Abstract
Background/Objectives: Clinical trials have investigated the efficacy of rehabilitation robotics for various pathological conditions, but the overall impact on rehabilitation practice remains unclear. We comprehensively examined and analyzed systematic reviews (SRs) of randomized controlled trials (RCTs) investigating rehabilitative interventions with robotic devices. Methods: Four databases were searched using term combinations of keywords related to robotic devices, rehabilitation, and SRs. The SR meta-analyses were categorized into “convincing”, “highly suggestive”, “suggestive”, “weak”, or “non-significant” depending on evidence strength and validity. Results: Overall, 62 SRs of 341 RCTs involving 14,522 participants were identified. Stroke was most frequently reported (40 SRs), followed by spinal cord injury (eight SRs), multiple sclerosis (four SRs), cerebral palsy (four SRs), Parkinson’s disease (three SRs), and neurological disease (any disease causing limited upper- and lower-limb functioning; three SRs). Furthermore, 38, 21, and 3 SRs focused on lower-limb devices, upper-limb devices, and both upper- and lower-limb devices, respectively. Quantitative synthesis of robotic intervention effects was performed by 51 of 62 SRs. Robot-assisted training was effective for various outcome measures per disease. Meta-analyses offering suggestive evidence were limited to studies on stroke. Upper-limb devices were effective for motor control and activities of daily living, and lower-limb devices for walking independence in stroke. Conclusions: Robotic devices are useful for improving impairments and disabilities in several diseases. Further high-quality SRs including RCTs with large sample sizes and meta-analyses of these RCTs, particularly on non-stroke-related diseases, are required. Further research should also ascertain which type of robotic device is the most effective for improving each specific impairment or disability.
1. Introduction
The number of people with physical impairments caused by diseases is increasing especially in countries with an aging population, leading to physical disabilities that substantially affect their activities of daily living (ADLs). It was recently reported that 2.41 billion individuals have conditions that would benefit from rehabilitation medicine [1]. In rehabilitation for physical impairments, an appropriate number of training programs that do not cause overuse and disuse should be provided to patients with optimal task difficulty in learning, repeated practice, and feedback. In addition to conventional physical and occupational therapy, interventions using technologies have been proposed to support the achievement of this essential goal. In particular, numerous novel robotic devices equipped with excellent features, such as high-precision sensing and accurate actuators without time delay, have been developed to establish better rehabilitation programs [2,3]. These robotic devices enable optimal assistance and load settings using feedback based on quantitative evaluation.
Many reviews concerned with the effect of robotic devices have been published for different diseases. Recent reviews have reported that various types of robotic devices for rehabilitative interventions could improve physical impairments and disabilities in patients with various diseases [4,5,6,7,8], and some clinical guidelines have suggested the use of robotic devices in the treatment of specific diseases [9,10]. Nevertheless, thus far, no review has provided a comprehensive overview of the cross-disease effectiveness of currently available robot-assisted training (RT). Through this umbrella review, we aimed to evaluate the comprehensive effectiveness of RT or robotic devices for medical rehabilitation, focusing on five areas: number of systematic reviews (SRs), randomized controlled trials (RCTs), and participants studied for each disease; quality of SRs for each disease; types of robotic devices used for each disease; outcome measures used in SRs for each disease; and effectiveness of RT for each outcome measure.
2. Materials and Methods
2.1. Study Design and Registration
The present study was designed as an umbrella review to comprehensively analyze SRs on the effectiveness of robotic devices for medical rehabilitation. The reporting of the key aspects of methods and results in this study adhered to the protocol for the Preferred Reporting Items for Overviews of Reviews (PRIOR) [11] guidelines. The review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/prospero/, accessed on 10 June 2024) (registration number: CRD42024553070).
2.2. Criteria for Considering Studies for This SR
2.2.1. Type of SRs
The present review considered SRs that assessed the effect of RT on upper- and lower-limb rehabilitation in patients with various symptoms and diseases and were published in English after 2009. SRs that met one of the following two criteria were included: (i) SRs of only RCTs with or without meta-analysis and (ii) SRs including at least one meta-analysis using only RCTs. The exclusion criteria were as follows: (i) SRs that did not include RCTs and (ii) SRs that did not perform a meta-analysis or that conducted a meta-analysis for only a mixture of both RCTs and non-RCTs in the case of SRs involving both RCTs and non-RCTs. (iii) SRs comparing effects through indirect comparison (network meta-analysis).
2.2.2. Types of Participants
The present review considered participants with various symptoms and diseases but without any specific exclusion criteria, such as pathological conditions, age, and sex.
2.2.3. Types of Interventions
The present review included interventions with robotic devices for rehabilitative treatment in patients with physical impairments and disabilities. Among various types of robotic devices developed for medical rehabilitation, robots involving interactive automation, sensors, and dynamic control mechanisms were included. However, (i) devices without features that deliver passive motion, such as treadmills, bicycles, and other simple mechanical trainers and (ii) a combination of robotics that utilized transcranial direct current stimulation and electromyography/electroencephalography during robotic gait training were excluded from analysis. The targeted robotic devices comprised different types of exoskeletons or end-effectors and unilateral or bilateral devices.
2.2.4. Types of Outcome Measures
The present review included all physical function measures such as gait function and upper-limb function measures; ADL measures such as the Functional Independence Measure (FIM) and Barthel Index; and quality of life (QOL) measures.
2.3. Search Methods for the Identification of Studies
MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, and PEDro were searched for studies published from 1 January 2009 to 31 December 2023 using term combinations of keywords (“rehabilitation” OR “physical therapy” OR “occupational therapy”) AND (“robotics” OR “robotic devices” OR “electromechanical” OR “robot-assisted”) AND (“systematic review” OR “meta-analysis”).
2.4. Data Collection Process and Analysis
2.4.1. Selection of SRs
Two reviewers (K.K. and Y.O.) independently screened the titles and abstracts of all records. Any discrepancies between the reviewers were resolved through discussion or consultation with a third reviewer (S.T.) until consensus was achieved.
2.4.2. Data Extraction and Management
Full texts were acquired, and two independent reviewers (K.K. and Y.O.) assessed them for inclusion. The first reviewer (K.K.) extracted the data from the SRs, and the second reviewer (Y.O.) subsequently checked the accuracy of these data. Errors were considered, and corrections were made. Disagreements regarding data extraction were resolved through discussion or by consulting a third reviewer (S.T.) until consensus was achieved.
2.4.3. Managing Overlap of Primary Studies
A list of the primary studies included in each SR was assembled, and a matrix table was created to determine overlap between SRs. To avoid double counting outcome data, the number of RCTs, study participants, and robotic devices were calculated from RCTs that excluded overlap between SRs contained in the present review.
2.5. Analysis and Synthesis of Results
2.5.1. Characteristics of Each SR
Data were extracted from each SR, and a table presenting characteristics such as participants, interventions, and comparators was created.
2.5.2. Number of the Included SRs, RCTs, and Participants
The number of SRs, RCTs included in each SR, and participants enrolled in each RCT was examined with respect to diseases (stroke, spinal cord injury, etc.) and type of devices (upper- or lower-limb devices).
2.6. Quality Assessment of SRs
Two reviewers (K.K. and Y.O.) independently evaluated all SRs using the A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2) checklist [12], which contains 16 items such as the literature search procedure, data extraction, quality assessment, and statistical analyses of meta-analyses. In line with recommendations, Items 2, 4, 7, 9, 11, 13, and 15 were extracted as critical domains for AMSTAR 2 [12]. The overall score of AMSTAR 2 was used to rate the quality of each SR included in this review on the grade of 4 scale (high, moderate, low, or critically low) [12].
2.7. Robotic Devices Used in the Included SRs
The type of robots utilized was investigated according to diseases (stroke, spinal cord injury, etc.), and the number of robotic devices used was summarized.
2.8. Outcome Measures Used in the Included SRs
Five reviewers (K.K., T.I., Y.N., K.T., and Y.O.) discussed the reported outcome measures in each SR and categorized them according to the International Classification of Functioning, Disability and Health (ICF) using linking rules [13,14].
2.9. Grading the Evidence for the Effectiveness of RT
Depending on the strength and validity of evidence, meta-analyses of all SRs were categorized into “convincing”, “highly suggestive”, “suggestive”, “weak”, or “non-significant” [15]. “Convincing” meta-analyses were defined as meta-analyses that satisfied all of the following criteria: statistical significance at p < 10−6 per the random-effects model; based on >1000 participants; without large between-study heterogeneity (I2 < 50%); 95% prediction intervals excluding the null value; and no evidence of small-study effects and excess significance bias. “Highly suggestive” meta-analyses were defined as meta-analyses based on >1000 participants that included the largest study presenting a statistically significant effect at p < 10−6. “Suggestive” meta-analyses referred to meta-analyses based on >1000 participants with a significant effect at p < 10−3. The remaining nominally significant associations (p < 0.05) were considered to provide “weak” evidence. No statistically significant differences were classified as “non-significant”.
The largest number of participants in the meta-analyses corresponding to each disease and outcome measure category was extracted and summarized as the effectiveness of robot-assisted rehabilitation on various outcome measures in each disease. Stroke was further categorized as acute, chronic, and overall (no-specific phase) stroke. The phases from onset were classified into the following four categories according to each paper: (i) <3 months or ≤3 months; (ii) >3 months or ≥3 months; (iii) <6 months or ≤6 months; and (iv) >6 months or ≥6 months. Additionally, proximal and distal motor control was summarized as a subclassification of upper-limb motor control in stroke.
3. Results
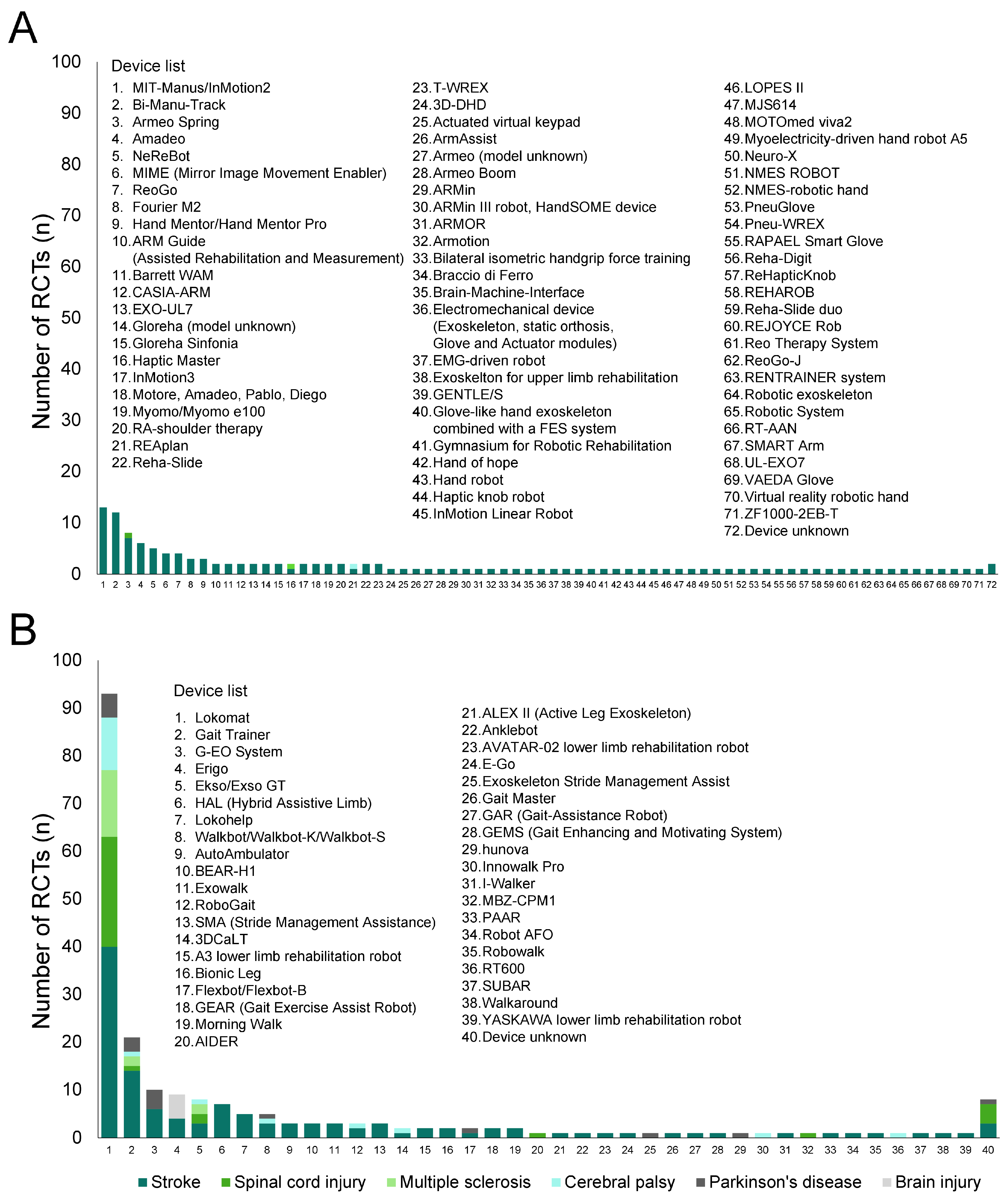
Figure 1 presents the PRIOR flow diagram. The database search resulted in 660 abstracts. The two reviewers identified 141 articles by independent screening, for which the full texts were obtained. Independent screening of these 141 articles led to the selection of 62 SRs satisfying all criteria. Table S1 summarizes the characteristics of the included studies, whereas Table S2 lists the 79 excluded studies along with the reasons for exclusion.
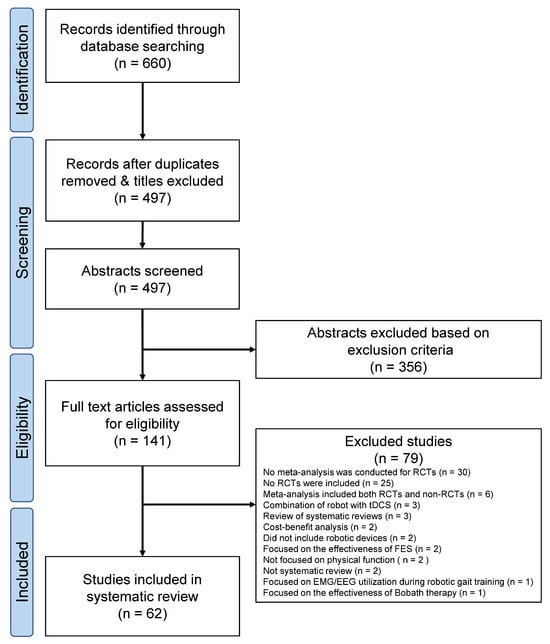
Figure 1.
PRIOR flowchart. EEG, electroencephalography; EMG, electromyography; RCT, randomized controlled trials; tDCS, transcranial direct current stimulation.
3.1. Number of Included SRs, RCTs, and Participants
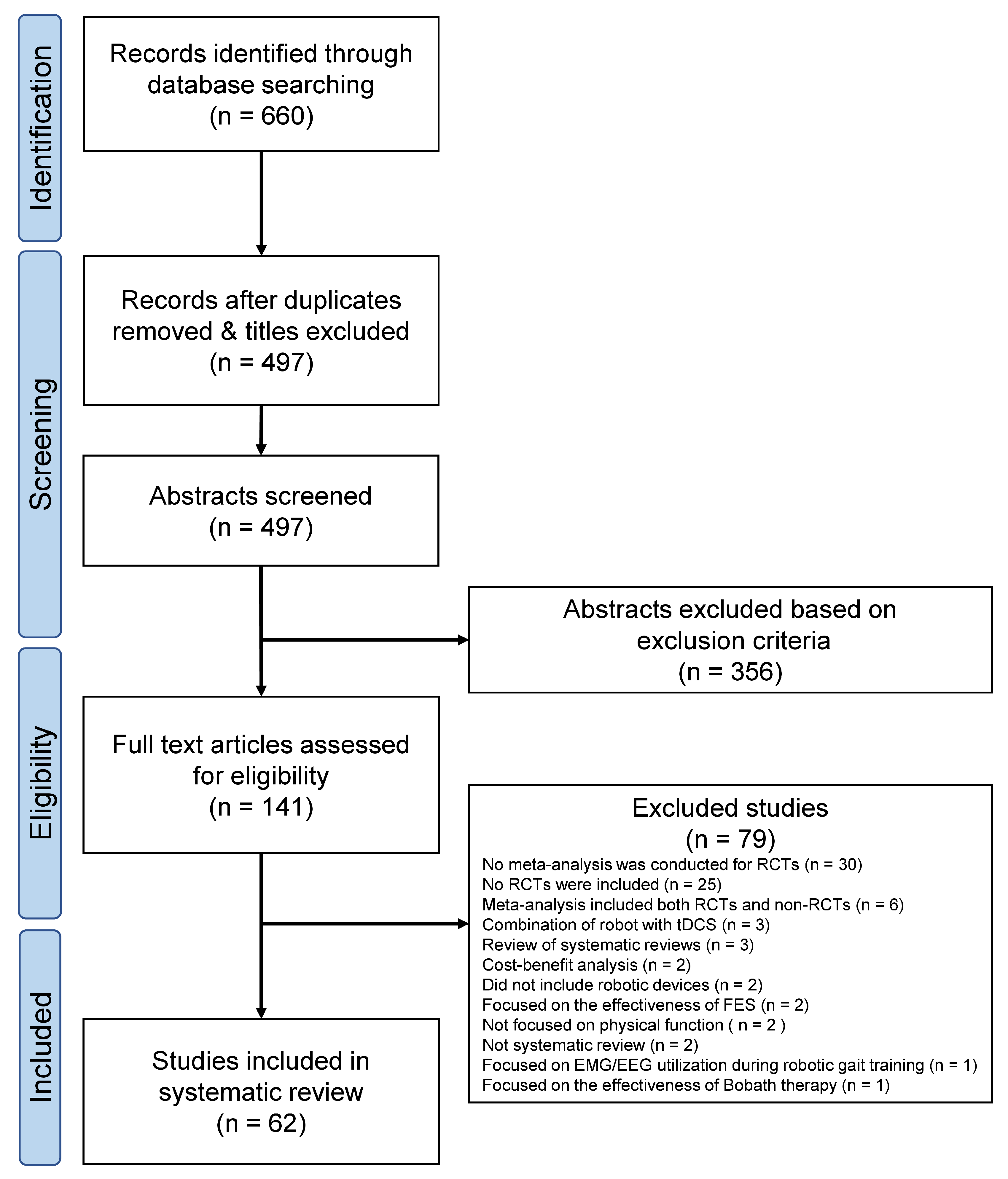
Figure 2 shows the number of included SRs, RCTs, and participants (excluding overlaps in RCTs and their participants) according to diseases. Among the 62 SRs identified, stroke was the most frequently reported disease, with 40 SRs (upper-limb devices, 19 SRs [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]; lower-limb devices, 19 SRs [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]; upper- and lower-limb devices, 2 SR [54,55]), followed by spinal cord injury with 8 SRs (lower-limb devices, 7 SRs [56,57,58,59,60,61,62]; upper- and lower-limb devices, 1 SR [63]), multiple sclerosis with 4 SRs (lower-limb devices, 4 SRs [64,65,66,67]), cerebral palsy with 4 SRs (lower-limb devices, 4 SRs [68,69,70,71]), Parkinson’s disease with 3 SR (lower-limb devices, 3 SR [72,73,74]), and neurological disease (any disease causing limited upper-, and lower-limb functioning) with 3 SRs (upper-limb devices, 2 SRs [75,76]; lower-limb device, 1 SR [77]).
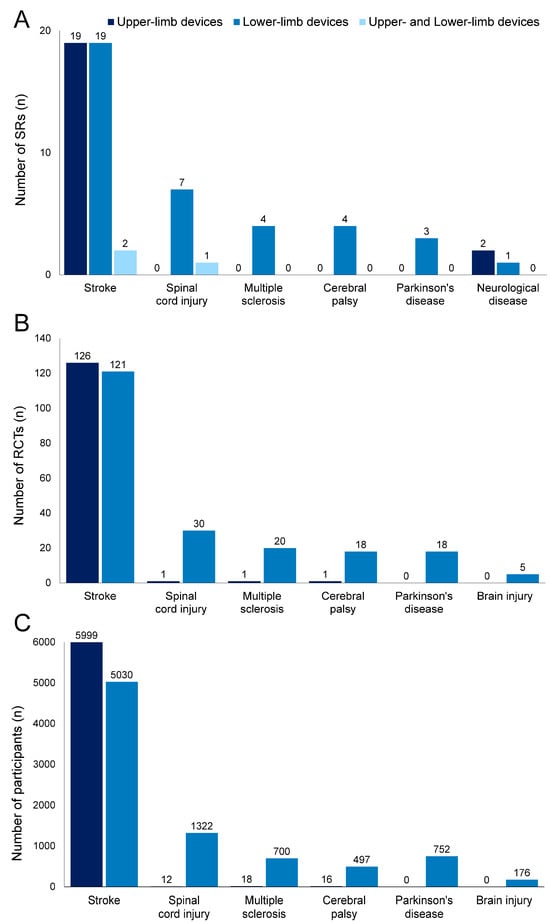
Figure 2.
Number of systematic reviews (SRs), randomized controlled trials (RCTs), and participants according to diseases. Dark blue, upper-limb devices; blue, lower-limb devices; light blue, upper- and lower-limb devices. Number of SRs (A), RCTs (B), and participants (C). SRs on neurological disease included RCTs investigating stroke, cerebral palsy, and brain injury. Duplicates were excluded from RCTs (B) and corresponding participants (C).
Table S3 (upper-limb devices) and Table S4 (lower-limb devices) show detailed information for all RCTs included in the SRs. The number of overlapping primary studies between SRs is extracted in Tables S3 and S4. The number of RCTs was 1110 and the number of participants was 48,099 before study overlap was removed. The most overlapping RCTs for upper and lower limb devices were used in 13 SRs and 12 SRs, respectively. After removing duplicates, 341 RCTs were included in SRs involving 14,522 participants. With respect to stroke, 126 RCTs (5999 participants) focused on upper-limb devices, whereas 121 RCTs (5030 participants) focused on lower-limb devices. Regarding spinal cord injury, one RCT (12 participants) focused on upper-limb devices, whereas 30 RCTs (1322 participants) focused on lower-limb devices. Regarding multiple sclerosis, one RCT (18 participants) focused on upper-limb devices, whereas 20 RCTs (700 participants) focused on lower-limb devices. Regarding cerebral palsy, one RCT (16 participants) focused on upper-limb devices, whereas 18 RCTs (497 participants) focused on lower-limb devices. Regarding Parkinson’s disease, 18 RCTs (752 participants) focused on lower-limb devices. SRs on neurological disease included RCTs investigating stroke, cerebral palsy, and brain injury. Among them, five RCTs on brain injury (176 participants) focused on lower-limb devices.
3.2. Quality Assessment of SRs
Table S5 shows the methodological quality of the 62 included SRs, as assessed using AMSTAR 2. A total of 51 SRs [19,20,21,22,23,24,25,26,27,28,29,30,32,33,34,35,37,38,39,40,41,42,44,45,46,47,48,50,51,52,53,54,55,56,57,58,59,60,61,63,64,66,67,68,69,71,72,73,74,75,77] performed meta-analyses, whereas the remaining 11 SRs [16,17,18,20,31,43,49,62,65,70,76] attempted qualitative synthesis only. The quality of included reviews ranged from critically low to high. Of the 62 SRs, 3, 3, 7, and 49 SRs were classified to be of high, moderate, low, and critically low quality, respectively. For patients with stroke, 21 SRs conducted assessment of the included trials on upper-limb devices, and their quality ranged from critically low to high (AMSTAR 2 assessment: high for 1 SR, moderate for 1 SR, low for 4 SRs, and critically low for 15 SRs); 21 SRs also performed assessment of the included trials on lower-limb devices, and their quality ranged from critically low to high (AMSTAR 2 assessment: high for 2 SRs, moderate for 1 SR, low for 2 SRs, and critically low for 16 SRs). The reviews conducted by Saragih [54] and Lo [55] were counted as both upper- and lower-limb device studies. For patients with spinal cord injury, eight SRs conducted an assessment of the included trials on lower-limb devices, and their quality was critically low to moderate (AMSTAR 2 assessment: moderate for one SR, low for one SR, critically low for six SRs); one SR performed an assessment of the included trials on upper-limb devices, and its quality was also determined to be critically low based on the AMSTAR 2 assessment. The reviews conducted by Cheung [63] counted as both upper- and lower-limb device studies. For patients with multiple sclerosis, four SRs conducted assessment of the included trials on lower-limb devices, and their quality was critically low and moderate (AMSTAR 2 assessment: moderate for one SR, critically low for three SRs). With respect to other conditions, all SRs on upper- and lower-limb devices for cerebral palsy, Parkinson’s disease, and neurological disease achieved a critically low value based on AMSTAR 2 assessment.
Various critical flaws were identified in the reporting of the included SRs. Specifically, 87% of the SRs did not perform an adequate literature search (Q4), and 79% did not provide justification for excluding studies (Q7). Conversely, 94% of the included SRs reported the assessed risk of bias (Q9) and 84% of the included SRs used appropriate meta-analytical methods (Q11).
3.3. Robotic Devices Used in the Included SRs
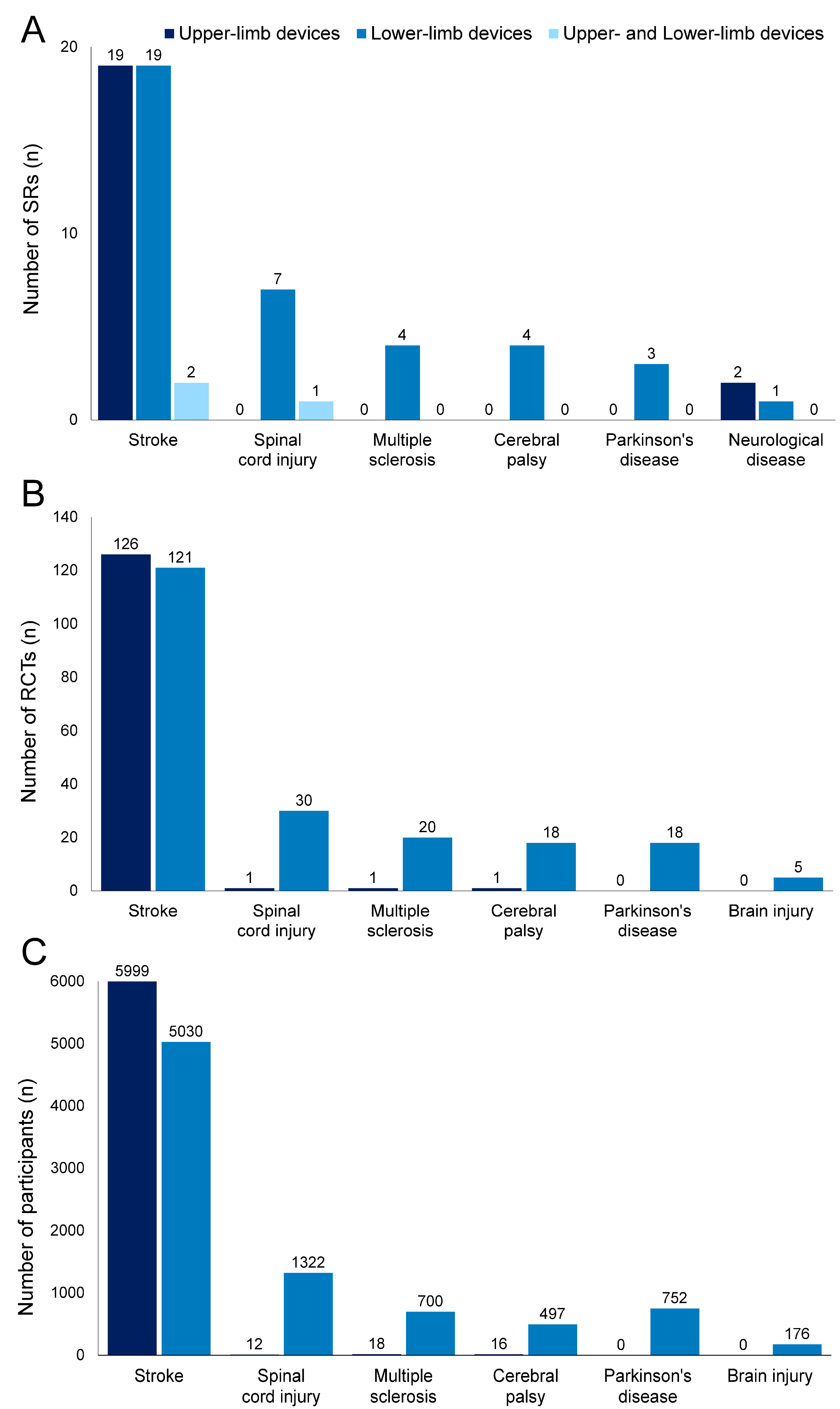
Figure 3 presents histograms of upper-limb (Figure 3A) and lower-limb (Figure 3B) robotic devices used in the RCTs included in the SRs (excluding overlaps). Detailed numbers are presented in Tables S6 and S7.
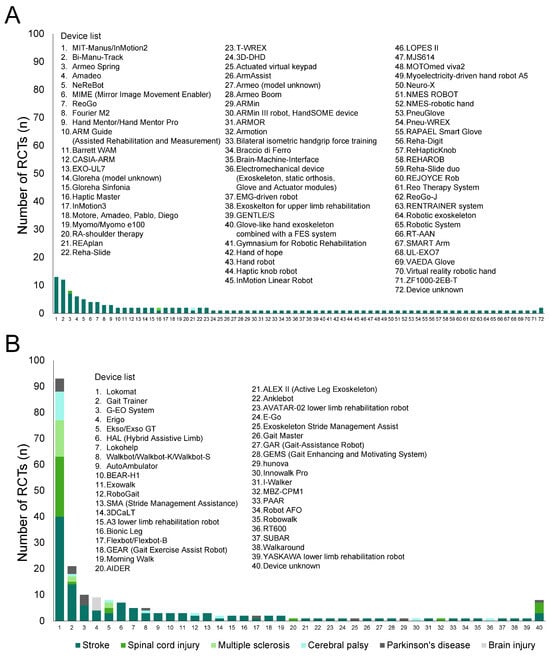
Figure 3.
Number of robotic devices according to diseases. (A) Upper-limb devices; (B) lower-limb devices.
In the 129 analyzed studies, 71 types of upper-limb robotic devices, except for unknown devices, were used. All types were used in studies on patients with stroke, and three of the devices were also utilized in studies on patients with spinal cord injury, multiple sclerosis, and cerebral palsy. The most frequently used device was MIT-Manus/InMotion2 (13 RCTs), followed by Bi-Manu-Track (12 RCTs).
In the 212 analyzed studies, 39 types of lower-limb robotic devices, except for unknown devices, were used. Among the devices, 33 types were used in studies on stroke, whereas 5, 3, 8, 7, and 1 types were used in studies on spinal cord injury, multiple sclerosis, cerebral palsy, Parkinson’s disease and brain injury, respectively. The most frequently used device was Lokomat (93 RCTs) followed by Gait Trainer (21 RCTs).
3.4. Outcome Measures Used in the Included SRs
Table 1 and Table 2 show the outcome measures used in the included SRs for upper-limb and lower-limb devices, respectively. Outcome measures were categorized as “Body functions”, “Activities and participation”, and “Other measures” according to ICF. “Body functions” included motor control, muscle strength, muscle tone, range of motion, sensory function, pain, and fatigue. “Activities and participation” included upper-limb capacity, walking independence, walking speed, walking capacity, gait index, balance capacity, ADLs, and comprehensive measure. Lastly, “Other measures” included outcomes that fit into neither category of “Body functions” and “Activities and participation”.

Table 1.
Outcome measures used in the included studies on upper-limb devices.

Table 2.
Outcome measures used in the included studies on lower-limb devices.
Regarding upper-limb devices, studies on patients with stroke utilized various outcome measures on various aspects, ranging from body functions to QOL. In particular, 18 and 10 SRs performed meta-analyses with the Fugl-Meyer Assessment for Upper Extremity (FMA-UE) and the FIM, respectively; the Stroke Impact Scale was used in eight SRs for meta-analyses. In contrast, studies on patients with neurological disease used few outcome measures for meta-analysis. No outcome measures were used for meta-analyses of spinal cord injury.
As for lower-limb devices, studies on patients with stroke frequently used gait-related outcomes. Specifically, the Functional Ambulation Scale (FAC) was used in 12 SRs for meta-analysis, the 6-Minute Walk Test was used in 8 SRs for meta-analysis. Studies on patients with spinal cord injury, multiple sclerosis, cerebral palsy, and Parkinson’s disease used various aspect components; the outcome measures frequently used for meta-analysis were gait-related outcomes, notably walking speed and capacity. Table S8 presents the outcome measures used in the included studies in detail.
3.5. Effectiveness of RT
Table 3 and Table 4 show the effectiveness of RT and grading of evidence in various outcome measures for each disease. With respect to upper-limb devices, RT showed “suggestive” effectiveness for overall (including proximal and distal) motor control and ADLs in patients with stroke and “weak” effectiveness for proximal motor control, muscle strength, muscle tone, pain, and upper-limb capacity. Regarding the effectiveness by phases from onset, “weak” effectiveness was found for motor control, muscle tone, and ADLs in several phases from onset; however, there was no “suggestive” effectiveness. As for lower-limb devices, RT showed “suggestive” effectiveness for walking independence and “weak” effectiveness for motor control, walking speed, and balance capacity in patients with stroke (no specific phase). By phases from onset, effectiveness on walking independence was “suggestive” even in SRs limited to the acute phase, and “weak” effectiveness was found for gait index regardless of phases. RT exhibited “weak” effectiveness for muscle strength, walking independence, balance capacity, and ADLs in patients with spinal cord injury; muscle tone, fatigue, walking speed, walking capacity, and balance capacity in patients with multiple sclerosis; and walking speed, walking capacity, gait index, and other measures in patients with Parkinson’s disease. In patients with cerebral palsy, RT showed no significant differences compared with conventional therapy.

Table 3.
Effectiveness of robot-assisted training with upper-limb devices.

Table 4.
Effectiveness of robot-assisted gait training with lower-limb devices.
4. Discussion
In the present study, we thoroughly reviewed SRs on the effectiveness of robotic devices for medical rehabilitation and successfully elucidated the current quantity and quality of evidence, types of interventions and outcome measures used, and knowledge regarding the efficacy of robotic devices on various outcome measures in several diseases.
4.1. Number of the Included SRs, RCTs, and Participants
A substantially greater number of SRs focused on stroke than on other diseases (as shown in Figure 2). Furthermore, more SRs and RCTs focused on lower-limb devices than on upper-limb devices.
Two possible reasons could explain the limited number of SRs and RCTs for diseases other than stroke. First, the number of patients with stroke is predominantly higher than that of patients with other diseases and conditions. As of 2019, the global prevalence of stroke was estimated to be 86 million people, whereas those of spinal cord injury, Parkinson’s disease, and multiple sclerosis were 21 million, 3.9 million, and 1.4 million people, respectively [1]. Cost–benefit issues when attempting to develop robots or modify existing robots, such as size-down for pediatric use, may have also contributed to the poor availability of robotic devices and smaller amount of evidence in relatively fewer diseases. Second, the adaptability of robotic devices differs among pathological conditions. For instance, limb paresis in patients with stroke may be relatively easy to target when considering the application of robotic devices to aid them; thus, designing a robot may be easier. In contrast, narrowing down the target of RT and designing a robot are both difficult for patients with Parkinson’s disease, who present with bradykinesia and rigidity as systemic symptoms, and patients with multiple sclerosis, where the site of impairment varies between time and among individuals. This background may also be the reason for the lack of robotic applications.
4.2. Quality Assessment of SRs
Quality assessment of the reviews indicated that most SRs included for each disease were of critically low quality (49 SRs). SRs deemed to be of high methodological quality could only be found for patients with stroke. Hence, high-quality reviews on patients with other diseases or conditions are required, and the quality of each SR should be improved by disclosing the adequacy of the literature search for each RCT and justification for excluding studies.
4.3. Robotic Devices Used in the Included SRs
This study also explored the types of robotic devices used in the RCTs included in the SRs. A total of 71 upper-limb robotic devices and 39 lower-limb robotic devices were utilized in the trials (as shown in Figure 3). Compared with upper-limb devices, the types of lower-limb devices used were limited. Among lower-limb devices, Lokomat followed by Gait Trainer were investigated and used in most RCTs. Although the SRs included several RCTs, most presented evidence was from trials with a limited number of robots. Therefore, the results strongly reflect the effects of specific types of robots rather than the effects of robots in general. If multiple robots are combined in a study, the robots used in only a small number of RCTs may not be well represented, and the effects could be overshadowed. Sub-analyses of the types of robots used provide some ideas [25,33,41,66,68]; nonetheless, current studies have not clarified the optimal matching of pathological conditions and appropriate type of robotic devices, and more studies focusing on each device are needed to determine which robotic devices are effective against each disease.
4.4. Outcome Measures Used in the Included SRs
For upper-limb devices, the most frequently investigated outcome measure was the FMA-UE, which is related to ICF’s control of voluntary movement functions [b760]. Robotic rehabilitation could enhance motor control by providing patients with intensive and repetitive rehabilitation training and by consequently boosting the use-dependent plasticity process [78,79]. Considering that upper-limb devices are suitable for performing repetitive motion tasks, it is reasonable that outcome measures related to the control of voluntary movement functions have been often used in SRs.
For lower-limb devices, the most frequently investigated outcome measures were the FAC, which is related to walking independence of ICF’s walking [b450] domain. This result may be attributed to the fact that most of lower-limb robots are designed to assist in the walking independence of patients who are unable to walk. Lower-limb devices aim to improve walking performance, given that the main role of the lower limbs is mobility. Walking independence has often been set as a goal because it is important for medical rehabilitation.
4.5. Effectiveness of RT and Implications to Future Studies
Based on the included SRs, RT including upper and lower-limb devices was found to be effective for patients with stroke, spinal cord injury, multiple sclerosis, and Parkinson’s disease compared to conventional therapy or other interventions (as shown in Table 3 and Table 4). Specifically, the effectiveness of RT was rated as “suggestive” for upper-limb motor control and ADLs on patients with stroke (no specific phase) and for walking independence on patients with acute stroke. Evidence supporting the use of RT for patients with spinal cord injury, multiple sclerosis, cerebral palsy, and Parkinson’s disease was rated as “weak” for several outcomes. Thus, further studies, including RCTs with large sample sizes and meta-analyses of RCTs, should be conducted. This is especially important for diseases identified in the present study other than stroke and for diseases not identified in the study, such as musculoskeletal disorders. Additionally, because the effectiveness depending on severity and length from onset is still unclear, further studies should assess the effects of each robotic device while taking the disease severity and time after onset into account. By focusing on exploring the mechanisms underlying their effects helps to determine which type of robotic device is the most effective tool for improving each specific type of impairment or disability.
Additionally, the users’ acceptance of robotic devices and cost effectiveness of robot-assisted rehabilitation should be investigated. Some studies analyzed the cost–benefit of the use of robot-assisted rehabilitation [80,81]; however, evidence in the related area was limited. A comprehensive cost–benefit analysis that considers various scenarios for the use of robotic devices in actual clinical settings is essential for the widespread use of robotic rehabilitation.
4.6. Study Limitations
There were a few limitations in the study. Only studies in English were included in the present review, which might have led to an overestimation of the effectiveness of robotic devices because papers with positive results were possibly more likely to be published in English than in a local language. Nonetheless, we included studies from various regions worldwide, and a previous report confirmed that there was no evidence of a systematic bias from the use of language restrictions in SR-based meta-analyses in conventional medicine [82]. Thus, we believe that the lack of SRs written in non-English languages did not have a major impact. Another limitation is that this review did not include more recent reviews. Robotic rehabilitation is a fast-paced advancing field with respect to scientific publications and development speed, and newer reviews are therefore constantly required.
5. Conclusions
There were more types of upper-limb devices than of lower-limb devices, and in contrast, there were more RCTs and SRs on lower-limb devices than on upper-limb devices. There was suggestive evidence of robotic rehabilitation’s effectiveness for upper-limb motor control and walking independence in patients with stroke. Further high-quality SRs including RCTs with large sample sizes and meta-analyses of these RCTs, particularly on diseases other than stroke, are required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13216616/s1, Table S1: Characteristics of the included studies; Table S2: Excluded studies and the reasons for exclusion (n = 79); Table S3: RCTs included in the SRs of upper-limb devices; Table S4: RCTs included in the SRs of lower-limb devices; Table S5: AMSTAR 2 quality assessment of the included reviews; Table S6: The list of upper-limb devices included in the RCTs; Table S7: The list of lower-limb devices included in the RCTs; Table S8: Outcome measures used in the included studies; Table S9: Summary of evidence grading and statistics used in meta-analyses of upper-limb devices; Table S10: Summary of evidence grading and statistics used in meta-analyses of lower-limb devices; Table S11: PRIOR Checklist.
Author Contributions
K.K., S.T., S.H., E.S. and Y.O. designed this study. K.K., S.T., T.I., Y.N., K.T. and Y.O. collected and analyzed the data. K.K., T.I., Y.N., K.T. and Y.O. interpreted the data and reviewed the manuscript. K.K., S.T., T.I., Y.N., K.T. and Y.O. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Agency for Medical Research and Development, grant number 19188854.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Y.O., otaka119@mac.com.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Suputtitada, A. Highlights in medical and surgical rehabilitation 2021/22. Front. Rehabil. Sci. 2023, 4, 1219924. [Google Scholar] [CrossRef] [PubMed]
- Everard, G.; Declerck, L.; Detrembleur, C.; Leonard, S.; Bower, G.; Dehem, S.; Lejeune, T. New technologies promoting active upper limb rehabilitation after stroke: An overview and network meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Li, C. Systematic review and network meta-analysis of robot-assisted gait training on lower limb function in patients with cerebral palsy. Neurol. Sci. 2023, 44, 3863–3875. [Google Scholar] [CrossRef]
- Zuccon, G.; Lenzo, B.; Bottin, M.; Rosati, G. Rehabilitation robotics after stroke: A bibliometric literature review. Expert Rev. Med. Devices 2022, 19, 405–421. [Google Scholar] [CrossRef]
- Stampacchia, G.; Gazzotti, V.; Olivieri, M.; Andrenelli, E.; Bonaiuti, D.; Calabro, R.S.; Carmignano, S.M.; Cassio, A.; Fundaro, C.; Companini, I.; et al. Gait robot-assisted rehabilitation in persons with spinal cord injury: A scoping review. NeuroRehabilitation 2022, 51, 609–647. [Google Scholar] [CrossRef] [PubMed]
- Carmignano, S.M.; Fundarò, C.; Bonaiuti, D.; Calabrò, R.S.; Cassio, A.; Mazzoli, D.; Bizzarini, E.; Campanini, I.; Cerulli, S.; Chisari, C.; et al. Robot-assisted gait training in patients with Parkinson’s disease: Implications for clinical practice. A systematic review. NeuroRehabilitation 2022, 51, 649–663. [Google Scholar] [CrossRef]
- Mannella, K.; Cudlip, A.C.; Holmes, M.W.R. Adaptations in Muscular Strength for Individuals with Multiple Sclerosis Following Robotic Rehabilitation: A Scoping Review. Front. Rehabil. Sci. 2022, 3, 882614. [Google Scholar] [CrossRef]
- Calabro, R.S.; Sorrentino, G.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E.; Campanini, I.; Carmignano, S.M.; Cerulli, S.; Chisari, C.; et al. Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 460–471. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., III; Johnson, R.; Keigher, K.M.; Mack, W.J. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Gates, M.; Gates, A.; Pieper, D.; Fernandes, R.M.; Tricco, A.C.; Moher, D.; Brennan, S.E.; Li, T.; Pollock, M.; Lunny, C.; et al. Reporting guideline for overviews of reviews of healthcare interventions: Development of the PRIOR statement. BMJ 2022, 378, e070849. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Fayed, N.; Bickenbach, J.; Prodinger, B. Refinements of the ICF Linking Rules to strengthen their potential for establishing comparability of health information. Disabil. Rehabil. 2019, 41, 574–583. [Google Scholar] [CrossRef]
- Cieza, A.; Brockow, T.; Ewert, T.; Amman, E.; Kollerits, B.; Chatterji, S.; Ustun, T.B.; Stucki, G. Linking health-status measurements to the international classification of functioning, disability and health. J. Rehabil. Med. 2002, 34, 205–210. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid.-Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef]
- Carrillo, C.; Tilley, D.; Horn, K.; Gonzalez, M.; Coffman, C.; Hilton, C.; Mani, K. Effectiveness of Robotics in Stroke Rehabilitation to Accelerate Upper Extremity Function: Systematic Review. Occup. Ther. Int. 2023, 2023, 7991765. [Google Scholar] [CrossRef]
- Doumen, S.; Sorba, L.; Feys, P.; Tedesco Triccas, L. Efficacy and Dose of Rehabilitation Approaches for Severe Upper Limb Impairments and Disability During Early Acute and Subacute Stroke: A Systematic Review. Phys. Ther. 2023, 103, pzad002. [Google Scholar] [CrossRef]
- Gnasso, R.; Palermi, S.; Picone, A.; Tarantino, D.; Fusco, G.; Messina, M.M.; Sirico, F. Robotic-Assisted Rehabilitation for Post-Stroke Shoulder Pain: A Systematic Review. Sensors 2023, 23, 8239. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.O.; Saragih, I.D.; Batubara, S.O. Robotic arm use for upper limb rehabilitation after stroke: A systematic review and meta-analysis. Kaohsiung J. Med. Sci. 2023, 39, 435–445. [Google Scholar] [CrossRef]
- Yang, X.; Shi, X.; Xue, X.; Deng, Z. Efficacy of Robot-Assisted Training on Rehabilitation of Upper Limb Function in Patients with Stroke: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2023, 104, 1498–1513. [Google Scholar] [CrossRef]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors: Systematic review and meta-analysis. Top. Stroke Rehabil. 2022, 29, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, G.; Ma, J.; Wang, S.; Cheng, L. Short and long-term effects of robot-assisted therapy on upper limb motor function and activity of daily living in patients post-stroke: A meta-analysis of randomized controlled trials. J. NeuroEng. Rehabil. 2022, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, G.; Wang, A.; Cheng, L.J.; Lau, Y. Robot-assisted distal training improves upper limb dexterity and function after stroke: A systematic review and meta-regression. Neurol. Sci. 2022, 43, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Rozevink, S.G.; Hijmans, J.M.; Horstink, K.A.; van der Sluis, C.K. Effectiveness of task-specific training using assistive devices and task-specific usual care on upper limb performance after stroke: A systematic review and meta-analysis. Disabil. Rehabil. Assist. Technol. 2021, 18, 1245–1258. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, H.; Zhang, J.; Yang, S.; Cai, S. Robot-Assisted Therapy for Upper Extremity Motor Impairment After Stroke: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab010. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Fan, W.; Gu, M.; Yasin, G.; Xiao, S.; Huang, J.; Huang, X. Robot-Assisted Arm Training versus Therapist-Mediated Training after Stroke: A Systematic Review and Meta-Analysis. J. Healthc. Eng. 2020, 2020, 8810867. [Google Scholar] [CrossRef]
- Chien, W.T.; Chong, Y.Y.; Tse, M.K.; Chien, C.W.; Cheng, H.Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef]
- Ferreira, F.; Chaves, M.E.A.; Oliveira, V.C.; Van Petten, A.; Vimieiro, C.B.S. Effectiveness of robot therapy on body function and structure in people with limited upper limb function: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0200330. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabro, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Kim, G.; Lim, S.; Kim, H.; Lee, B.; Seo, S.; Cho, K.; Lee, W. Is robot-assisted therapy effective in upper extremity recovery in early stage stroke?—A systematic literature review. J. Phys. Ther. Sci. 2017, 29, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.E.; Meskers, C.G.; Kwakkel, G. Effects of Robot-Assisted Therapy for the Upper Limb After Stroke. Neurorehabil Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li-Tsang, C.W.; Au, R.K. Robotic approaches for the rehabilitation of upper limb recovery after stroke: A systematic review and meta-analysis. Int. J. Rehabil. Res. 2017, 40, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Norouzi-Gheidari, N.; Archambault, P.S.; Fung, J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: Systematic review and meta-analysis of the literature. J. Rehabil. Res. Dev. 2012, 49, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Leow, X.R.G.; Ng, S.L.A.; Lau, Y. Overground Robotic Exoskeleton Training for Patients with Stroke on Walking-Related Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2023, 104, 1698–1710. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Yu, L.; Peng, L.; Cui, Y.; Huang, H. Effect of exoskeleton robot-assisted training on gait function in chronic stroke survivors: A systematic review of randomised controlled trials. BMJ Open 2023, 13, e074481. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Ruan, M.; Yun, R.S.; Zhong, Y.X.; Zhang, Y.X.; Wang, Y.J.; Sun, Y.L.; Cui, J.W. Is Leg-Driven Treadmill-Based Exoskeleton Robot Training Beneficial to Poststroke Patients: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2023, 102, 331–339. [Google Scholar] [CrossRef]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; de Sire, A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: A systematic review. Eur. J. Phys. Rehabil. Med. 2022, 58, 1–8. [Google Scholar] [CrossRef]
- Baronchelli, F.; Zucchella, C.; Serrao, M.; Intiso, D.; Bartolo, M. The Effect of Robotic Assisted Gait Training with Lokomat (R) on Balance Control After Stroke: Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 661815. [Google Scholar] [CrossRef]
- Nedergard, H.; Arumugam, A.; Sandlund, M.; Brandal, A.; Hager, C.K. Effect of robotic-assisted gait training on objective biomechanical measures of gait in persons post-stroke: A systematic review and meta-analysis. J. NeuroEng. Rehabil. 2021, 18, 64. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Dang, Y.; Teng, M.; Zhang, X.; Cheng, Y.; Zhang, X.; Yu, Q.; Yin, A.; Lu, X. Effects of robot-assisted training on balance function in patients with stroke: A systematic review and meta-analysis. J. Rehabil. Med. 2021, 53, jrm00174. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Cheng, Y.H.; Lai, C.H.; Lin, Y.N. Clinical non-superiority of technology-assisted gait training with body weight support in patients with subacute stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2019, 63, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, E.; Riccardi, G.R.; Di Donna, V.; Di Rosa, M.; Fabbietti, P.; Luzi, R.; Pranno, L.; Lattanzio, F.; Bevilacqua, R. Effectiveness of Intervention Based on End-effector Gait Trainer in Older Patients with Stroke: A Systematic Review. J. Am. Med. Dir. Assoc. 2019, 21, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of robotic gait training after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2020, 63, 518–534. [Google Scholar] [CrossRef]
- Postol, N.; Marquez, J.; Spartalis, S.; Bivard, A.; Spratt, N.J. Do powered over-ground lower limb robotic exoskeletons affect outcomes in the rehabilitation of people with acquired brain injury? Disabil. Rehabil. Assist. Technol. 2019, 14, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.X.; Ge, L.; Wang, C.C.; Ma, Q.S.; Liao, Y.T.; Huang, P.P.; Wang, G.D.; Xie, Q.L.; Rask, M. Robot-assisted therapy for balance function rehabilitation after stroke: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2019, 95, 7–18. [Google Scholar] [CrossRef]
- Bruni, M.F.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabro, R.S. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J. Clin. Neurosci. 2018, 48, 11–17. [Google Scholar] [CrossRef]
- Cho, J.E.; Yoo, J.S.; Kim, K.E.; Cho, S.T.; Jang, W.S.; Cho, K.H.; Lee, W.H. Systematic Review of Appropriate Robotic Intervention for Gait Function in Subacute Stroke Patients. BioMed Res. Int. 2018, 2018, 4085298. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Werner, C.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2017, 5, CD006185. [Google Scholar] [CrossRef]
- Hesse, S.; Schattat, N.; Mehrholz, J.; Werner, C. Evidence of end-effector based gait machines in gait rehabilitation after CNS lesion. NeuroRehabilitation 2013, 33, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M. Electromechanical-assisted gait training after stroke: A systematic review comparing end-effector and exoskeleton devices. J. Rehabil. Med. 2012, 44, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ada, L.; Dean, C.M.; Vargas, J.; Ennis, S. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: A systematic review. J. Physiother. 2010, 56, 153–161. [Google Scholar] [CrossRef]
- Saragih, I.D.; Everard, G.; Tzeng, H.M.; Saragih, I.S.; Lee, B.O. Efficacy of Robots-Assisted Therapy in Patients with Stroke: A Meta-Analysis Update. J. Cardiovasc. Nurs. 2023, 38, E192–E217. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M.; Lockwood, C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 3049–3091. [Google Scholar] [CrossRef]
- Huang, L.; Huang, H.L.; Dang, X.W.; Wang, Y.J. Effect of Body Weight Support Training on Lower Extremity Motor Function in Patients with Spinal Cord Injury: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2024, 103, 149–157. [Google Scholar] [CrossRef]
- Wan, C.; Huang, S.; Wang, X.; Ge, P.; Wang, Z.; Zhang, Y.; Li, Y.; Su, B. Effects of robot-assisted gait training on cardiopulmonary function and lower extremity strength in individuals with spinal cord injury: A systematic review and meta-analysis. J. Spinal Cord. Med. 2024, 47, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ding, M.; Wang, J.; Pan, H.; Sun, X.; Huang, L.; Fu, C.; He, C.; Wei, Q. Effectiveness of robotic-assisted gait training on cardiopulmonary fitness and exercise capacity for incomplete spinal cord injury: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2023, 37, 312–329. [Google Scholar] [CrossRef]
- Fang, C.Y.; Tsai, J.L.; Li, G.S.; Lien, A.S.; Chang, Y.J. Effects of Robot-Assisted Gait Training in Individuals with Spinal Cord Injury: A Meta-Analysis. BioMed Res. Int. 2020, 2020, 2102785. [Google Scholar] [CrossRef]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. NeuroEng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef]
- Mehrholz, J.; Harvey, L.A.; Thomas, S.; Elsner, B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord. 2017, 55, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Fisahn, C.; Aach, M.; Jansen, O.; Moisi, M.; Mayadev, A.; Pagarigan, K.T.; Dettori, J.R.; Schildhauer, T.A. The Effectiveness and Safety of Exoskeletons as Assistive and Rehabilitation Devices in the Treatment of Neurologic Gait Disorders in Patients with Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2016, 6, 822–841. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.Y.Y.; Ng, T.K.W.; Yu, K.K.K.; Kwan, R.L.C.; Cheing, G.L.Y. Robot-Assisted Training for People with Spinal Cord Injury: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2320–2331.e12. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.A.; Lin, C.L.; Huang, W.C.; Wang, H.Y.; Peng, C.W.; Chen, H.C. Effect of Robot-Assisted Gait Training on Multiple Sclerosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Neurorehabil Neural Repair 2023, 37, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.; Gervasoni, E.; Amico, A.P.; Antenucci, R.; Benanti, P.; Boldrini, P.; Bonaiuti, D.; Burini, A.; Castelli, E.; Draicchio, F.; et al. What is the impact of robotic rehabilitation on balance and gait outcomes in people with multiple sclerosis? A systematic review of randomized control trials. Eur. J. Phys. Rehabil. Med. 2021, 57, 246–253. [Google Scholar] [CrossRef]
- Yeh, S.W.; Lin, L.F.; Tam, K.W.; Tsai, C.P.; Hong, C.H.; Kuan, Y.C. Efficacy of robot-assisted gait training in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2020, 41, 102034. [Google Scholar] [CrossRef]
- Sattelmayer, M.; Chevalley, O.; Steuri, R.; Hilfiker, R. Over-ground walking or robot-assisted gait training in people with.multiple sclerosis: Does the effect depend on baseline walking speed and disease related disabilities? A systematic review and meta-regression. BMC Neurol. 2019, 19, 93. [Google Scholar] [CrossRef]
- Conner, B.C.; Remec, N.M.; Lerner, Z.F. Is robotic gait training effective for individuals with cerebral palsy? A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2022, 36, 873–882. [Google Scholar] [CrossRef]
- Cortes-Perez, I.; Gonzalez-Gonzalez, N.; Peinado-Rubia, A.B.; Nieto-Escamez, F.A.; Obrero-Gaitan, E.; Garcia-Lopez, H. Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Sensor 2022, 22, 9910. [Google Scholar] [CrossRef]
- Llamas-Ramos, R.; Sanchez-Gonzalez, J.L.; Llamas-Ramos, I. Robotic Systems for the Physiotherapy Treatment of Children with Cerebral Palsy: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 5116. [Google Scholar] [CrossRef]
- Lefmann, S.; Russo, R.; Hillier, S. The effectiveness of robotic-assisted gait training for paediatric gait disorders: Systematic review. J. NeuroEng. Rehabil. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhou, J.; Chen, Q.; Xu, Q.; Wang, S.; Yuan, L.; Zhang, D.; Bi, H.; Li, H. Effect of robot-assisted gait training on motor dysfunction in Parkinson’s patients: A systematic review and meta-analysis. J. Back Musculoskelet. Rehabil. 2024, 37, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yang, X.; Deng, Z. Efficacy of rehabilitation robot-assisted gait training on lower extremity dyskinesia in patients with Parkinson’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 85, 101837. [Google Scholar] [CrossRef]
- Alwardat, M.; Etoom, M.; Al Dajah, S.; Schirinzi, T.; Di Lazzaro, G.; Sinibaldi Salimei, P.; Biagio Mercuri, N.; Pisani, A. Effectiveness of robot-assisted gait training on motor impairments in people with Parkinson’s disease: A systematic review and meta-analysis. Int. J. Rehabil. Res. 2018, 41, 287–296. [Google Scholar] [CrossRef]
- Ferreira, F.; Chaves, M.E.A.; Oliveira, V.C.; Martins, J.S.R.; Vimieiro, C.B.S.; Van Petten, A. Effect of Robot-Assisted Therapy on Participation of People with Limited Upper Limb Functioning: A Systematic Review with GRADE Recommendations. Occup. Ther. Int. 2021, 2021, 6649549. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Tedla, J.S. Effectiveness of robotics in improving upper extremity functions among people with neurological dysfunction: A systematic review. Int. J. Neurosci. 2019, 129, 369–383. [Google Scholar] [CrossRef]
- Garlet, A.B.; Righi, N.C.; Schardong, J.; Della Méa Plentz, R. Effects of robotic rehabilitation using the Erigo® device on patients with neurological injury: A systematic review and meta-analysis of randomized clinical trials. Disabil. Rehabil. Assist. Technol. 2024, 19, 1135–1144. [Google Scholar] [CrossRef]
- Singh, N.; Saini, M.; Kumar, N.; Srivastava, M.; Mehndiratta, A. Evidence of neuroplasticity with robotic hand exoskeleton for post-stroke rehabilitation: A randomized controlled trial. J. NeuroEng. Rehabil. 2021, 18, 76. [Google Scholar] [CrossRef]
- Xing, Y.; Bai, Y. A review of exercise-induced neuroplasticity in ischemic stroke: Pathology and mechanisms. Mol. Neurobiol. 2020, 57, 4218–4231. [Google Scholar] [CrossRef]
- Carpino, G.; Pezzola, A.; Urbano, M.; Guglielmelli, E. Assessing Effectiveness and Costs in Robot-Mediated Lower Limbs Rehabilitation: A Meta-Analysis and State of the Art. J. Healthc. Eng. 2018, 2018, 7492024. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M.; Lockwood, C. The economic cost of robotic rehabilitation for adult stroke patients: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 520–547. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).