Reduced Salivary Gustin and Statherin in Long-COVID Cohort with Impaired Bitter Taste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Taste Test Administration
2.3. Sense of Smell Test
2.4. Saliva Collection and Isolation of Epithelial Cells
2.5. Immunofluorescence Staining of SEC
2.6. ELISA for sACE2 and Anti-ACE2 IgG
2.7. Determination of Salivary SHH, Gustin, and Statherin
2.8. ELISA for Cytokines and sCD14
2.9. Quantitative Real Time Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results
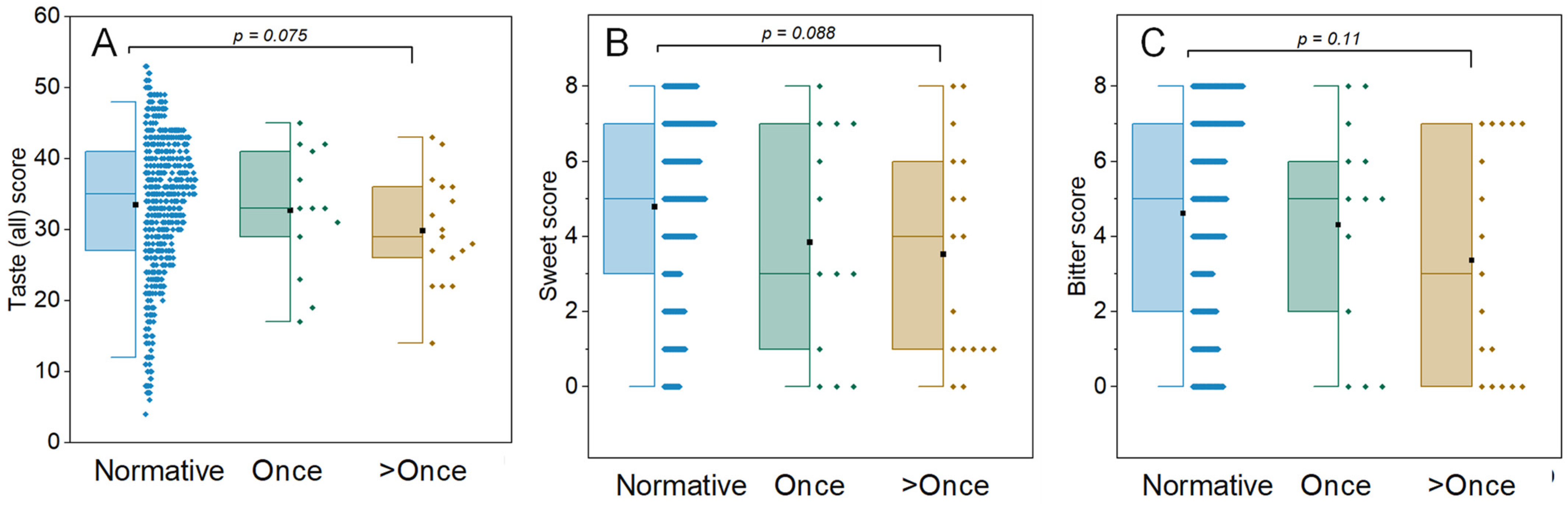
3.1. Participant Details and Objective Taste and Smell Scores
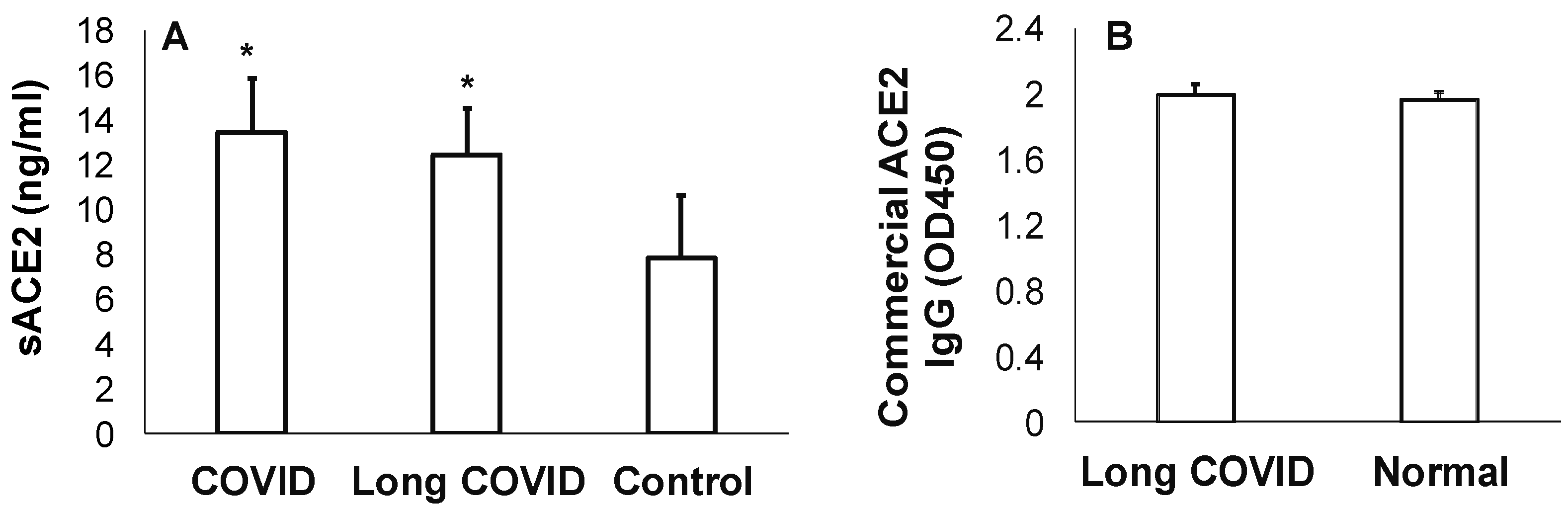
3.2. Measures of sACE2 and Anti-ACE2 in Long COVID
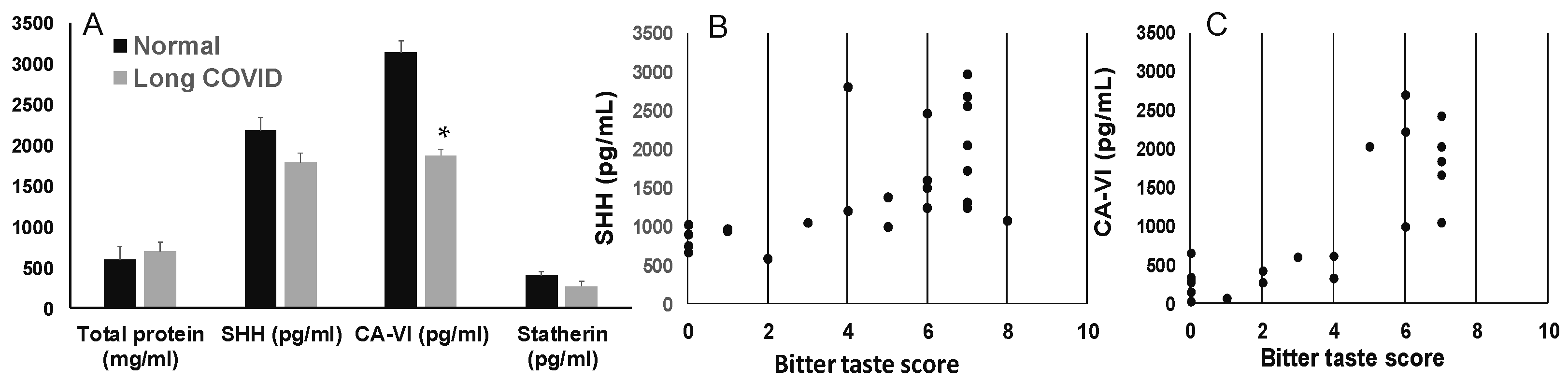
3.3. Salivary SHH, Gustin, and Statherin in Long-COVID
3.4. Bitter Taste Receptor, T2R38, in Long COVID
3.4.1. Epithelial Cells in Saliva Express SHH and Occludin
3.4.2. Expression of T2R38 and Cytokines in Long-COVID Saliva
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, L.; Gazit, S.; Pinto, Y.; Perez, G.; Mizrahi Reuveni, M.; Yehoshua, I.; Hoffman, R.; Azuri, J.; Patalon, T. Long-COVID in patients with a history of mild or asymptomatic SARS-CoV-2 infection: A Nationwide Cohort Study. Scand. J. Prim. Health Care 2022, 40, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Epsi, N.J.; Chenoweth, J.G.; Blair, P.W.; Lindholm, D.A.; Ganesan, A.; Lalani, T.; Smith, A.; Mody, R.M.; Jones, M.U.; Colombo, R.E.; et al. Precision symptom phenotyping identifies early clinical and proteomic predictors of distinct COVID-19 sequelae. J. Infect. Dis. 2024, jiae318. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Long-Term Consequences of Asymptomatic SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1613. [Google Scholar] [CrossRef]
- Doyle, M.E.; Premathilake, H.U.; Yao, Q.; Mazucanti, C.H.; Egan, J.M. Physiology of the tongue with emphasis on taste transduction. Physiol. Rev. 2023, 103, 1193–1246. [Google Scholar] [CrossRef]
- Rogn, A.; Jensen, J.L.; Iversen, P.O.; Singh, P.B. Post-COVID-19 patients suffer from chemosensory, trigeminal, and salivary dysfunctions. Sci. Rep. 2024, 14, 3455. [Google Scholar] [CrossRef]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef]
- Zhao, D.; Cheng, T.; Koohi-Moghadam, M.; Wu, M.-Z.; Yu, S.Y.; Ding, X.; Pelekos, G.; Yiu, K.H.; Jin, L. Salivary ACE2 and TMPRSS2 link to periodontal status and metabolic parameters. Clin. Transl. Discov. 2022, 2, e37. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. WHO COVID-19 Dashboard; World Health Organization: Geneva, Swizerland, 2023; Available online: https://covid19.who.int/WHO (accessed on 8 August 2024).
- Daniell, H.; Nair, S.K.; Shi, Y.; Wang, P.; Montone, K.T.; Shaw, P.A.; Choi, G.H.; Ghani, D.; Weaver, J.; Rader, D.J.; et al. Decrease in Angiotensin-Converting Enzyme activity but not concentration in plasma/lungs in COVID-19 patients offers clues for diagnosis/treatment. Mol. Ther. Methods Clin. Dev. 2022, 26, 266–278. [Google Scholar] [CrossRef]
- Kawabe, M.; Nakashima, A.; Yamamoto, I.; Ohkido, I.; Yokoo, T.; Urashima, M. Higher Soluble ACE2 Levels and Increased Risk of Infection-Related Hospitalization in Patients on Maintenance Hemodialysis. Front. Med. 2022, 9, 791284. [Google Scholar] [CrossRef]
- Ermel, A.; Thyvalikakath, T.P.; Foroud, T.; Khan, B.; Srinivasan, M. Can Salivary Innate Immune Molecules Provide Clue on Taste Dysfunction in COVID-19? Front. Microbiol. 2021, 12, 727430. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, L.; Qian, X.; Su, K.; Huang, Y.; Qu, Y.; Zhang, Z.; Liu, W. Tongue coating microbiome composition reflects disease severity in patients with COVID-19 in Nanjing, China. J. Oral Microbiol. 2023, 15, 2236429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, J.; Liu, Y.; Chen, K.; Huang, L.; Liu, Y. Oral, Tongue-Coating Microbiota, and Metabolic Disorders: A Novel Area of Interactive Research. Front. Cardiovasc. Med. 2021, 8, 730203. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; So, P.W.; Carpenter, G.H. Intraoral Microbial Metabolism and Association with Host Taste Perception. J. Dent. Res. 2020, 99, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Bradley, E.; Zeamer, A.L.; Cincotta, L.; Salive, M.C.; Dutta, P.; Mutaawe, S.; Anya, O.; Meza-Segura, M.; Moormann, A.M.; et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight 2021, 6, e152346. [Google Scholar] [CrossRef]
- Lingering COVID Virus in Tongue Linked to Long-Term Taste Loss. Available online: https://www.nia.nih.gov/news/lingering-covid-virus-tongue-linked-long-term-taste-loss#:~:text=A%20recent%20study%20estimated%20that,a%20decreased%20sense%20of%20smell (accessed on 9 September 2024).
- Srinivasan, M. Taste Dysfunction and Long COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 716563. [Google Scholar] [CrossRef]
- Fabian, T.K.; Beck, A.; Fejerdy, P.; Hermann, P.; Fabian, G. Molecular mechanisms of taste recognition: Considerations about the role of saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, X.; Wang, Z.; Zhang, Y.; Zhang, Y.; Chen, J.; Liu, Y. Umami Altering Salivary Proteome: A Study across a Sensitivity Spectrum on Subjects. J. Agric. Food Chem. 2024, 72, 13451–13464. [Google Scholar] [CrossRef]
- Ozturk, E.E.; Dikmen, D. Is sonic hedgehog expression in saliva related to taste sensitivity in adults? Physiol. Behav. 2021, 236, 113412. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling. Annu. Rev. Physiol. 2017, 79, 335–356. [Google Scholar] [CrossRef]
- Tsuchiya, H. Gustatory and Saliva Secretory Dysfunctions in COVID-19 Patients with Zinc Deficiency. Life 2022, 12, 353. [Google Scholar] [CrossRef]
- Padiglia, A.; Zonza, A.; Atzori, E.; Chillotti, C.; Calo, C.; Tepper, B.J.; Barbarossa, I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010, 92, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Ponnusamy, V.; Vasanthakumar, K.; Panneerselvan, P.; Krishnan, V.; Subramaniam, S. The gustin gene variation at rs2274333 and PROP taster status affect dietary fat perception: A stepwise multiple regression model study. J. Nutr. Biochem. 2024, 128, 109619. [Google Scholar] [CrossRef] [PubMed]
- Parlak, H.M.; Buber, E.; Gur, A.T.; Karabulut, E.; Akalin, F.A. Statherin and alpha-amylase levels in saliva from patients with gingivitis and periodontitis. Arch. Oral Biol. 2023, 145, 105574. [Google Scholar] [CrossRef]
- Baima, G.; Marruganti, C.; Sanz, M.; Aimetti, M.; Romandini, M. Periodontitis and COVID-19: Biological Mechanisms and Meta-analyses of Epidemiological Evidence. J. Dent. Res. 2022, 101, 1430–1440. [Google Scholar] [CrossRef]
- Casarin, M.; Silva, F.H.; Pontes, A.F.L.; Lima, B.D.; Pirih, F.Q.; Muniz, F. Association between sequelae of COVID-19 with periodontal disease and obesity: A cross-sectional study. J. Periodontol. 2024, 95, 688–698. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Jaramillo, M.; Thyvalikakath, T.P.; Eckert, G.; Srinivasan, M. Characteristics of Chemosensory Perception in Long COVID and COVID Reinfection. J.Clin. Med. 2023, 12, 3598. [Google Scholar] [CrossRef]
- Doty, R.L.; Wylie, C.; Potter, M. Validation of the Waterless Empirical Taste Test (WETT((R))). Behav. Res. Methods 2021, 53, 864–873. [Google Scholar] [CrossRef]
- Doty, R.L. TheWaterless Empirical Taste Test (WETT) Administration Manual; Sensonics International: Haddon Heights, NJ, USA, 2019. [Google Scholar]
- Doty, R.L. Olfactory dysfunction and its measurement in the clinic and workplace. Int. Arch. Occup. Environ. Health 2006, 79, 268–282. [Google Scholar] [CrossRef]
- Doty, R.L.; Frye, R.E.; Agrawal, U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. 1989, 45, 381–384. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Janardhanam, S.B.; Zunt, S.L.; Srinivasan, M. Quality assessment of saliva bank samples. Biopreserv. Biobank. 2012, 10, 282–287. [Google Scholar] [CrossRef]
- Bru, S.; Brotons, P.; Jordan, I.; Alsina, L.; Henares, D.; Carballar, R.; de Sevilla, M.F.; Barrabeig, I.; Fumado, V.; Baro, B.; et al. Association between soluble angiotensin-converting enzyme 2 in saliva and SARS-CoV-2 infection: A cross-sectional study. Sci. Rep. 2023, 13, 5985. [Google Scholar] [CrossRef]
- Geanes, E.S.; McLennan, R.; LeMaster, C.; Bradley, T. Autoantibodies to ACE2 and immune molecules are associated with COVID-19 disease severity. Commun. Med. 2024, 4, 47. [Google Scholar] [CrossRef]
- Aidoukovitch, A.; Bodahl, S.; Tufvesson, E.; Nilsson, B.O. Desquamated Epithelial Cells of Unstimulated Human Whole Saliva Express Both EGF Transcript and Protein. Int. J. Dent. 2022, 2022, 3194703. [Google Scholar] [CrossRef]
- Dawes, C. Estimates, from salivary analyses, of the turnover time of the oral mucosal epithelium in humans and the number of bacteria in an edentulous mouth. Arch. Oral Biol. 2003, 48, 329–336. [Google Scholar] [CrossRef]
- Risso, D.; Carmagnola, D.; Morini, G.; Pellegrini, G.; Canciani, E.; Antinucci, M.; Henin, D.; Dellavia, C. Distribution of TAS2R38 bitter taste receptor phenotype and haplotypes among COVID-19 patients. Sci. Rep. 2022, 12, 7381. [Google Scholar] [CrossRef]
- Risso, D.S.; Mezzavilla, M.; Pagani, L.; Robino, A.; Morini, G.; Tofanelli, S.; Carrai, M.; Campa, D.; Barale, R.; Caradonna, F.; et al. Global diversity in the TAS2R38 bitter taste receptor: Revisiting a classic evolutionary PROPosal. Sci. Rep. 2016, 6, 25506. [Google Scholar] [CrossRef]
- Barham, H.P.; Taha, M.A.; Broyles, S.T.; Stevenson, M.M.; Zito, B.A.; Hall, C.A. Association Between Bitter Taste Receptor Phenotype and Clinical Outcomes Among Patients With COVID-19. JAMA Netw. Open 2021, 4, e2111410. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Islam, M.A.; Abdullah, B. Prevalence and Characteristics of Taste Disorders in Cases of COVID-19: A Meta-analysis of 29,349 Patients. Otolaryngol. Head Neck Surg. 2021, 165, 33–42. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020, 11, 2944–2961. [Google Scholar] [CrossRef] [PubMed]
- Huart, C.; Philpott, C.; Konstantinidis, I.; Altundag, A.; Whitcroft, K.L.; Trecca, E.M.C.; Cassano, M.; Rombaux, P.; Hummel, T. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology 2020, 58, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Cooper, K.W.; Di Pizio, A.; Joseph, P.V.; Bhutani, S.; Parma, V. Corona Viruses and the Chemical Senses: Past, Present, and Future. Chem. Senses 2020, 45, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.P.; Moreira de Freitas, P.; de Paula Eduardo, C.; Hiramatsu Azevedo, L. COVID-19-Related Long-Term Taste Impairment: Symptom Length, Related Taste, Smell Disturbances, and Sample Characteristics. Cureus 2023, 15, e38055. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, J.; Jin, B.; He, S.; Dang, Y.; Zhao, T.; Jin, Z. The Emerging Role of Hedgehog Signaling in Viral Infections. Front. Microbiol. 2022, 13, 870316. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation. Int. J. Mol. Sci. 2019, 20, 1341. [Google Scholar] [CrossRef]
- Henkin, R.I.; Knoppel, A.B.; Abdelmeguid, M.; Stateman, W.A.; Hosein, S. Theophylline increases saliva sonic hedgehog and improves taste dysfunction. Arch. Oral Biol. 2017, 82, 263–270. [Google Scholar] [CrossRef]
- Henkin, R.I.; Knoppel, A.B.; Abdelmeguid, M.; Stateman, W.A.; Hosein, S. Sonic hedgehog is present in parotid saliva and is decreased in patients with taste dysfunction. J. Oral Pathol. Med. 2017, 46, 829–833. [Google Scholar] [CrossRef]
- Harding, J.L.; Oviedo, S.A.; Ali, M.K.; Ofotokun, I.; Gander, J.C.; Patel, S.A.; Magliano, D.J.; Patzer, R.E. The bidirectional association between diabetes and long-COVID-19—A systematic review. Diabetes Res. Clin. Pract. 2023, 195, 110202. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef]



| (A) Subjective Complaints | Same as Pre-COVID-29 | Worse After COVID-19 |
| Taste | 16 | 14 |
| Smell | 18 | 12 |
| Mean total taste score | 33.7 ± 7.36 | 28.7 ± 8.8 |
| Mean total smell score | 36.2 ± 4.5 | 35.2 ± 4 |
| Objective Chemosensory Tests (B) # of Positive COVID-19 Tests | Once | >Once |
| Number (male: female) | 13 (6:7) | 17 (8:9) |
| Age | 56 ± 12.9 | 46.5 ± 11.6 |
| Mean total taste score | 31.2 ± 7.8 | 30.8 ± 9.1 |
| Mean total smell score | 35.3 ± 2.9 | 36.6 ± 2.2 |
| Objective Chemosensory Tests (C) # of Positive COVID-19 Tests | Once | >Once |
| Number | 13 | 17 |
| Impaired bitter taste | 16% | 26% |
| Impaired sweet taste | 6.50% | 12.90% |
| Impaired salt taste | 6.50% | 3% |
| Impaired sour taste | 6.50% | 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdary, H.; Riley, N.; Patel, P.; Gossweiler, A.G.; Running, C.A.; Srinivasan, M. Reduced Salivary Gustin and Statherin in Long-COVID Cohort with Impaired Bitter Taste. J. Clin. Med. 2024, 13, 6816. https://doi.org/10.3390/jcm13226816
Chowdary H, Riley N, Patel P, Gossweiler AG, Running CA, Srinivasan M. Reduced Salivary Gustin and Statherin in Long-COVID Cohort with Impaired Bitter Taste. Journal of Clinical Medicine. 2024; 13(22):6816. https://doi.org/10.3390/jcm13226816
Chicago/Turabian StyleChowdary, Harika, Naomi Riley, Parul Patel, Ana G. Gossweiler, Cordelia A. Running, and Mythily Srinivasan. 2024. "Reduced Salivary Gustin and Statherin in Long-COVID Cohort with Impaired Bitter Taste" Journal of Clinical Medicine 13, no. 22: 6816. https://doi.org/10.3390/jcm13226816
APA StyleChowdary, H., Riley, N., Patel, P., Gossweiler, A. G., Running, C. A., & Srinivasan, M. (2024). Reduced Salivary Gustin and Statherin in Long-COVID Cohort with Impaired Bitter Taste. Journal of Clinical Medicine, 13(22), 6816. https://doi.org/10.3390/jcm13226816






