MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury
Abstract
:1. Introduction
2. Methods
2.1. Human Subjects
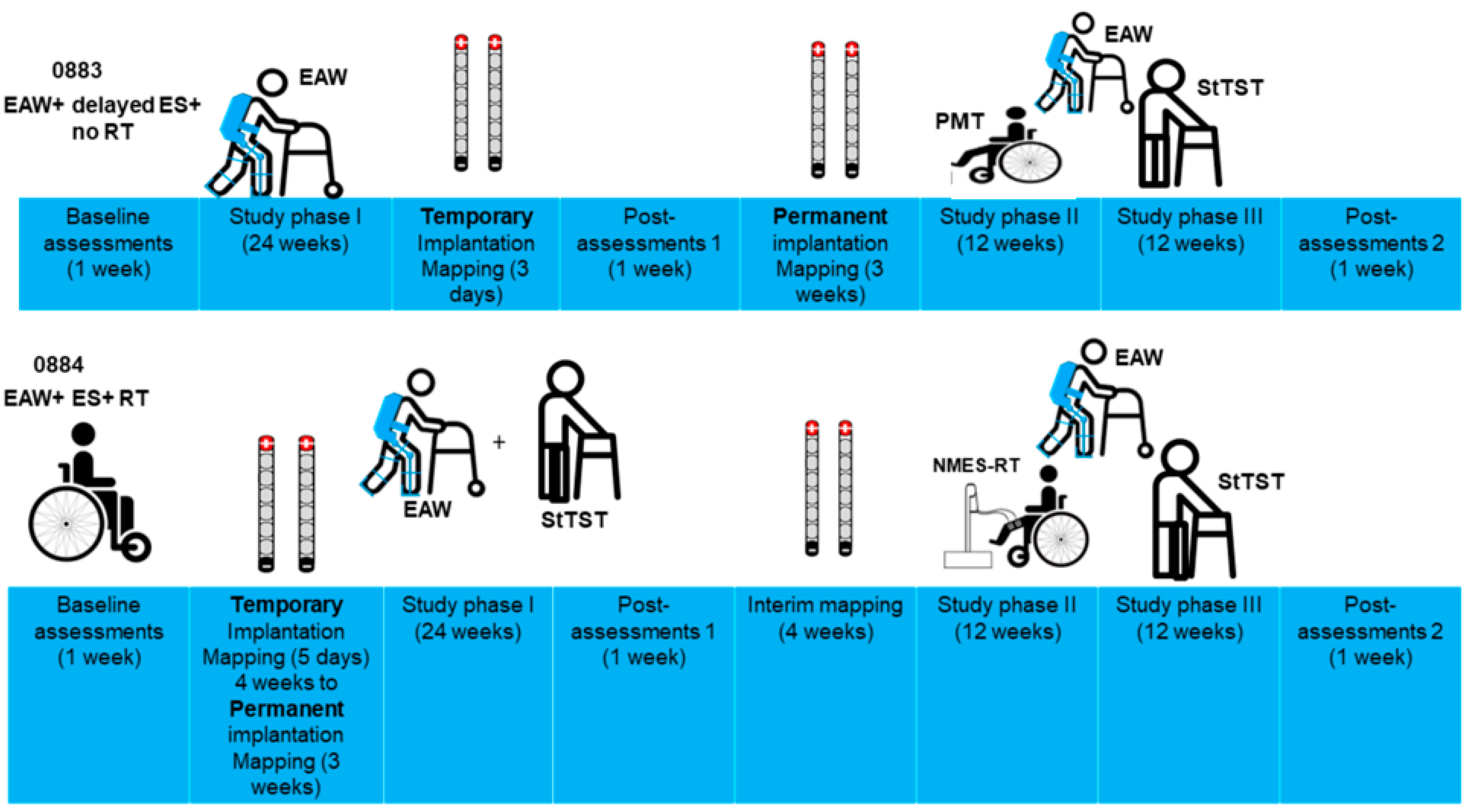
2.2. Timeline of the Study
2.3. Inclusion and Exclusion Criteria
2.4. Magnetic Resonance Imaging (MRI)
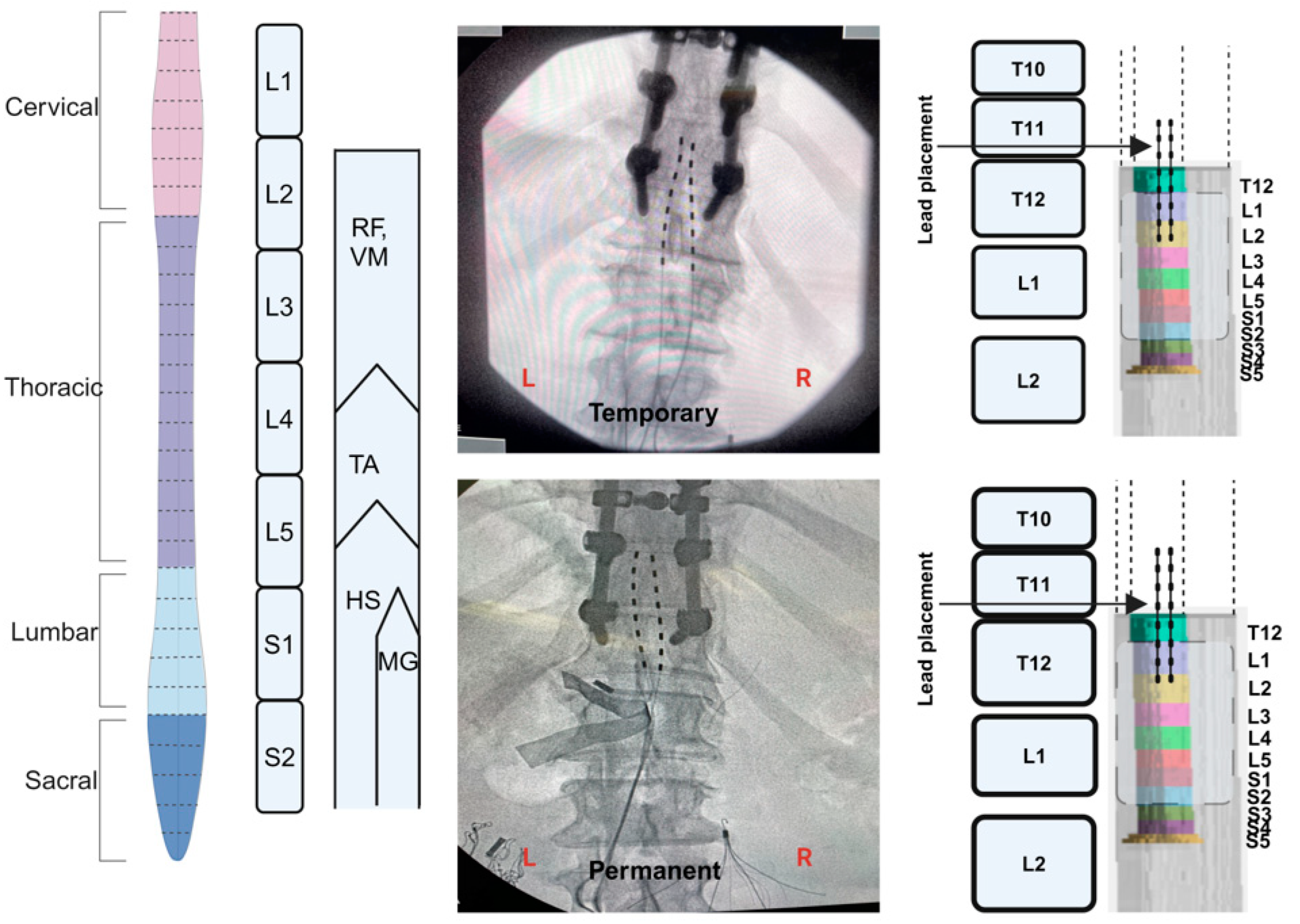
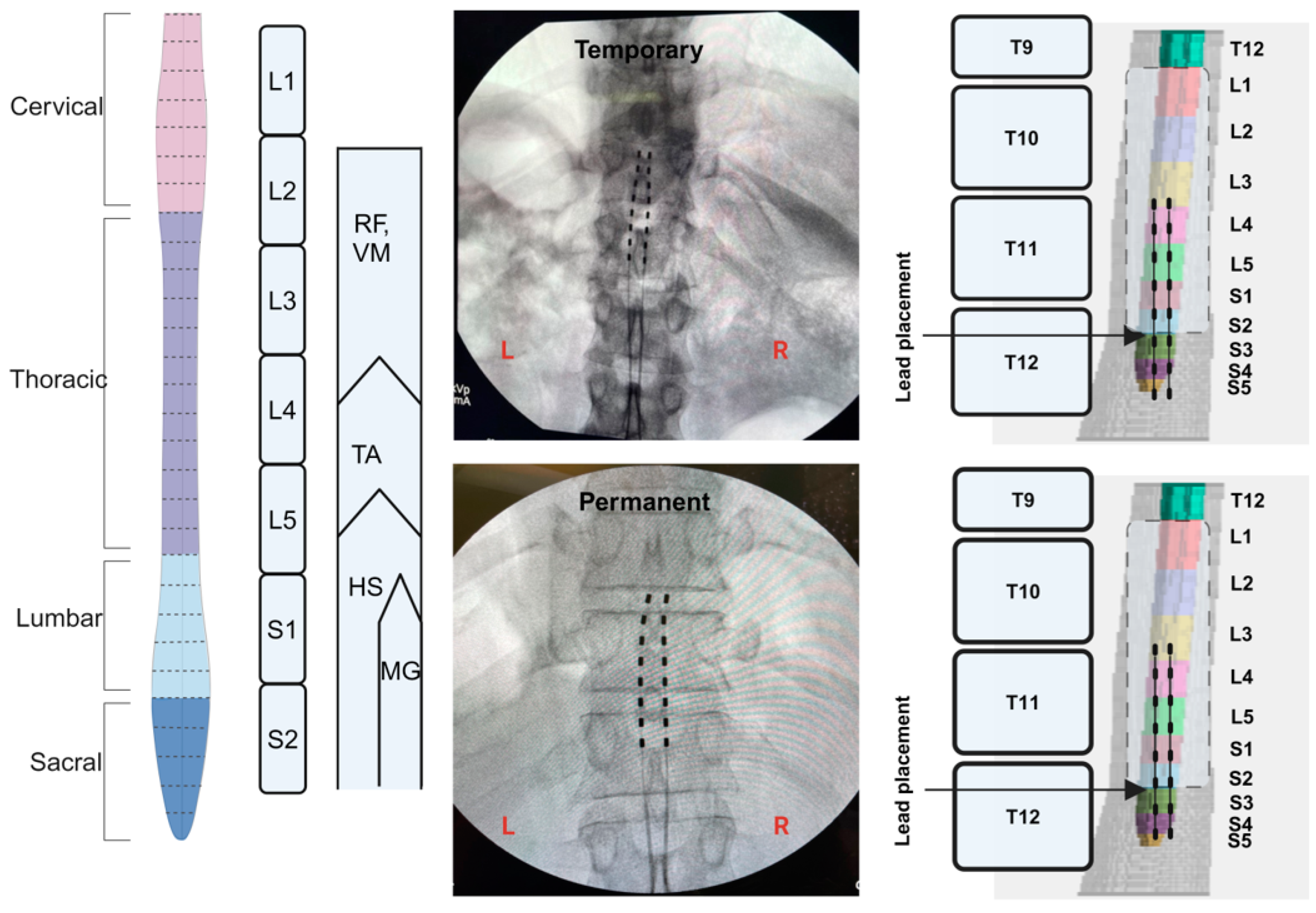
2.5. Implantation of Percutaneous SCES
2.6. Temporary Implantation
2.7. Permanent Implantation
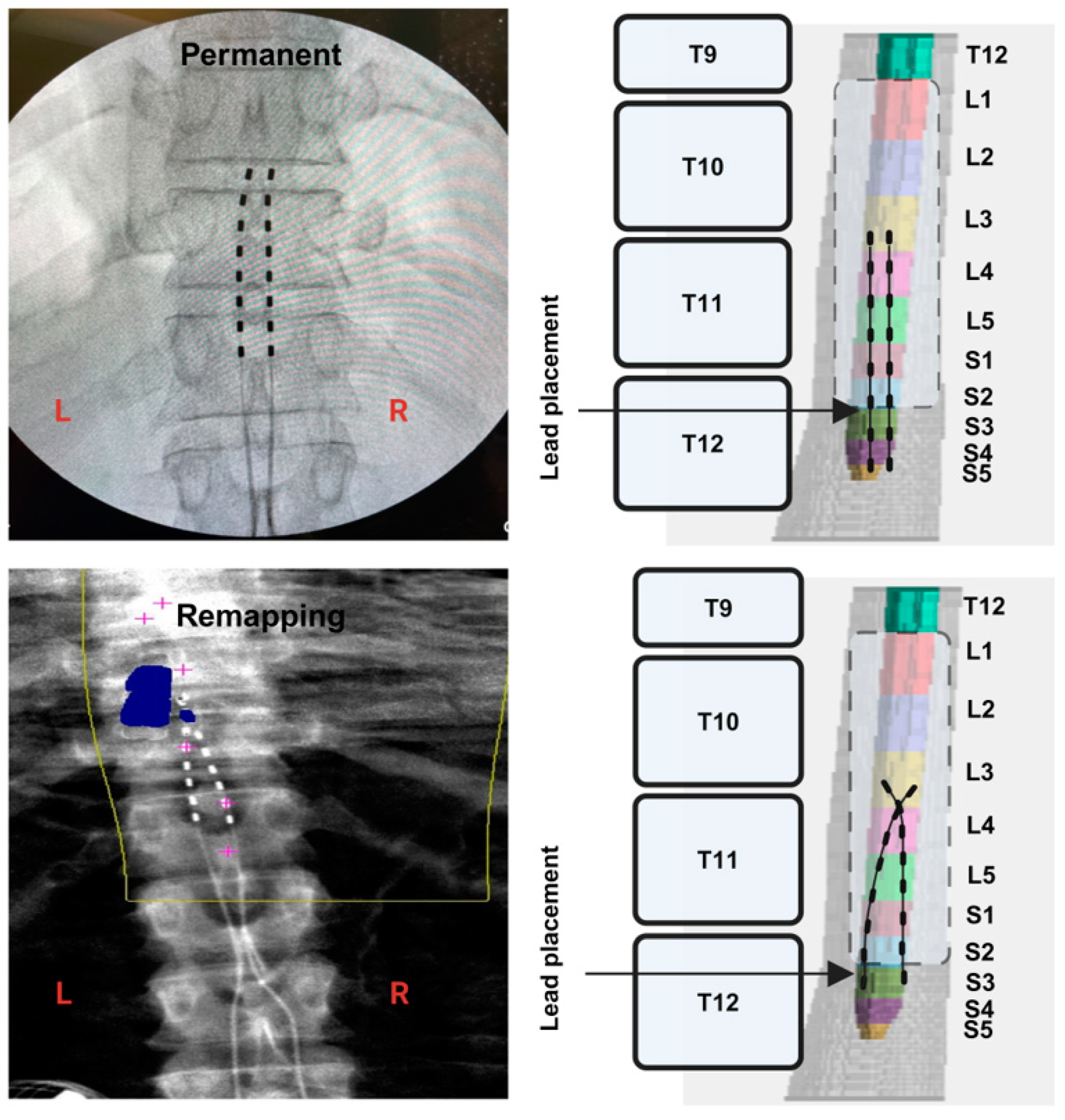
2.8. Migration of the Leads
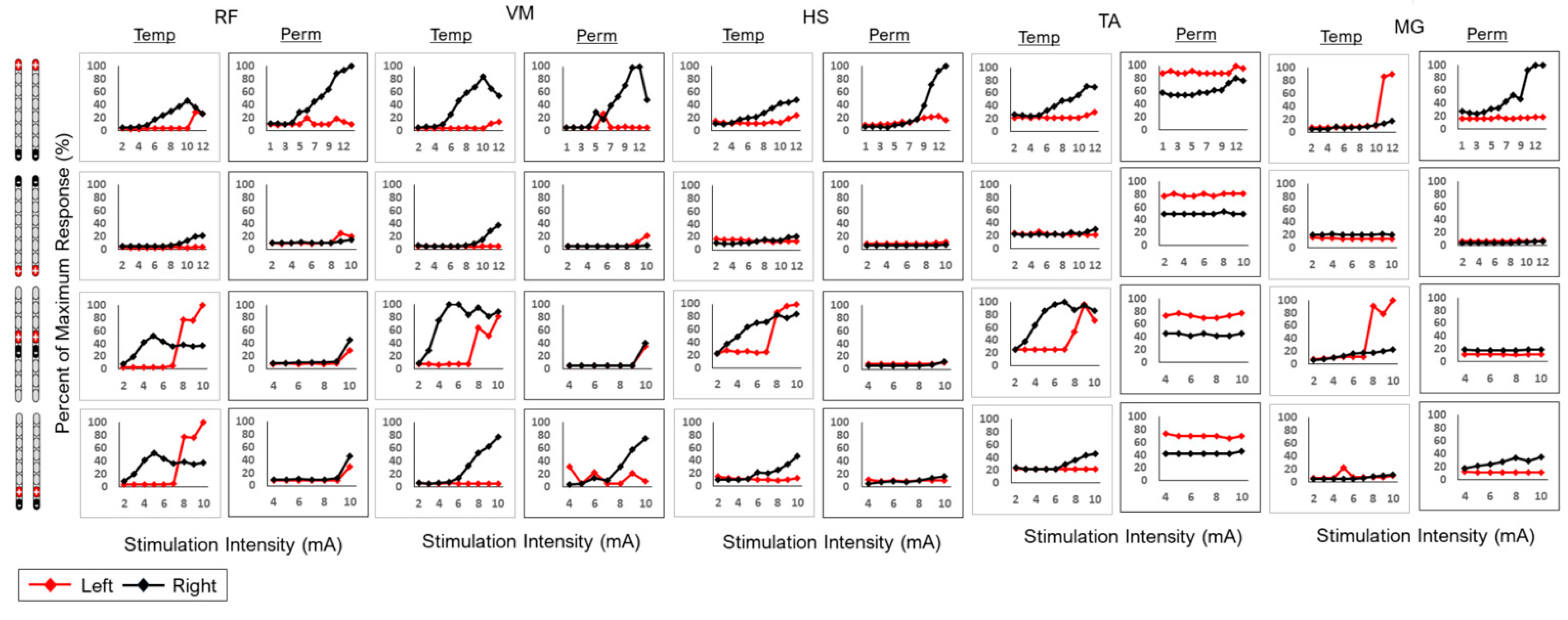
2.9. Spinal Mapping
2.10. EMG Data Analysis
2.11. Temporary Versus Permanent Mapping
2.12. MRI Spinal Cord Reconstruction
3. Results
3.1. Percutaneous Lead Migration in Participant 0884
3.2. Remapping of the Spinal Cord Following Implantation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.M.; Chen, S.Y.; Xu, Q.J.; Fu, S.P.; Yang, Y.C.; Zou, W.H.; Zhang, M.; Liu, J.; Wan, W.H.; Peng, J.C.; et al. Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front. Immunol. 2021, 12, 751021. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R., Jr. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Epstein, D. A Systematic Review of the Impact of Spinal Cord Injury on Costs and Health-Related Quality of Life. PharmacoEconomics Open 2024, 8, 793–808. [Google Scholar] [CrossRef]
- Turczyn, P.; Wojdasiewicz, P.; Poniatowski, Ł.A.; Purrahman, D.; Maślińska, M.; Żurek, G.; Romanowska-Próchnicka, K.; Żuk, B.; Kwiatkowska, B.; Piechowski-Jóźwiak, B.; et al. Omega-3 fatty acids in the treatment of spinal cord injury: Untapped potential for therapeutic intervention? Mol. Biol. Rep. 2022, 49, 10797–10809. [Google Scholar] [CrossRef]
- Huang, Q.; Duan, W.; Sivanesan, E.; Liu, S.; Yang, F.; Chen, Z.; Ford, N.C.; Chen, X.; Guan, Y. Spinal Cord Stimulation for Pain Treatment After Spinal Cord Injury. Neurosci. Bull. 2019, 35, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Brief History of Neuromodulation. Available online: https://www.neuromodulation.com/brief-history-of-neuromodulation (accessed on 30 August 2024).
- Shandybina, N.D.; Kuropatenko, M.V.; Moshonkina, T.R. Regulation of Human Respiration by Electrical Stimulation. J. Evol. Biochem. Physiol. 2022, 58, 1879–1891. [Google Scholar] [CrossRef]
- Herrity, A.N.; Aslan, S.C.; Mesbah, S.; Siu, R.; Kalvakuri, K.; Ugiliweneza, B.; Mohamed, A.; Hubscher, C.H.; Harkema, S.J. Targeting bladder function with network-specific epidural stimulation after chronic spinal cord injury. Sci. Rep. 2022, 12, 11179. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Goldsmith, J.; Alazzam, A.; Trainer, R. Effects of percutaneously-implanted epidural stimulation on cardiovascular autonomic function and spasticity after complete spinal cord injury: A case report. Front. Neurosci. 2023, 17, 1112853. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Trainer, R.; Sutor, T.W.; Goldsmith, J.A.; Alazzam, A.; Goetz, L.L.; Lester, D.; Lavis, T.D. A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury. Nat. Commun. 2023, 14, 2064. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.C.; Legg Ditterline, B.E.; Park, M.C.; Angeli, C.A.; Rejc, E.; Chen, Y.; Ovechkin, A.V.; Krassioukov, A.; Harkema, S.J. Epidural Spinal Cord Stimulation of Lumbosacral Networks Modulates Arterial Blood Pressure in Individuals with Spinal Cord Injury-Induced Cardiovascular Deficits. Front. Physiol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Darrow, D.; Balser, D.; Netoff, T.I.; Krassioukov, A.; Phillips, A.; Parr, A.; Samadani, U. Epidural Spinal Cord Stimulation Facilitates Immediate Restoration of Dormant Motor and Autonomic Supraspinal Pathways after Chronic Neurologically Complete Spinal Cord Injury. J. Neurotrauma 2019, 36, 2325–2336. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Sutor, T.W.; Goldsmith, J.A.; Ennasr, A.N.; Lavis, T.D.; Cifu, D.X.; Trainer, R. Epidural stimulation with locomotor training ameliorates unstable blood pressure after tetraplegia. A case report. Ann. Clin. Transl. Neurol. 2022, 9, 232–238. [Google Scholar] [CrossRef]
- Angeli, C.; Rejc, E.; Boakye, M.; Herrity, A.; Mesbah, S.; Hubscher, C.; Forrest, G.; Harkema, S. Targeted Selection of Stimulation Parameters for Restoration of Motor and Autonomic Function in Individuals with Spinal Cord Injury. Neuromodulation 2024, 27, 645–660. [Google Scholar] [CrossRef]
- Alazzam, A.M.; Ballance, W.B.; Smith, A.C.; Rejc, E.; Weber, K.A., 2nd; Trainer, R.; Gorgey, A.S. Peak Slope Ratio of the Recruitment Curves Compared to Muscle Evoked Potentials to Optimize Standing Configurations with Percutaneous Epidural Stimulation after Spinal Cord Injury. J. Clin. Med. 2024, 13, 1344. [Google Scholar] [CrossRef]
- Chalif, J.I.; Chavarro, V.S.; Mensah, E.; Johnston, B.; Fields, D.P.; Chalif, E.J.; Chiang, M.; Sutton, O.; Yong, R.; Trumbower, R.; et al. Epidural Spinal Cord Stimulation for Spinal Cord Injury in Humans: A Systematic Review. J. Clin. Med. 2024, 13, 1090. [Google Scholar] [CrossRef]
- Calvert, J.S.; Darie, R.; Parker, S.R.; Shaaya, E.; Syed, S.; McLaughlin, B.L.; Fridley, J.S.; Borton, D.A. Spatiotemporal Distribution of Electrically Evoked Spinal Compound Action Potentials During Spinal Cord Stimulation. Neuromodulation 2023, 26, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Venigalla, S.; Rehman, M.U.; George, B.; Rejc, E.; Gouda, J.J. Interleaved configurations of percutaneous epidural stimulation enhanced overground stepping in a person with chronic paraplegia. Front. Neurosci. 2023, 17, 1284581. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Perret, I.; Bayart, A.; Lackner, P.; Binder, H.; Freundl, B.; Minassian, K. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience 2020, 24, 101930. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Gouda, J.J. Single Lead Epidural Spinal Cord Stimulation Targeted Trunk Control and Standing in Complete Paraplegia. J. Clin. Med. 2022, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, S.; Herrity, A.; Ugiliweneza, B.; Angeli, C.; Gerasimenko, Y.; Boakye, M.; Harkema, S. Neuroanatomical mapping of the lumbosacral spinal cord in individuals with chronic spinal cord injury. Brain Commun. 2022, 5, fcac330. [Google Scholar] [CrossRef]
- Mendez, A.; Islam, R.; Latypov, T.; Basa, P.; Joseph, O.J.; Knudsen, B.; Siddiqui, A.M.; Summer, P.; Staehnke, L.J.; Grahn, P.J.; et al. Segment-specific orientation of the dorsal and ventral roots for precise therapeutic targeting of human spinal cord. Mayo Clin. Proc. 2021, 96, 1426–1437. [Google Scholar] [CrossRef]
- Lempka, S.F.; Zander, H.J.; Anaya, C.J.; Wyant, A.; Ozinga, J.G.; Machado, A.G. Patient-specific analysis of neural activation during spinal cord stimulation for pain. Neuromodulation 2020, 23, 572–581. [Google Scholar] [CrossRef]
- Zander, H.J.; Graham, R.D.; Anaya, C.J.; Lempka, S.F. Anatomical and technical factors affecting the neural response to epidural spinal cord stimulation. J. Neural Eng. 2020, 17, 036019. [Google Scholar] [CrossRef]
- Hara, S.; Andresen, H.; Solheim, O.; Carlsen, S.M.; Jakola, A.S.; Salvesen, Ø.O.; Gulati, S. Six-Month Follow-up of a Trial of Spinal Cord Burst Stimulation vs Placebo Stimulation and Disability in Patients With Chronic Radicular Pain After Lumbar Spine Surgery. JAMA 2023, 329, 1985–1986. [Google Scholar] [CrossRef]
- North, R.B.; Kidd, D.H.; Olin, J.C.; Sieracki, J.M. Spinal cord stimulation electrode design: Prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I: Technical outcomes. Neurosurgery 2002, 51, 381–389; discussion 389–390. [Google Scholar]
- Lee, C.L.; ShakerKhavidaki, S.; Sitthinamsuwan, B.; Jirachaipitak, S.; Srirabheebhat, P. Retrograde Epidural Spinal Cord Stimulation for the Treatment of Intractable Neuropathic Pain Following Spinal Cord and Cauda Equina Injuries: A Case Report and Literature Review. Asian J. Neurosurg. 2024, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.Y.; Park, J.H.; Shin, Y.B.; Baek, S.Y. Gross quantitative measurements of spinal cord segments in human. Spinal Cord 2004, 42, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Fradet, L.; Arnoux, P.J.; Ranjeva, J.P.; Petit, Y.; Callot, V. Morphometrics of the entire human spinal cord and spinal canal measured from in vivo high-resolution anatomical magnetic resonance imaging. Spine 2014, 39, E262–E269. [Google Scholar] [CrossRef]
- Smith, A.C.; Albin, S.R.; O’Dell, D.R.; Berliner, J.C.; Dungan, D.; Sevigny, M.; Draganich, C.; Elliott, J.M.; Weber, K.A., II. Axial MRI biomarkers of spinal cord damage to predict future walking and motor function: A retrospective study. Spinal Cord 2021, 59, 693–699. [Google Scholar] [CrossRef]
- Smith, A.C.; Angeli, C.A.; Ugiliweneza, B.; Weber, K.A.; Bert, R.J.; Negahdar, M.; Mesbah, S.; Boakye, M.; Harkema, S.J.; Rejc, E. Spinal cord imaging markers and recovery of standing with epidural stimulation in individuals with clinically motor complete spinal cord injury. Exp. Brain Res. 2022, 240, 279–288. [Google Scholar] [CrossRef]
- Dombovy-Johnson, M.L.; D’Souza, R.S.; Ha, C.T.; Hagedorn, J.M. Incidence and Risk Factors for Spinal Cord Stimulator Lead Migration With or Without Loss of Efficacy: A Retrospective Review of 91 Consecutive Thoracic Lead Implants. Neuromodulation 2022, 25, 731–737. [Google Scholar] [CrossRef]
- Frigo, C.; Pavan, E.E.; Brunner, R. A dynamic model of quadriceps and hamstrings function. Gait Posture. 2010, 31, 100–103. [Google Scholar] [CrossRef]
- Mironer, Y.E.; Brown, C.; Satterthwaite, J.R.; Cohen, M.; Tonder, L.M.; Grumman, S. A new technique of “midline anchoring” in spinal cord stimulation dramatically reduces lead migration. Neuromodulation 2004, 7, 32–37. [Google Scholar] [CrossRef]
- Shaparin, N.; Gritsenko, K.; Agrawal, P.; Kim, S.; Wahezi, S.; Gitkind, A.; Hascalovici, J.; Vydyanathan, A.; Bernstein, J.; Dizdarevic, A.; et al. A Retrospective Case Series of a Novel Spinal Cord Stimulator Trial Technique with Less Displacement and Migration of the Trial Leads. Pain Res. Manag. 2019, 2019, 1236430. [Google Scholar] [CrossRef]
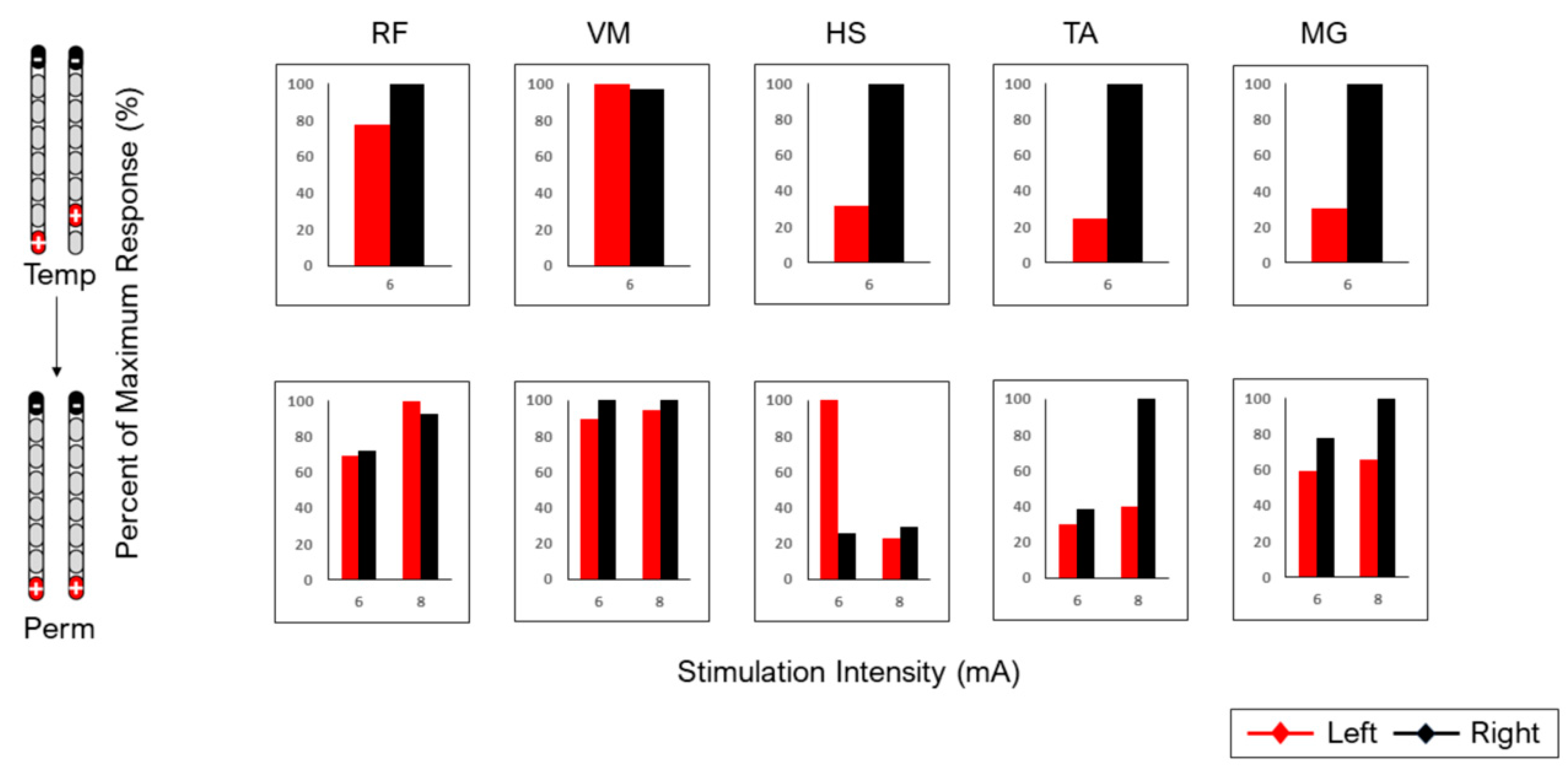

| Configuration | Muscle | Agreement Between Temporary and Permanent Spinal Mappings |
|---|---|---|
| Subject 0883 | ||
 | RF | X |
| VM | A (R2: 0.02, 0.81) | |
| HS | A (R2: 0.07, 0.85) | |
| TA | X | |
| MG | X | |
 | RF | NR |
| VM | NR | |
| HS | NR | |
| TA | X | |
| MG | NR | |
 | RF | X |
| VM | X | |
| HS | X | |
| TA | X | |
| MG | NR | |
 | RF | X |
| VM | A (R2: 0.21, 0.88) | |
| HS | X | |
| TA | X | |
| MG | X | |
| Subject 0884 | ||
 | RF | A |
| VM | A | |
| HS | X | |
| TA | A | |
| MG | A |
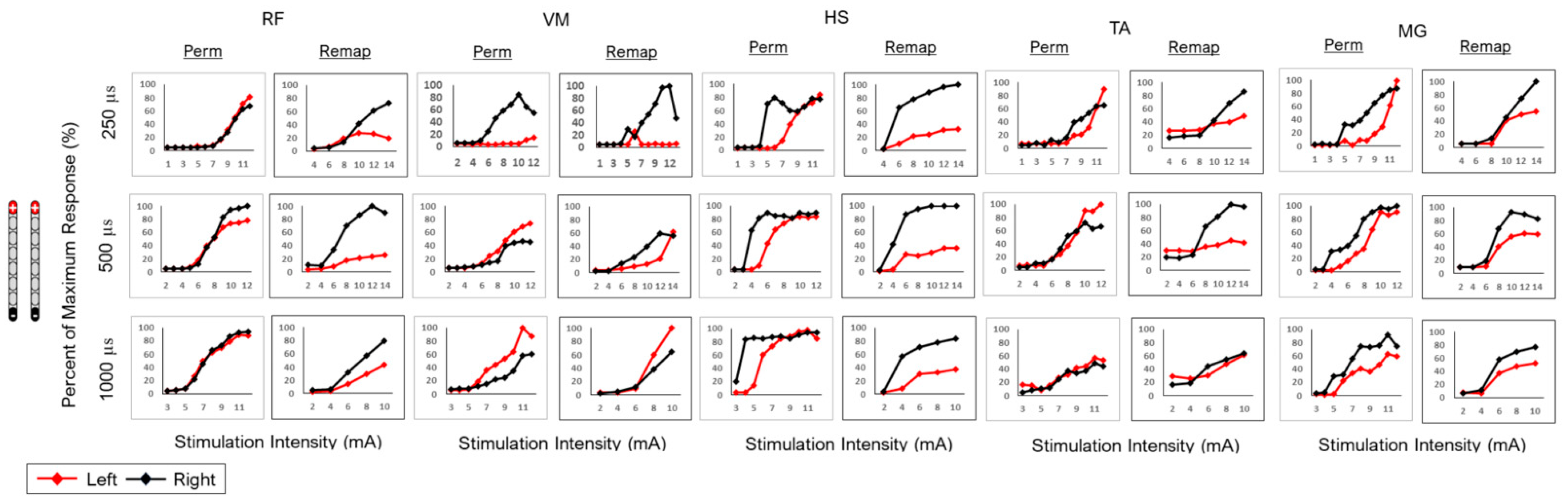
| Configuration | Muscle | Agreement Between Permanent and Spinal Remapping |
|---|---|---|
| Subject 0884 | ||
 | RF | A [250 µs: (R2: 0.75, 0.99); 500 µs: (R2: 0.99, 0.95); 1000 µs: (R2: 0.98, 0.98)] |
| VM | A [250 µs: (R2: 0.99, 0.94); 500 µs: (R2: 0.95, 0.91); 1000 µs: (R2: 0.97, 0.99)] | |
| HS | X | |
| TA | A [250 µs: (R2: 0.76, 0.74); 500 µs: (R2: 0.88, 0.94); 1000 µs: (R2: 0.99, 0.75)] | |
| MG | A [250 µs; (R2: 0.77, 0.79); 500 µs: (R2: 0.93, 0.93); 1000 µs: (R2: 0.95, 0.86)] | |
 | RF | X |
| VM | A [250 µs: (R2: 0.99, 0.99); 500 µs: (R2: 0.94, 0.73); 1000 µs: (R2: 0.98, 0.92)] | |
| HS | A [250 µs: (R2: 0.55, 0.52); 500 µs: (R2: 0.56, 0.93); 1000 µs: (R2: 0.66, 0.91)] | |
| TA | X | |
| MG | X | |
 | RF | X |
| VM | X | |
| HS | X | |
| TA | X | |
| MG | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venigalla, S.; Rehman, M.U.; Deitrich, J.N.; Trainer, R.; Gorgey, A.S. MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury. J. Clin. Med. 2024, 13, 6826. https://doi.org/10.3390/jcm13226826
Venigalla S, Rehman MU, Deitrich JN, Trainer R, Gorgey AS. MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury. Journal of Clinical Medicine. 2024; 13(22):6826. https://doi.org/10.3390/jcm13226826
Chicago/Turabian StyleVenigalla, Siddharth, Muhammad Uzair Rehman, Jakob N. Deitrich, Robert Trainer, and Ashraf S. Gorgey. 2024. "MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury" Journal of Clinical Medicine 13, no. 22: 6826. https://doi.org/10.3390/jcm13226826
APA StyleVenigalla, S., Rehman, M. U., Deitrich, J. N., Trainer, R., & Gorgey, A. S. (2024). MRI Spinal Cord Reconstruction Provides Insights into Mapping and Migration Following Percutaneous Epidural Stimulation Implantation in Spinal Cord Injury. Journal of Clinical Medicine, 13(22), 6826. https://doi.org/10.3390/jcm13226826









