & McIntosh, C. S. (2024). A Patient Case of Malan Syndrome Involving 19p13.2 Deletion of NFIX with Longitudinal Follow-Up and Future Prospectives. Journal of Clinical Medicine, 13(21), 6575. https://doi.org/10.3390/jcm13216575






