Enhancing ICU Outcomes Through Intelligent Monitoring Systems: A Comparative Study on Ventilator-Associated Events
Abstract
:1. Introduction
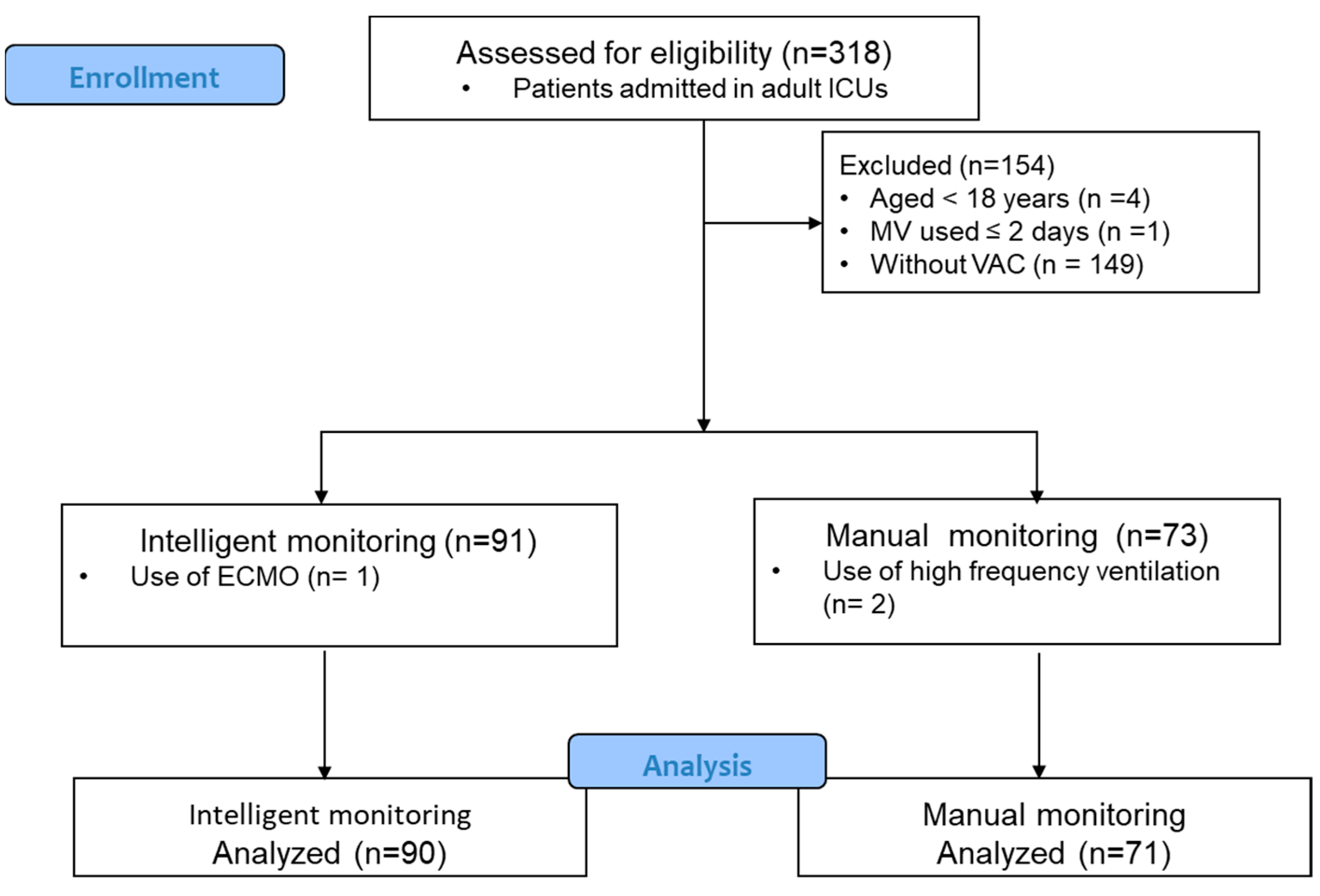
2. Materials and Methods
2.1. Implementation of the VAE Monitoring System
2.2. Data Collection
- Demographic characteristics: data collected included the diagnosis, age, sex, weight, body mass index (BMI), acute diagnosis upon ICU admission, reason for intubation, medical history, and ICU type.
- VAE-related characteristics: the type of VAC (FiO2 increase by ≥0.20, PEEP increase by >3 cm H2O, or simultaneous increases in both FiO2 and PEEP), occurrences of IVAC and PVAP, Acute Physiology and Chronic Health Evaluation (APACHE) II scores at the onset and resolution of VAE, Sequential Organ Failure Assessment (SOFA) scores, the Charlson Comorbidity Index (CCI), and the number and frequency of VAC occurrences and duration were recorded.
- Infection-related characteristics: These included suspected infection sites (e.g., pulmonary, intra-abdominal, urinary, bacteremia, or unidentified infections). Pulmonary-related conditions, such as respiratory tract infections, atelectasis/sputum congestion, pulmonary embolism, pleural effusion, and aspiration pneumonia, were also documented. Extrapulmonary issues such as systemic inflammatory response syndrome, sepsis, cardiovascular complications, volume overload, heart failure, and abdominal distension were recorded.
- Clinical outcomes: this study recorded the number of days on a ventilator; extubation failures; successful ventilator liberation; antibiotic use; and 7-day, 14-day, and 90-day mortality rates.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, J.G.; Day, A.; Heyland, D.K. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. S1), S120–S125. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Smyer, J.; Flaherty, J.J.A.J.O.I.C. Development and validation of an automated ventilator associated event electronic surveillance system: It works! Am. J. Infect. Control 2016, 44, S20–S21. [Google Scholar] [CrossRef]
- Klompas, M.; Khan, Y.; Kleinman, K.; Evans, R.S.; Lloyd, J.F.; Stevenson, K.; Samore, M.; Platt, R.; Program, F.T.C.P.E. Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS ONE 2011, 6, e18062. [Google Scholar] [CrossRef]
- Klompas, M. Ventilator-Associated Events: What They Are and What They Are Not. Respir. Care 2019, 64, 953–961. [Google Scholar] [CrossRef]
- Esper, A.M.; Arabi, Y.M.; Cecconi, M.; Du, B.; Giamarellos-Bourboulis, E.J.; Juffermans, N.; Machado, F.; Peake, S.; Phua, J.; Rowan, K.; et al. Systematized and efficient: Organization of critical care in the future. Crit. Care 2022, 26, 366. [Google Scholar] [CrossRef]
- Shenoy, E.S.; Rosenthal, E.S.; Shao, Y.P.; Biswal, S.; Ghanta, M.; Ryan, E.E.; Suslak, D.; Swanson, N.; Moura, V., Jr.; Hooper, D.C.; et al. Real-Time, Automated Detection of Ventilator-Associated Events: Avoiding Missed Detections, Misclassifications, and False Detections Due to Human Error. Infect. Control Hosp. Epidemiol. 2018, 39, 826–833. [Google Scholar] [CrossRef]
- Mann, T.; Ellsworth, J.; Huda, N.; Neelakanta, A.; Chevalier, T.; Sims, K.L.; Dhar, S.; Robinson, M.E.; Kaye, K.S. Building and Validating a Computerized Algorithm for Surveillance of Ventilator-Associated Events. Infect. Control. Hosp. Epidemiol. 2015, 36, 999–1003. [Google Scholar] [CrossRef]
- Wolffers, O.; Faltys, M.; Thomann, J.; Jakob, S.M.; Marschall, J.; Merz, T.M.; Sommerstein, R. An automated retrospective VAE-surveillance tool for future quality improvement studies. Sci. Rep. 2021, 11, 22264. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.; Zhu, S.; Wang, M.; Kang, Y.; Zhang, R.; Zou, K.; Zong, Z.; Sun, X. The epidemiology and clinical outcomes of ventilator-associated events among 20,769 mechanically ventilated patients at intensive care units: An observational study. Crit. Care 2021, 25, 44. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Ramírez-Estrada, S.; Romero, A.; Arvaniti, K.; Koulenti, D.; Nseir, S.; Oztoprak, N.; Bouadma, L.; Vidaur, L.; Lagunes, L.; et al. Factors associated with ventilator-associated events: An international multicenter prospective cohort study. Clin. Microbiol. Infect. 2019, 38, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.F.; Fang, Y.T.; Huang, C.H.; Chen, Y.M.; Chang, Y.C.; Lin, C.Y.; Hung, K.Y.; Chang, Y.T.; Chen, H.C.; Huang, K.T.; et al. Risk factors and associated outcomes of ventilator-associated events developed in 28 days among sepsis patients admitted to intensive care unit. Sci. Rep. 2020, 10, 12702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, W.; Kang, Y.; He, Q.; Zhang, H.; Deng, Y.; Cai, L.; Zhang, R.; Sun, X.; Zong, Z. Clinical outcomes and risk factors for mortality from ventilator-associated events: A registry-based cohort study among 30,830 intensive care unit patients. Infect. Control. Hosp. Epidemiol. 2022, 43, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Uchino, S.; Takinami, M.; Uezono, S. The Impact of Ventilator-Associated Events in Critically Ill Subjects With Prolonged Mechanical Ventilation. Respir. Care 2017, 62, 1379–1386. [Google Scholar] [CrossRef]
- Seaver, J.; Grant, K.; Lunn, J.; Sandor, P.; Moran, P.; Shapiro, D.S. A multidisciplinary approach to reducing ventilator-associated events in a busy urban hospital. Am. J. Infect. Control 2020, 48, 828–830. [Google Scholar] [CrossRef]
- Hebert, C.; Flaherty, J.; Smyer, J.; Ding, J.; Mangino, J.E. Development and validation of an automated ventilator-associated event electronic surveillance system: A report of a successful implementation. Am. J. Infect. Control 2018, 46, 316–321. [Google Scholar] [CrossRef]
- Peña-López, Y.; Campins-Martí, M.; Slöcker-Barrio, M.; Bustinza, A.; Alejandre, C.; Jordán-García, I.; Ortiz-Álvarez, A.; López-Castilla, J.D.; Pérez, E.; Schüffelmann, C.; et al. Ventilator-associated events in children: A multicentre prospective cohort study. Anaesth. Crit. Care Pain. Med. 2022, 41, 101072. [Google Scholar] [CrossRef]
- Dudeck, M.A.; Horan, T.C.; Peterson, K.D.; Allen-Bridson, K.; Morrell, G.; Pollock, D.A.; Edwards, J.R. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am. J. Infect. Control 2011, 39, 798–816. [Google Scholar] [CrossRef]
- Dudeck, M.A.; Weiner, L.M.; Allen-Bridson, K.; Malpiedi, P.J.; Peterson, K.D.; Pollock, D.A.; Sievert, D.M.; Edwards, J.R. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am. J. Infect. Control 2013, 41, 1148–1166. [Google Scholar] [CrossRef]
- Chahoud, J.; Semaan, A.; Almoosa, K.F. Ventilator-associated events prevention, learning lessons from the past: A systematic review. Heart Lung 2015, 44, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orto, V.; Raschetti, R.; Centorrino, R.; Montane, A.; Tissieres, P.; Yousef, N.; De Luca, D. Short-and long-term respiratory outcomes in neonates with ventilator-associated pneumonia. Pediatr. Pulmonol. 2019, 54, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Iosifidis, E.; Pitsava, G.; Roilides, E. Ventilator-associated pneumonia in neonates and children: A systematic analysis of diagnostic methods and prevention. Future Microbiol. 2018, 13, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Intelligent Monitoring (n = 90) | Manual Monitoring (n = 71) | p-Value |
|---|---|---|---|
| Age (years) | 66.6 ± 13.96 | 68.46 ± 14.04 | 0.42 b |
| BMI (kg/m2) | 25.07 ± 6.18 | 28.58 ± 24.31 | 0.18 b |
| Gender; man, n (%) | 25.07 ± 6.18 | 28.58 ± 24.31 | 0.18 b |
| Diagnosis at admission | |||
| Acute kidney injury | 11(12.2) | 13 (18.3) | 0.28 a |
| Congestive heart failure | 8 (8.9) | 7 (9.9) | 0.83 a |
| Cerebrovascular disease | 8 (8.9) | 4 (5.6) | 0.43 a |
| Chronic liver disease | 14 (15.6) | 12 (16.9) | 0.83 a |
| COPD | 11 (12.2) | 10 (14.1) | 0.52 a |
| Collagen disease | 4 (4.4) | 2 (2.8) | 0.69 a |
| DM | 12 (13.3) | 13 (18.3) | 0.39 a |
| Ischemic heart disease | 8 (8.9) | 6 (8.5) | 0.92 a |
| Malignancy | 10 (11.1) | 14 (19.7) | 0.18 a |
| Medical history | 0.47 a | ||
| Heart disease, n (%) | 5 (5.6) | 3 (4.2) | |
| Liver disease, n (%) | 9 (10.0) | 5 (7.0) | |
| Renal disease, n (%) | 24 (26.7) | 20 (28.2) | |
| Hematologic diseases, n (%) | 24 (26.7) | 18 (25.4) | |
| Respiratory disease, n (%) | 15 (16.7) | 15 (21.1) | |
| Diabetes mellitus, n (%) | 10 (11.1) | 6 (8.5) | |
| Cerebrovascular disease, n (%) | 3 (3.3) | 4 (5.6) | |
| Reason for intubation | 0.66 a | ||
| Inability to keep airway open, n (%) | 0 | 1 (1.4) | |
| Failure to protect airway from aspiration, n (%) | 8 (8.9) | 4 (5.6) | |
| Ventilation failure, n (%) | 4 (4.4) | 2 (2.8) | |
| Insufficiency in oxygenation, n (%) | 14 (15.6) | 12 (16.9) | |
| Possible conditions that may lead to respiratory failure, n (%) | 31 (34.4) | 36 (50.7) | |
| Other, n (%) | 33 (36.7) | 16 (22.5) | |
| Type of ICU | 0.55 a | ||
| GSICU, n (%) | 12 (13.3) | 16 (22.5) | |
| CVSICU, n (%) | 5 (5.5) | 3 (4.2) | |
| NSICU, n (%) | 6 (6.6) | 4 (5.6) | |
| NICU, n (%) | 15 (16.6) | 4 (5.6) | |
| MICU, n (%) | 38 (42.2) | 30 (42.3) | |
| RICU, n (%) | 24 (26.6) | 14 (19.8) |
| Parameters | Intelligent Monitoring (n = 90) | Manual Monitoring (n = 71) | p-Value |
|---|---|---|---|
| Incidence of VAC | |||
| Increased FiO2 > 0.2, n (%) | 17 (18.9) | 17 (23.9) | 0.44 a |
| Increased PEEP 9 cm H2O, n (%) | 81 (90.0) | 52 (81.7) | 0.17 a |
| Increased both FiO2 0.2 and PEEP 3 cm H2O, n (%) | 21 (23.3) | 10 (14.1) | 0.16 a |
| Number of IVAC occurrences, n (%) | 51 (56.7) | 33 (46.5) | 0.21 a |
| Number of PVAP occurrences, n (%) | 42 (46.7) | 21 (29.6) | 0.03 * a |
| APACE score at the time of VAE occurrence | 29.01 ± 10.7 | 28.72 ± 9.82 | 0.86 b |
| APACE score at the end of the VAE | 22.02 ± 6.35 | 22.01 ± 5.99 | 0.99 b |
| SOFA score at the time of VAE occurrence | 10.89 ± 7.4 | 10.48 ± 5.38 | 0.69 b |
| SOFA score at the end of the VAE | 6.76 ± 2.9 | 7.55 ± 3.48 | 0.13 b |
| CCI score at the time of VAE occurrence | 6.26 ± 3.14 | 6.59 ± 3.34 | 0.51 b |
| CCI score at the end of the VAE | 5.53 ± 2.65 | 5.70 ± 2.81 | 0.69 b |
| Days of VAC occurred from admission | 25.97 ± 23.61 | 26.73 ± 18.74 | 0.82 b |
| Days of first VAC detection following ICU admission | 4.96 ± 1.86 | 7.77 ± 3.35 | <0.001 ** b |
| Number of VAC occurrence | 1.37 ± 0.59 | 1.83 ± 0.76 | <0.001 ** b |
| Number of VAC days | 14.3 ± 14.19 | 19.14 ± 13.38 | 0.03 * b |
| Parameters | Intelligent Monitoring (n = 90) | Manual Monitoring (n = 71) | p-Value |
|---|---|---|---|
| Site of infection | |||
| Pulmonary, n (%) | 66 (73.3) | 43 (60.6) | 0.44 a |
| Intra-abdominal, n (%) | 19 (21.1) | 10 (14.1) | 0.31 a |
| Urinary tract, n (%) | 18 (20.1) | 23 (32.4) | 0.07 a |
| Bacteremia, n (%) | 16 (17.8) | 16 (22.5) | 0.55 a |
| Unidentified infection, n (%) | 5 (5.6) | 2 (2.8) | 0.47 b |
| Intrapulmonary-related | 0.39 a | ||
| None, n (%) | 24 (26.7) | 28 (39.4) | |
| Respiratory tract infection, n (%) | 24 (26.7) | 14 (19.7) | |
| Atelectasis/sputum plug, n (%) | 15 (16.7) | 13 (18.3) | |
| Pneumothorax, n (%) | 7 (7.8) | 3 (4.2) | |
| Pulmonary embolus, n (%) | 2 (2.2) | 1 (1.4) | |
| Pleural effusion, n (%) | 15 (16.7) | 7 (12.7) | |
| Aspiration, n (%) | 3 (3.3) | 5 (7.0) | |
| Extrapulmonary-related | 0.39 a | ||
| None, n (%) | 31 (34.4) | 32 (45.1) | |
| New onset of SIRS/sepsis, n (%) | 26 (28.9) | 20 (28.2) | |
| Cardiac/circulatory, n (%) | 8 (8.9) | 4 (5.6) | |
| Volume overload, n (%) | 7 (7.8) | 9 (7.1) | |
| Heart failure, n (%) | 11 (12.2) | 2 (2.8) | |
| Abdominal distension, n (%) | 4 (4.4) | 1 (1.4) | |
| No reason for VAC identified, n (%) | 3 (3.3) | 3 (4.2) |
| Parameters | Intelligent Monitoring (n = 90) | Manual Monitoring (n = 71) | p-Value |
|---|---|---|---|
| Ventilator days | 26.17 (25.24) | 34.14 (24.68) | 0.04 * b |
| Extubation failure | 0.67 (0.75) | 0.79 (1.05) | 0.062 b |
| Reintubation, n (%) | 30 (33.3) | 31 (43.7) | 0.19 a |
| Successful weaning from the ventilator, n (%) | 71 (78.9) | 50 (70.4) | 0.27 a |
| Days of antibiotic use | 22.04 (22.78) | 32.37 (28.57) | 0.01 * b |
| 7-day mortality, n (%) | 8 (8.9) | 12 (16.9) | 0.15 a |
| 14-day mortality, n (%) | 18 (20.0) | 25 (35.2) | 0.03 * a |
| 90-day mortality, n (%) | 20 (44.4) | 25 (55.6) | 0.79 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-F.; Kang, M.-Y.; Lin, H.-L.; Liu, S.-F. Enhancing ICU Outcomes Through Intelligent Monitoring Systems: A Comparative Study on Ventilator-Associated Events. J. Clin. Med. 2024, 13, 6600. https://doi.org/10.3390/jcm13216600
Liu J-F, Kang M-Y, Lin H-L, Liu S-F. Enhancing ICU Outcomes Through Intelligent Monitoring Systems: A Comparative Study on Ventilator-Associated Events. Journal of Clinical Medicine. 2024; 13(21):6600. https://doi.org/10.3390/jcm13216600
Chicago/Turabian StyleLiu, Jui-Fang, Mei-Ying Kang, Hui-Ling Lin, and Shih-Feng Liu. 2024. "Enhancing ICU Outcomes Through Intelligent Monitoring Systems: A Comparative Study on Ventilator-Associated Events" Journal of Clinical Medicine 13, no. 21: 6600. https://doi.org/10.3390/jcm13216600
APA StyleLiu, J.-F., Kang, M.-Y., Lin, H.-L., & Liu, S.-F. (2024). Enhancing ICU Outcomes Through Intelligent Monitoring Systems: A Comparative Study on Ventilator-Associated Events. Journal of Clinical Medicine, 13(21), 6600. https://doi.org/10.3390/jcm13216600







