Advancements in 3D Transoesophageal Echocardiography (TOE) and Computed Tomography (CT) for Stroke Prevention in Left Atrial Appendage Occlusion Interventions
Abstract
1. Introduction
1.1. Stroke Prevention in AF
1.2. Types of LAAO
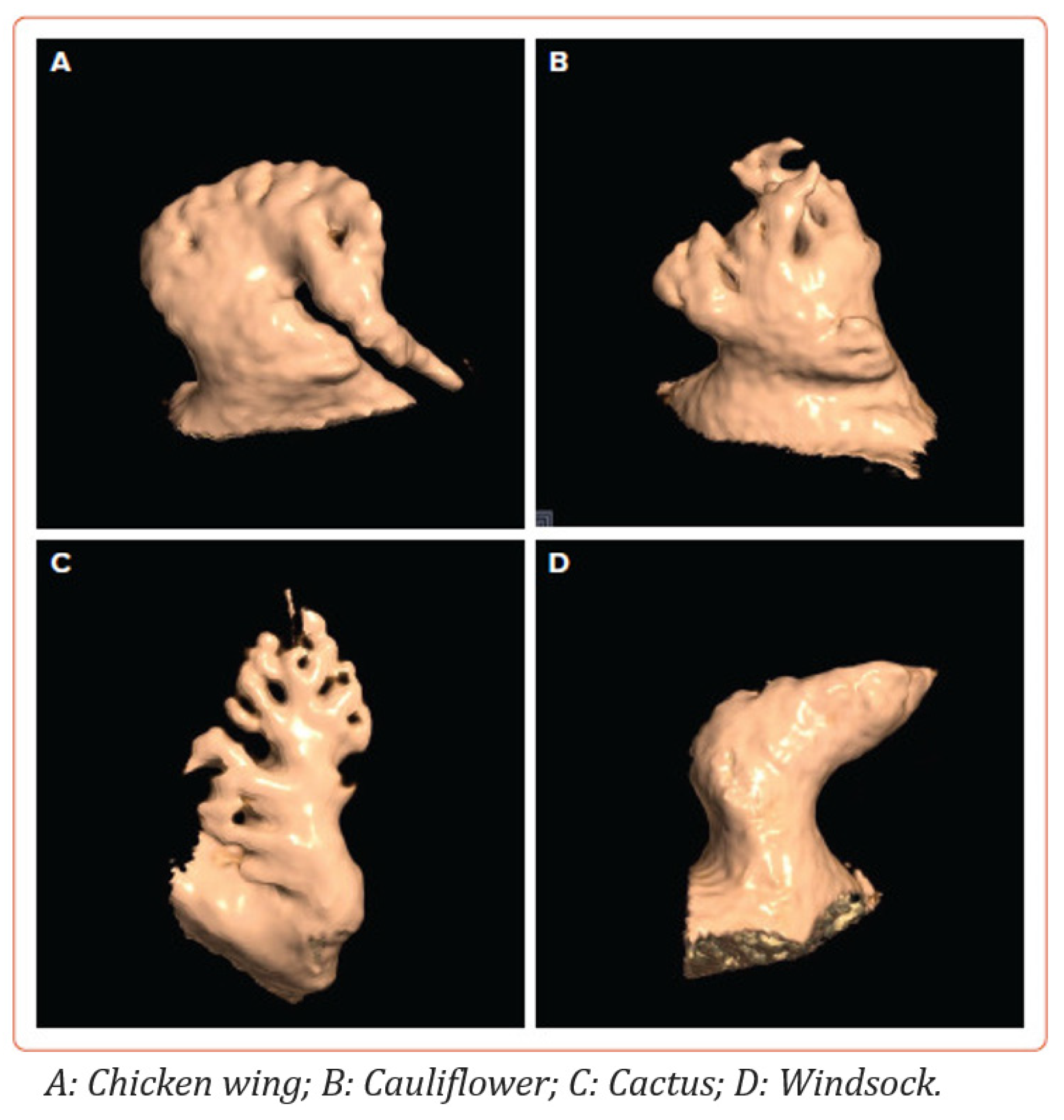
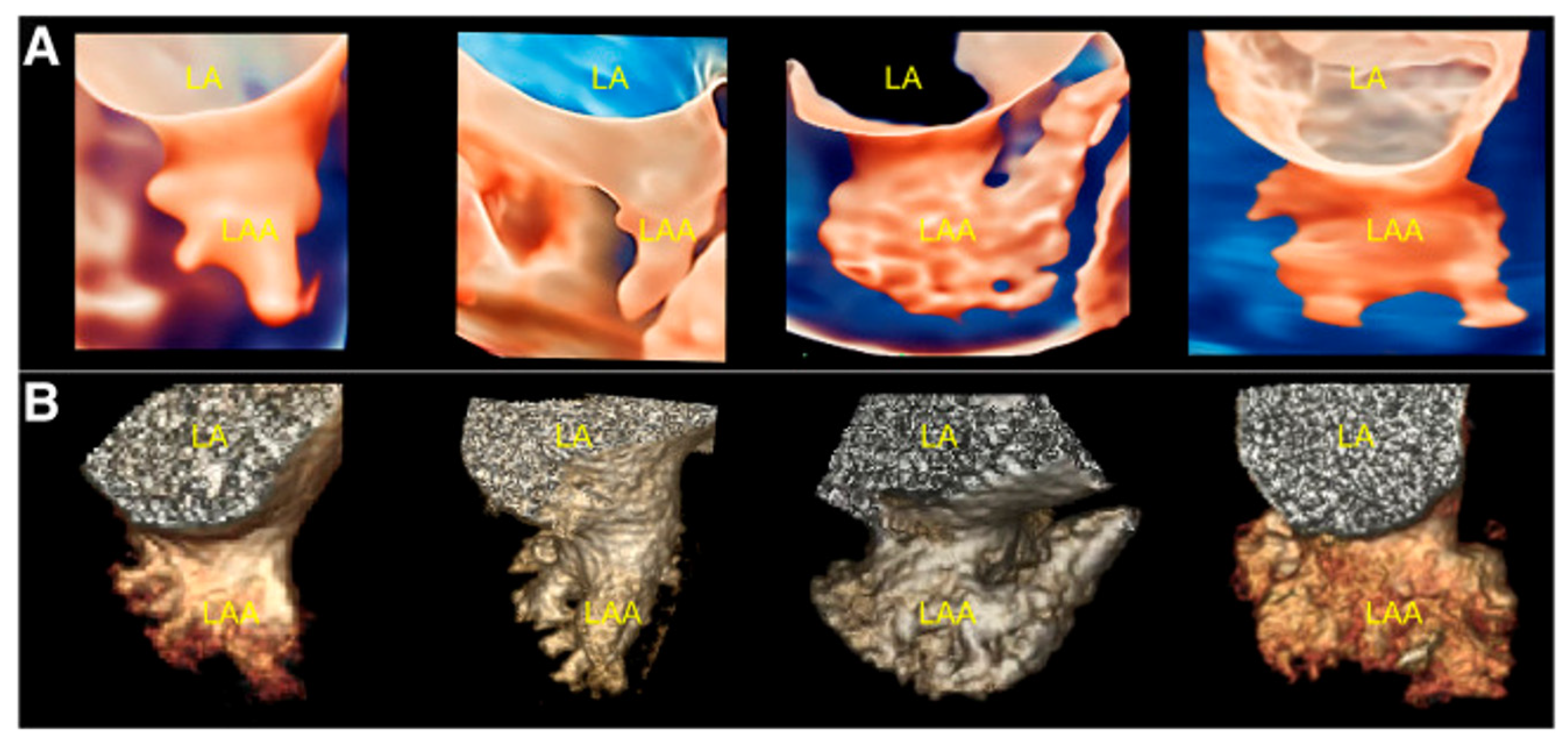
1.3. Left Atrial Appendage Anatomy and Morphology
2. Pre-Implantation Imaging
2.1. Cardiac CT
2.1.1. Anatomical Contraindications
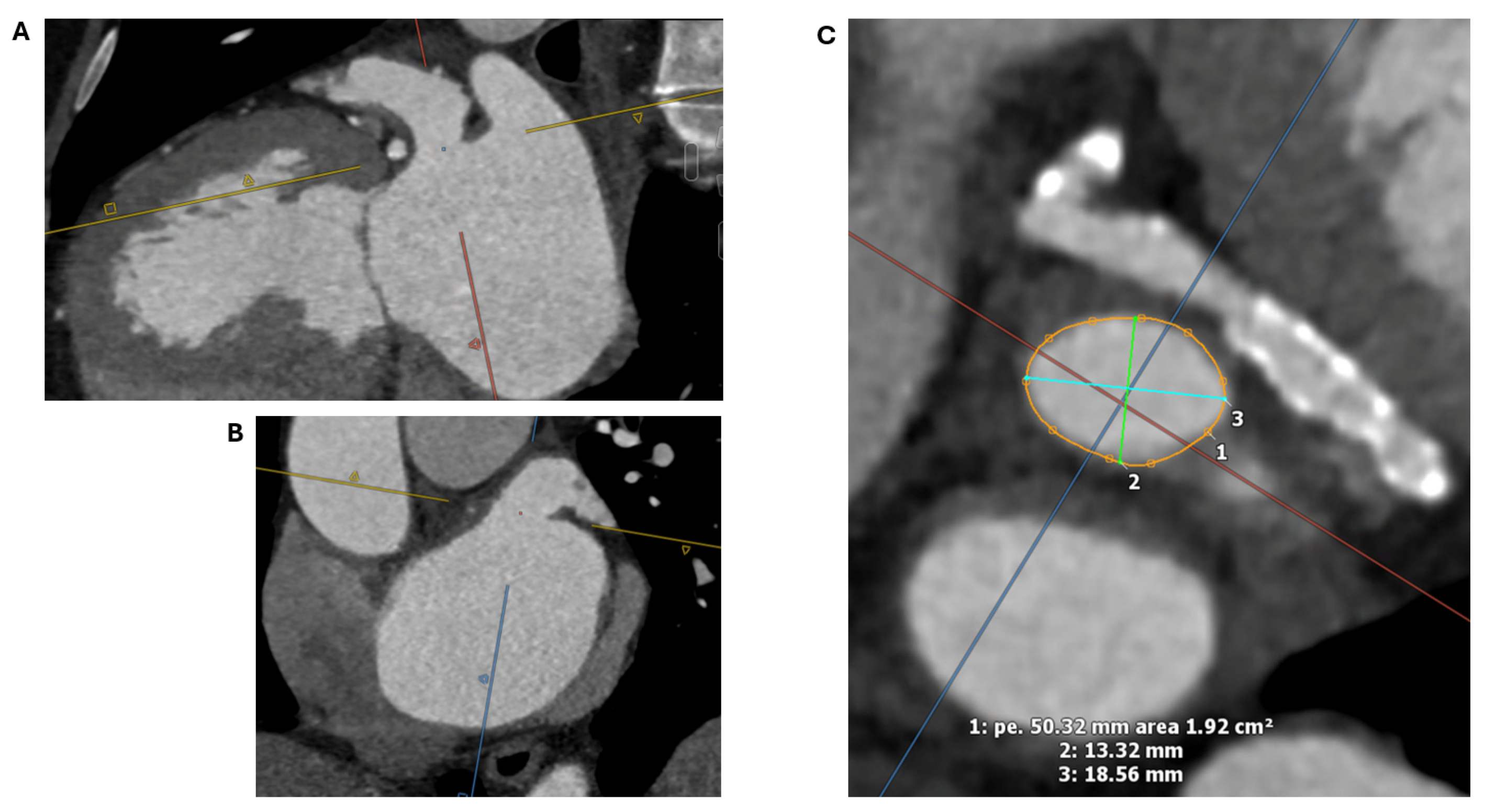
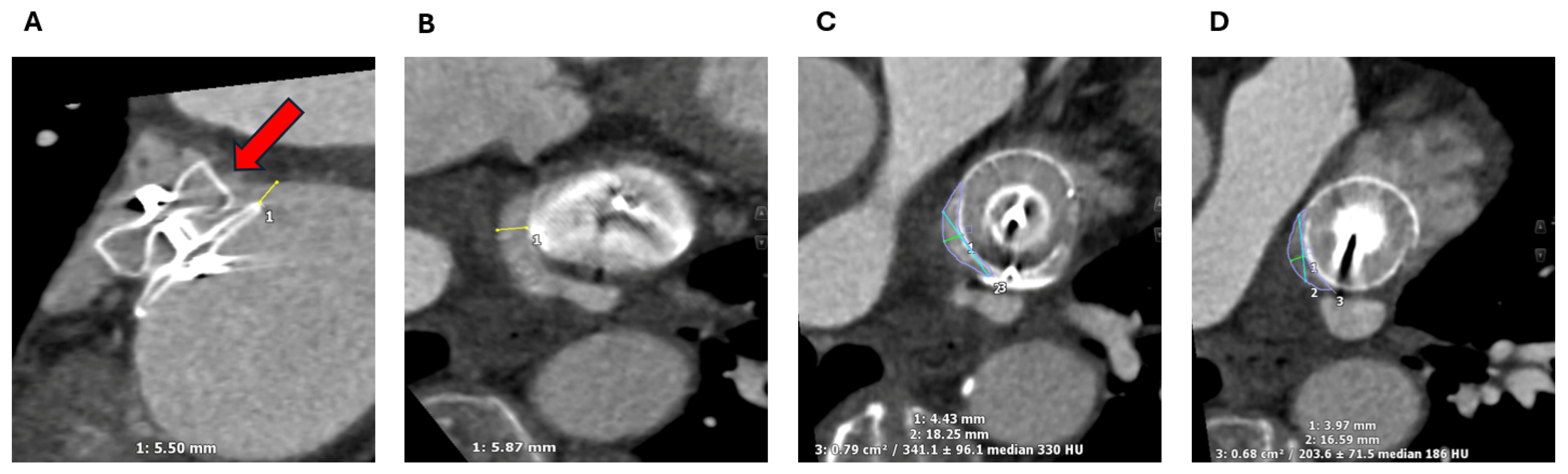
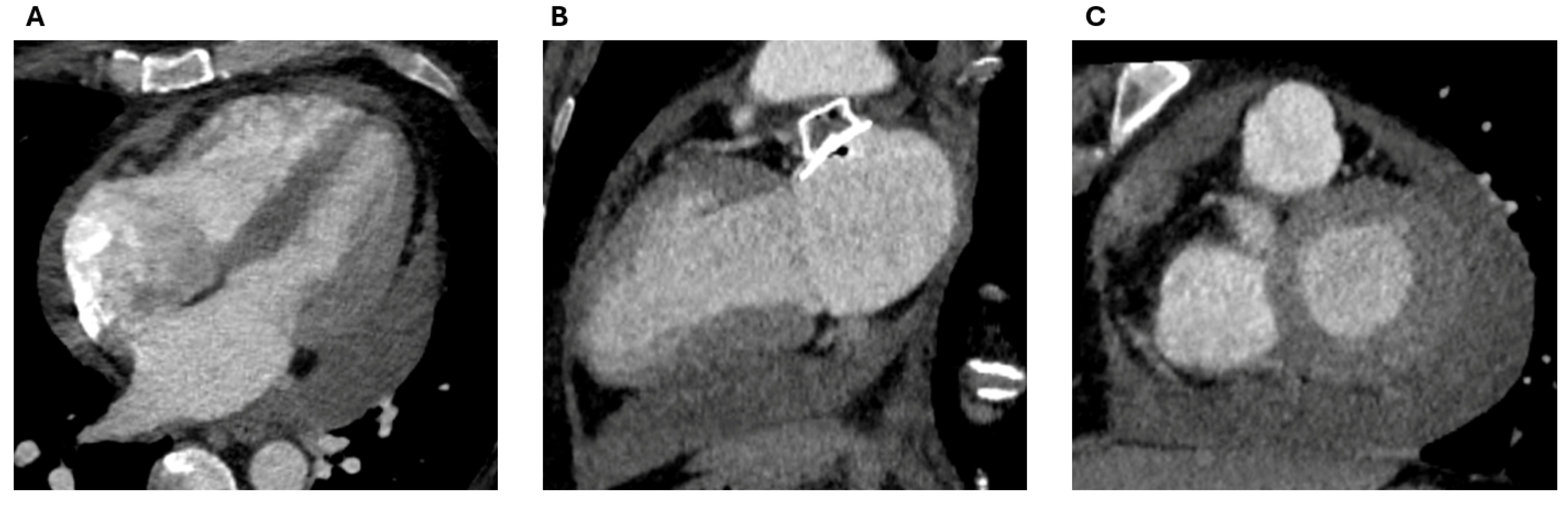
2.1.2. LAA Measurements
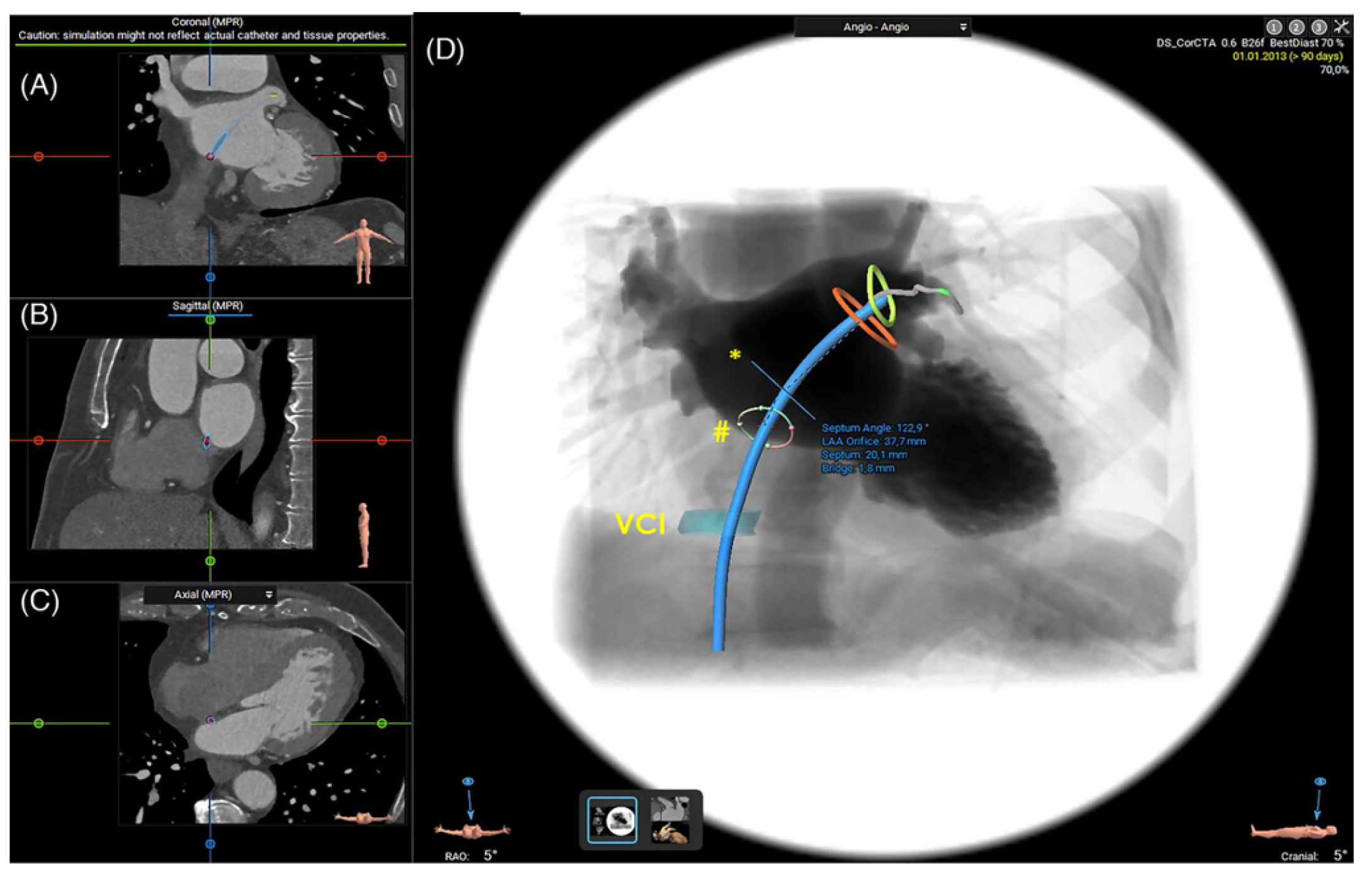
2.1.3. Assessment of the Inter-Atrial Septum
2.1.4. Other Anatomical Considerations
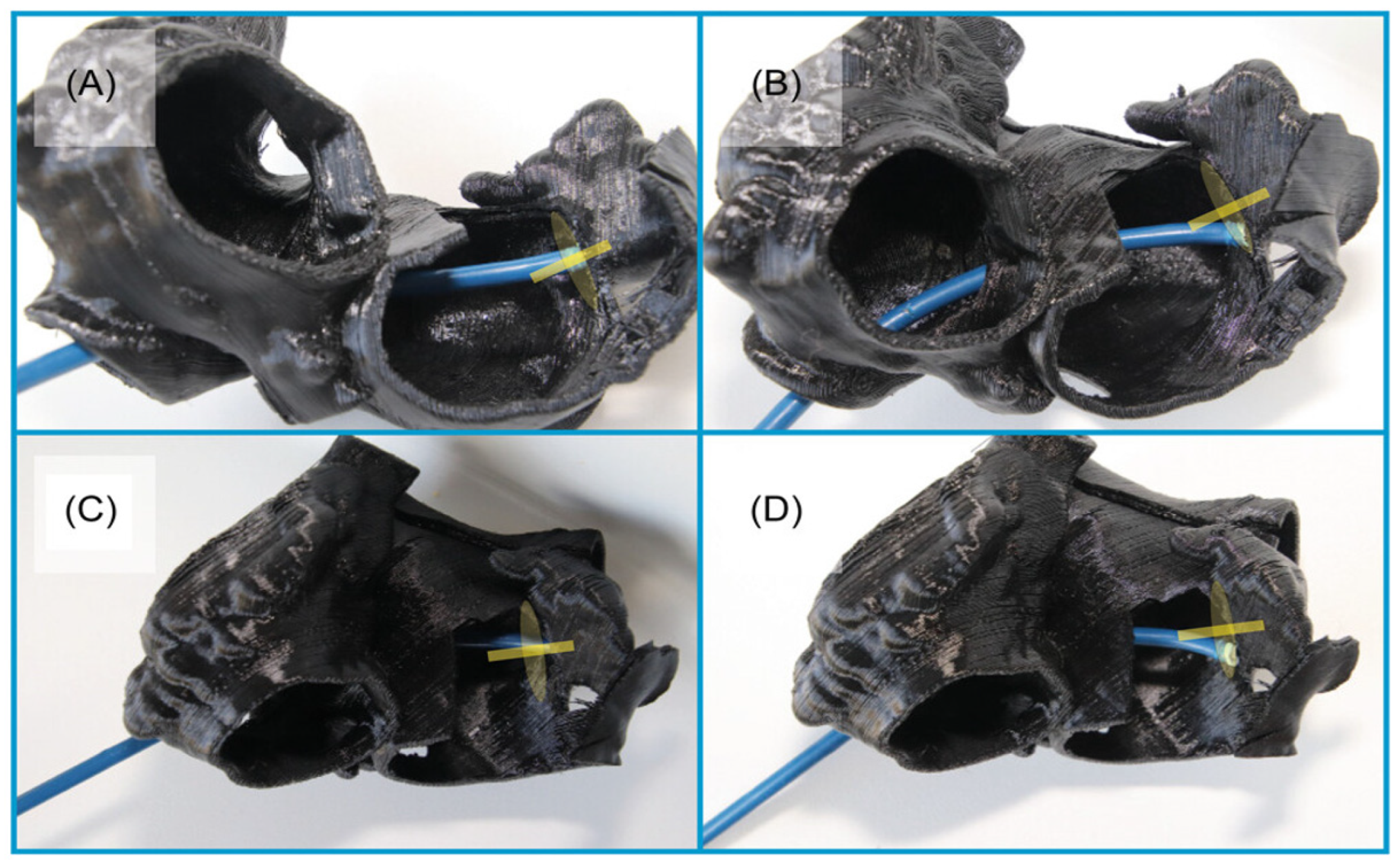
2.1.5. 3D Printing and Virtual Reality
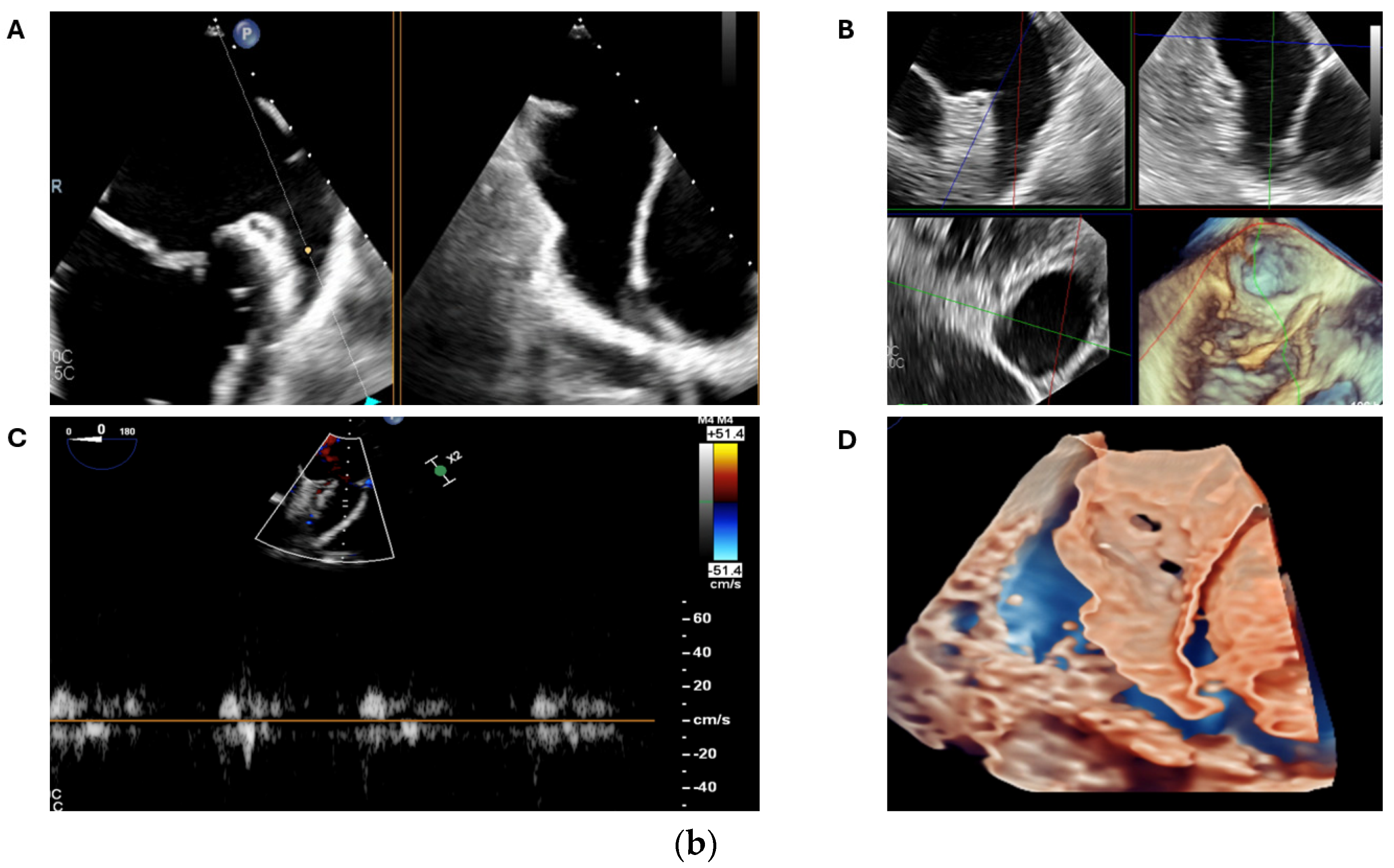
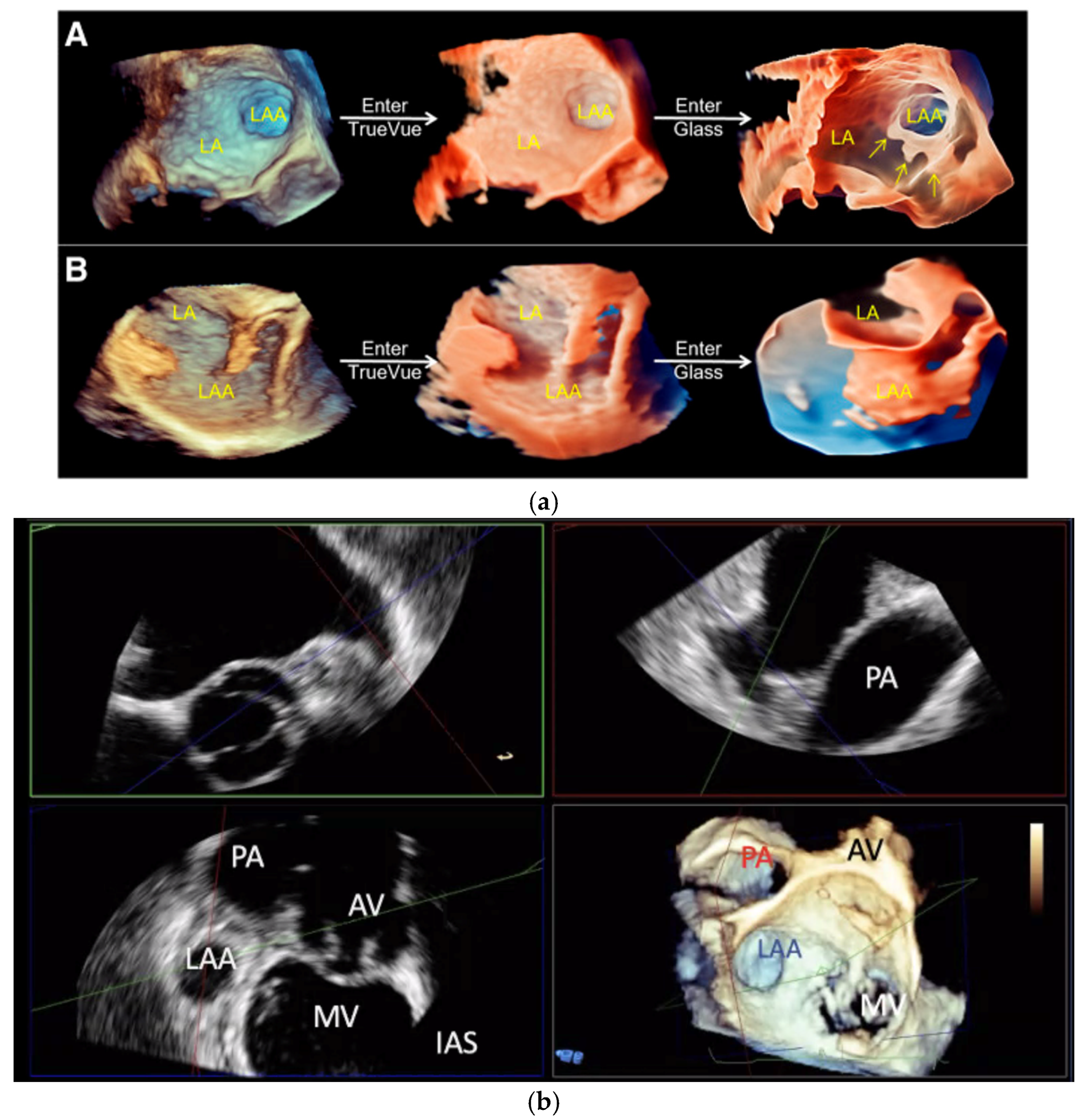
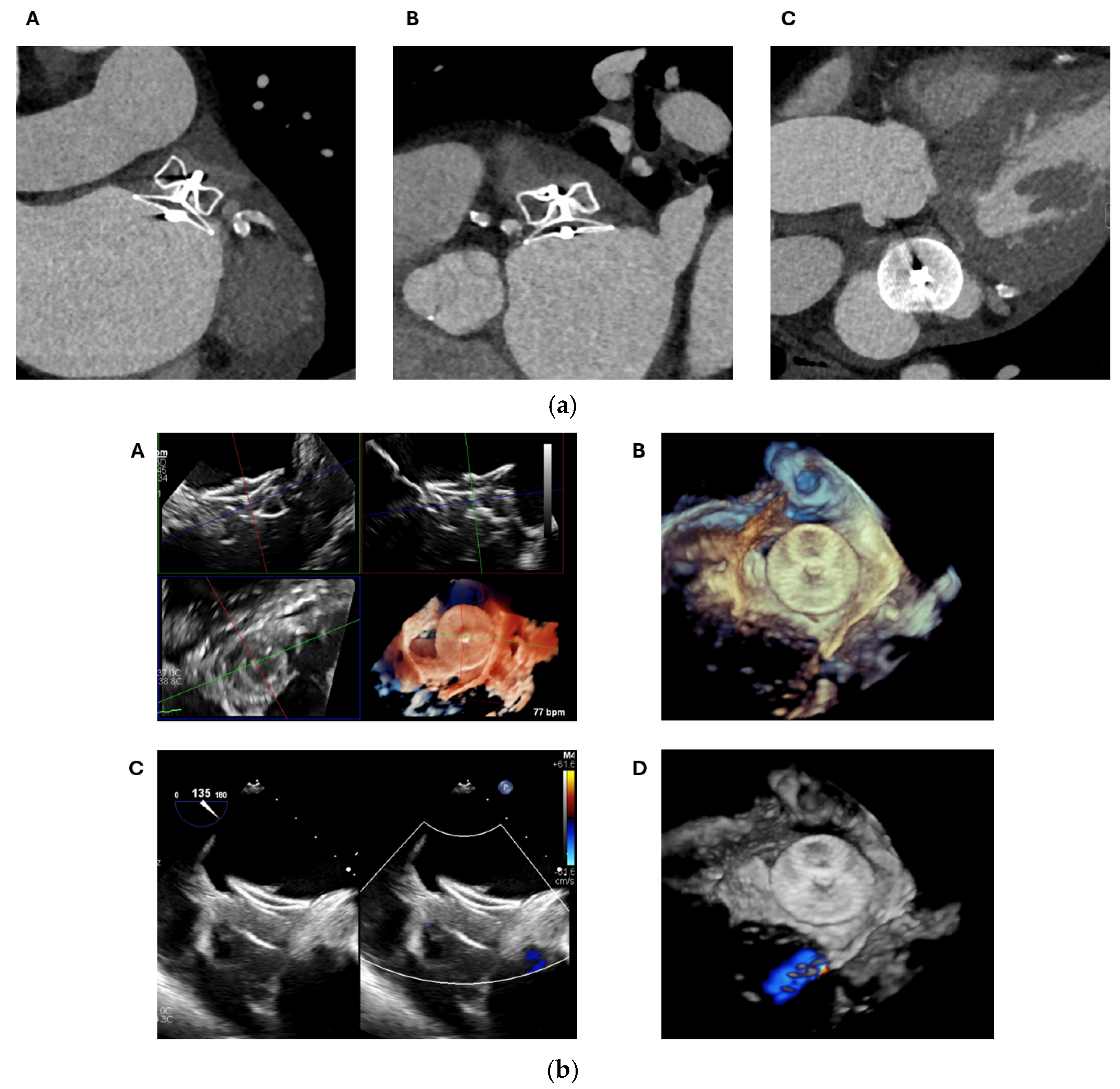
2.2. Transoesophageal Echocardiography
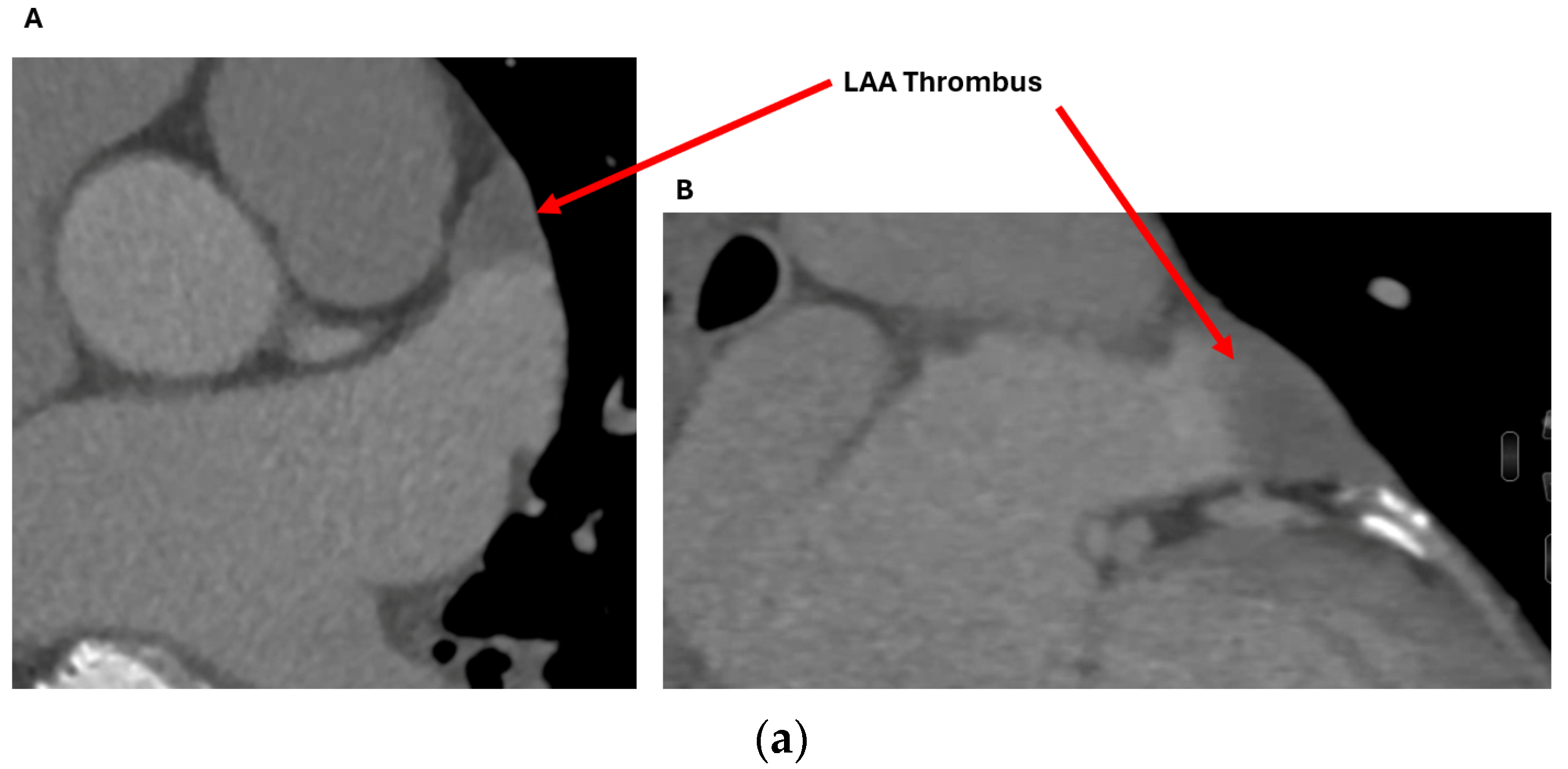
2.2.1. LAA Thrombus Assessment
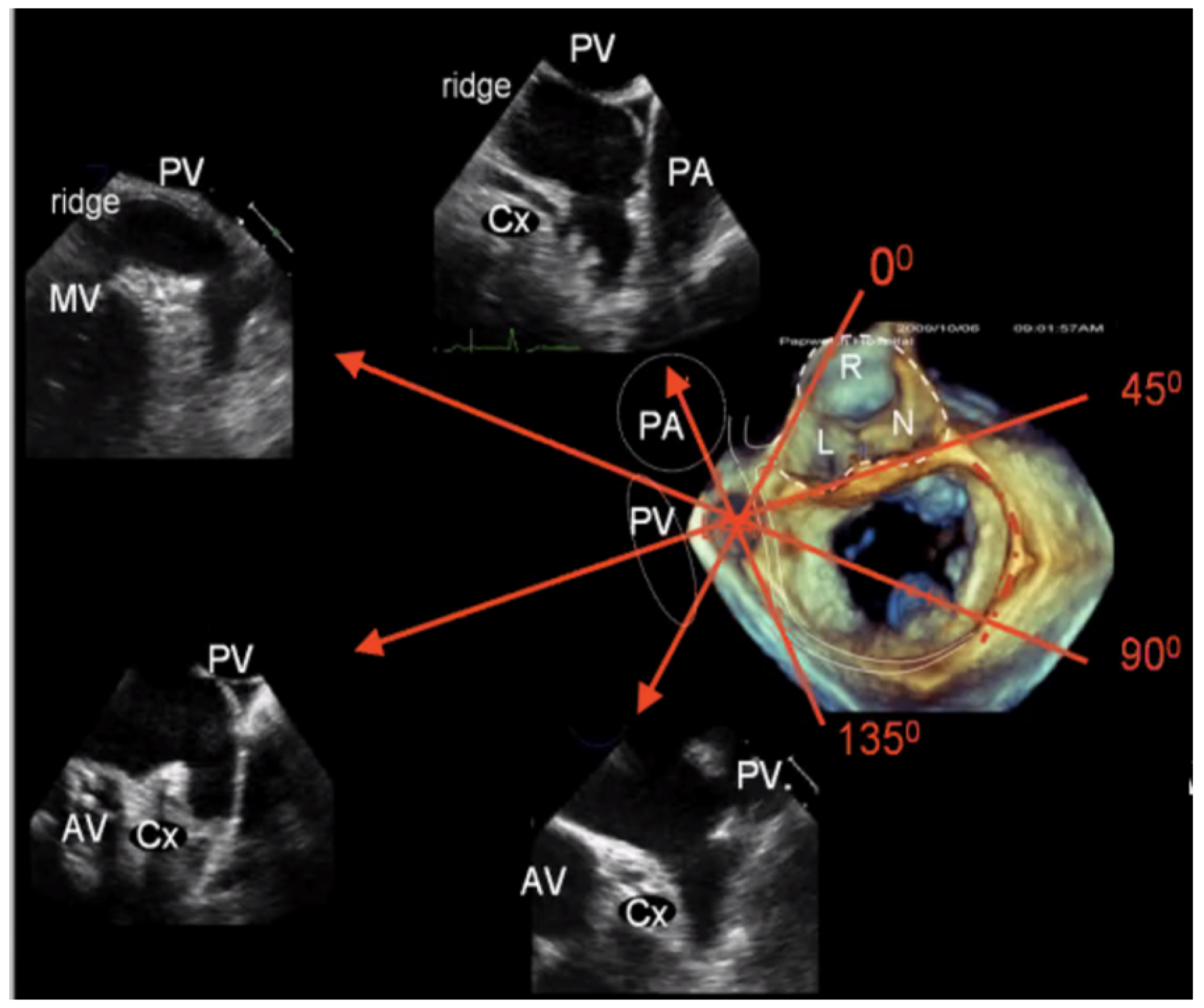
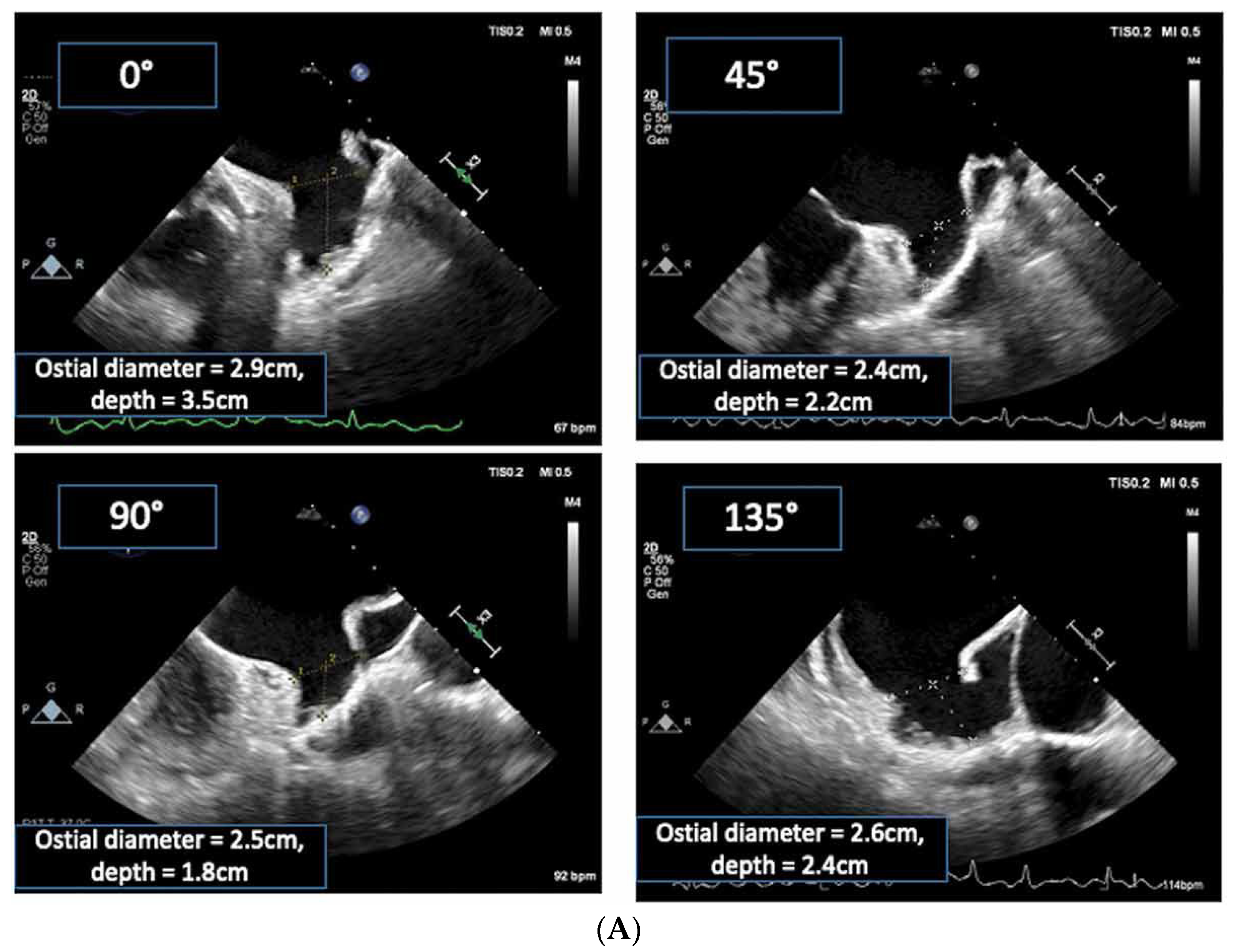
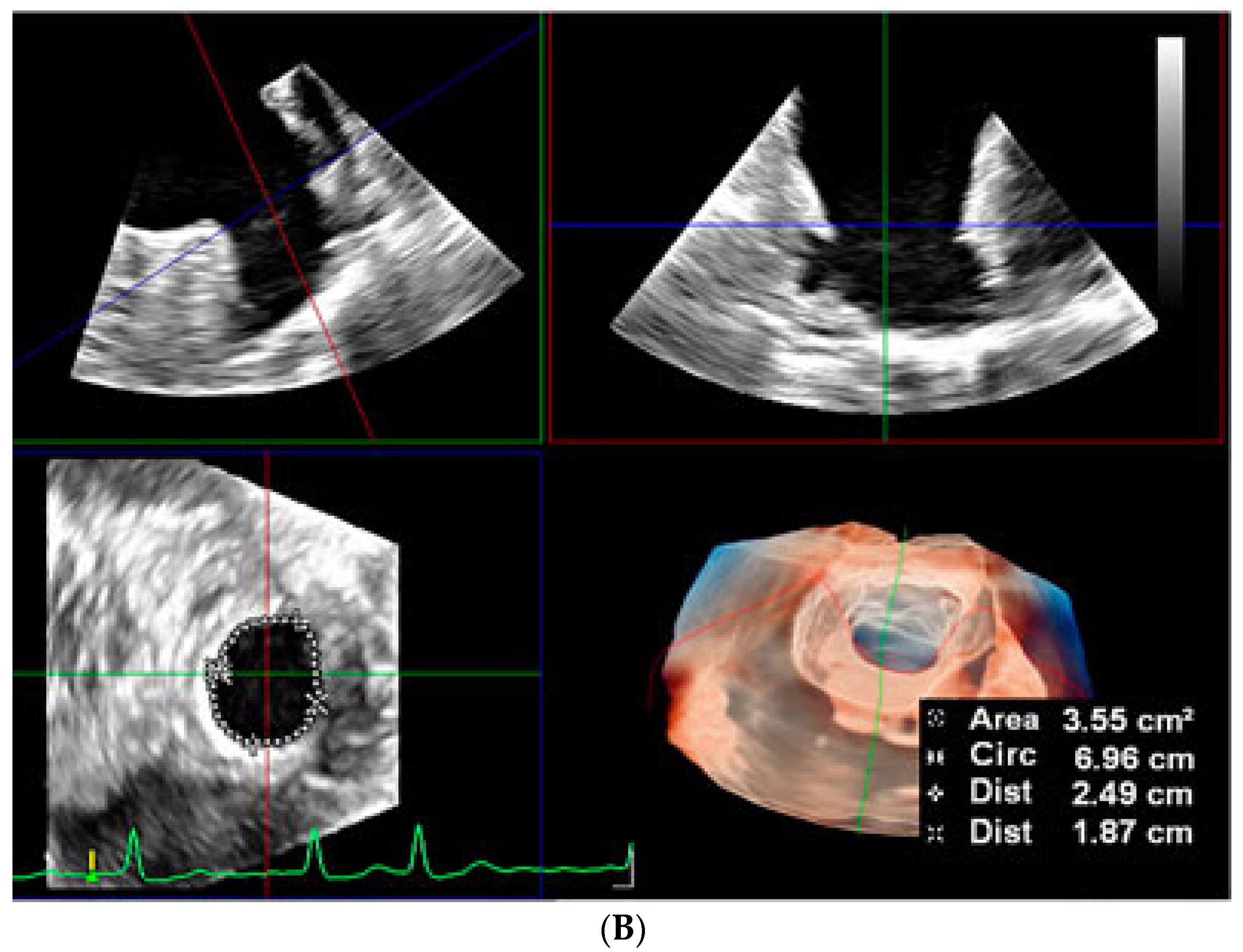
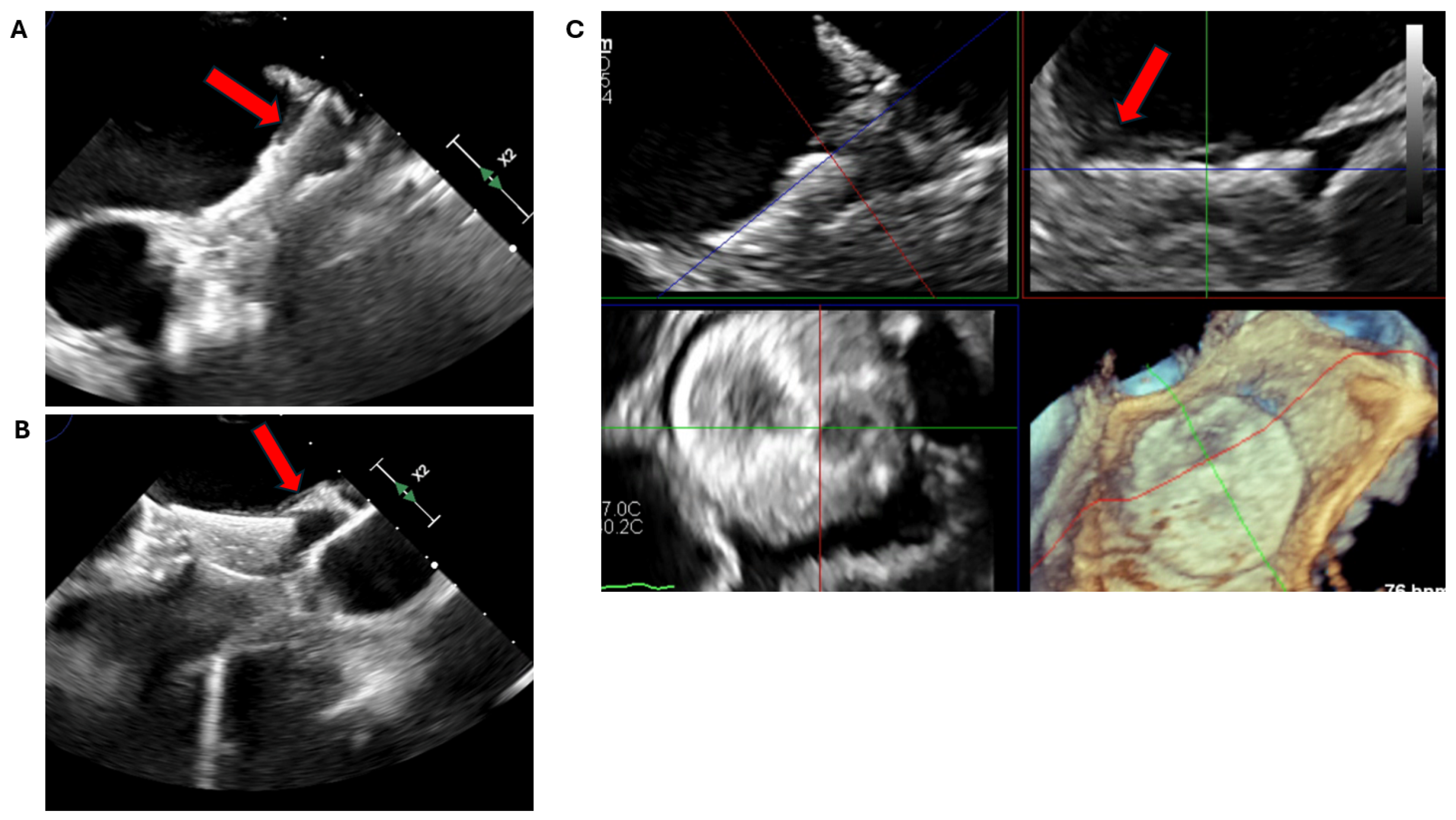
2.2.2. LAA Measurements (Anatomy and Morphology)
2.2.3. Intra-Procedural TOE
2.3. Intracardiac Echocardiography (ICE)
3. Post-Implantation Imaging
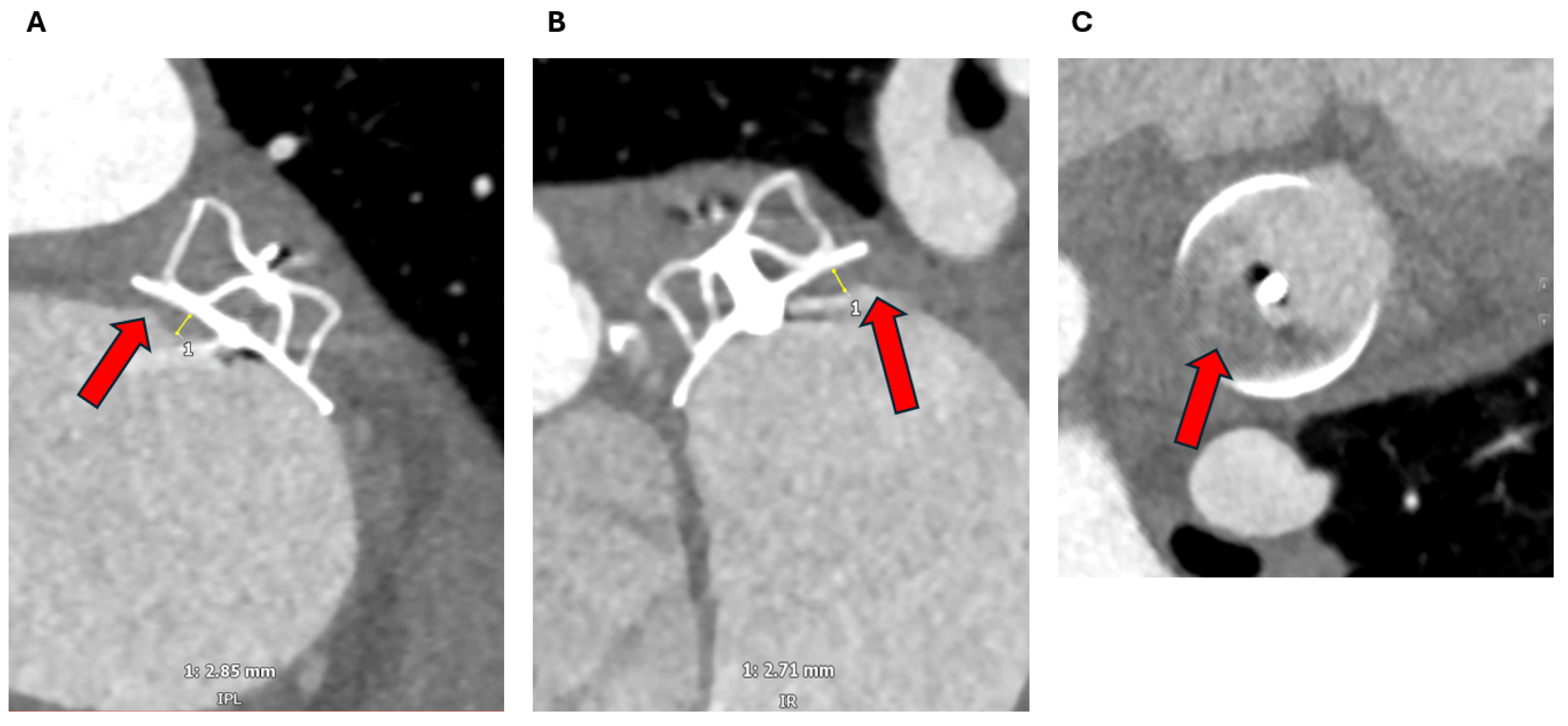
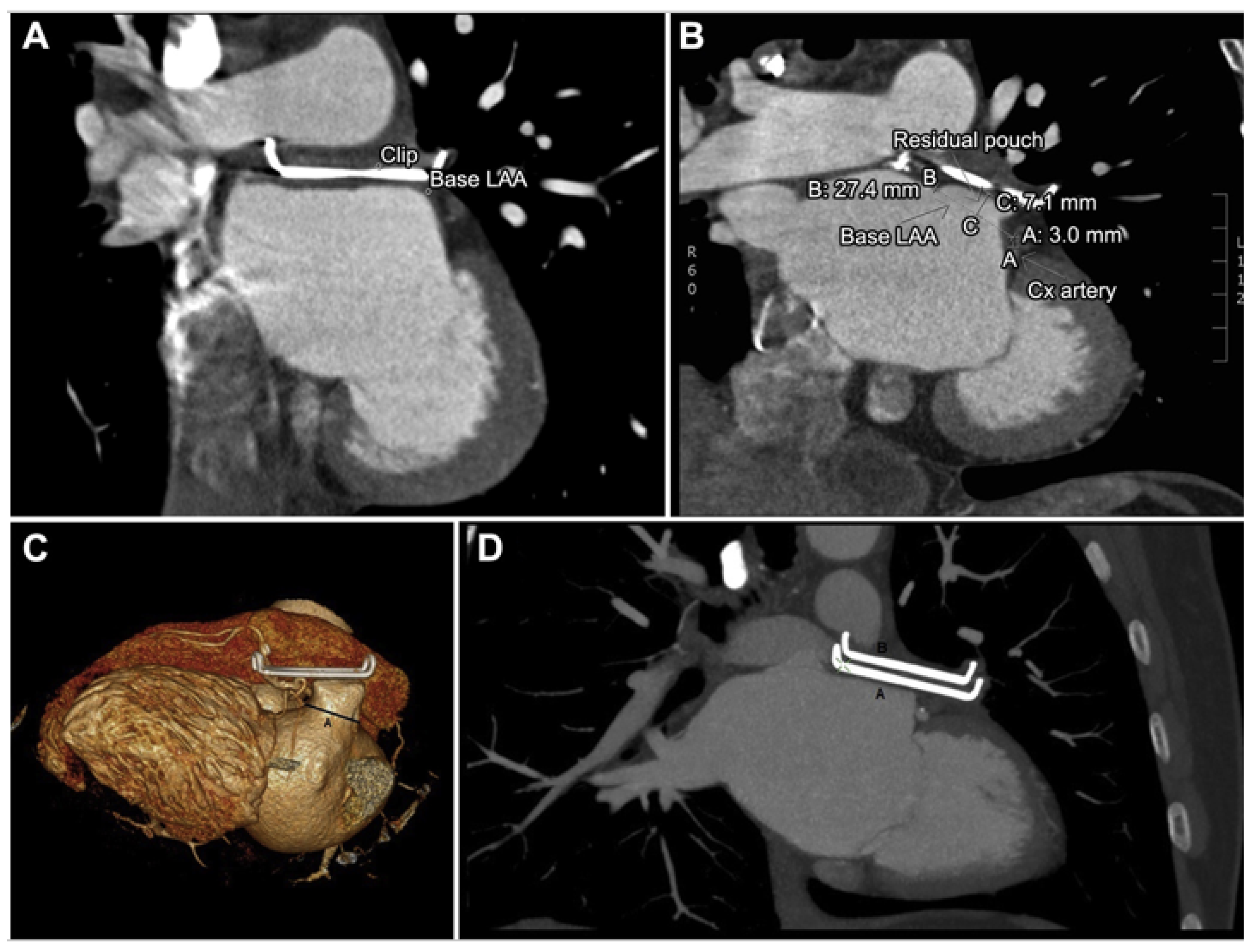
3.1. Device Position and Embolization
3.2. Peri-Device Leaks
3.3. Device-Related Thrombus
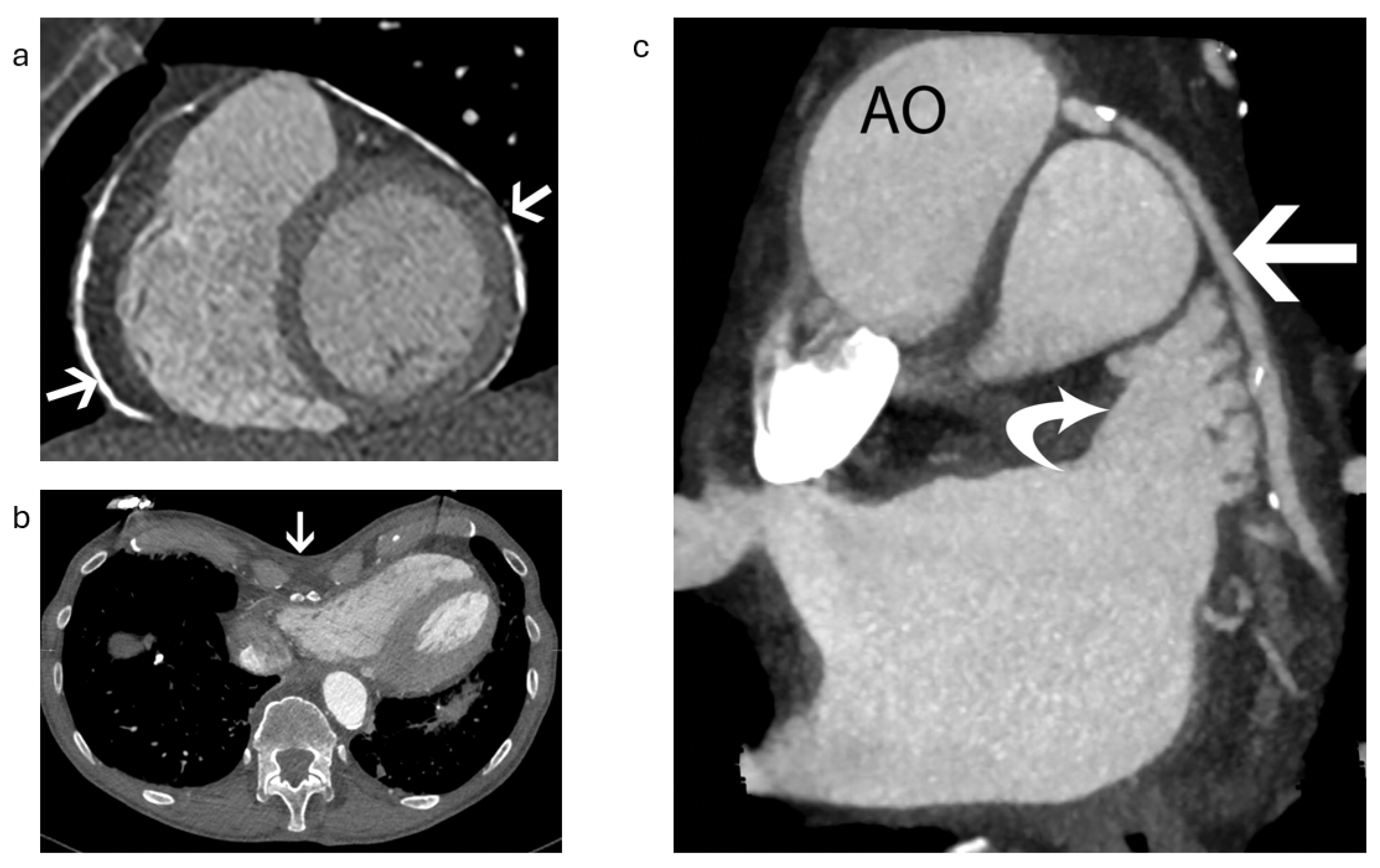
3.4. Pericardial Effusion and Device Erosion
3.5. Epicardial Devices
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.H.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial Fibrillation: Epidemiology, Screening and Digital Health. Lancet Reg. Health—Eur. 2024, 37, 100786. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous Closure of the Left Atrial Appendage versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation: A Randomised Non-Inferiority Trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ebeling, M.; Ziegler, L.; Wennberg, A.; Modig, K. Time Trends in Atrial Fibrillation-Related Stroke during 2001–2020 in Sweden: A Nationwide, Observational Study. Lancet Reg. Health—Eur. 2023, 28, 100596. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.S.; Kase, C.S.; Benjamin, E.J.; D’Agostino, R.B. Stroke Severity in Atrial Fibrillation. The Framingham Study. Stroke 1996, 27, 1760–1764. [Google Scholar] [CrossRef]
- Investigators, T.A.F.F.I. of R.M. (AFFIRM) A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation versus Long-Term Warfarin Therapy: The PREVAIL Trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Albors, C.; Mill, J.; Olivares Id, A.L.; Iriart, X.; Cochet, H.; Camaraid, O. Impact of Occluder Device Configurations in In-Silico Left Atrial Hemodynamics for the Analysis of Device-Related Thrombus. PLoS Comput. Biol. 2024, 20, e1011546. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, B.; Srimahachota, S.; de Backer, O.; Boonyartavej, S.; Lertsuwunseri, V.; Tumkosit, M.; Søndergaard, L. First-in-Human Results of the Omega Left Atrial Appendage Occluder for Patients with Non-Valvular Atrial Fibrillation. EuroIntervention 2021, 17, 376–379. [Google Scholar] [CrossRef]
- Rajiah, P.; Alkhouli, M.; Thaden, J.; Foley, T.; Williamson, E.; Ranganath, P. Pre- and Postprocedural CT of Transcatheter Left Atrial Appendage Closure Devices. Radiographics 2021, 41, 680–698. [Google Scholar] [CrossRef]
- Veinot, J.P.; Harrity, P.J.; Gentile, F.; Khandheria, B.K.; Bailey, K.R.; Eickholt, J.T.; Seward, J.B.; Tajik, A.J.; Edwards, W.D. Anatomy of the Normal Left Atrial Appendage: A Quantitative Study of Age-Related Changes in 500 Autopsy Hearts: Implications for Echocardiographic Examination. Circulation 1997, 96, 3112–3115. [Google Scholar] [CrossRef]
- Dudzińska-Szczerba, K.; Kułakowski, P.; Michałowska, I.; Baran, J. Association Between Left Atrial Appendage Morphology and Function and the Risk of Ischaemic Stroke in Patients with Atrial Fibrillation. Arrhythm. Electrophysiol. Rev. 2022, 11, e09. [Google Scholar] [CrossRef]
- Lupercio, F.; Carlos Ruiz, J.; Briceno, D.F.; Romero, J.; Villablanca, P.A.; Berardi, C.; Faillace, R.; Krumerman, A.; Fisher, J.D.; Ferrick, K.; et al. Left Atrial Appendage Morphology Assessment for Risk Stratification of Embolic Stroke in Patients with Atrial Fibrillation: A Meta-Analysis. Heart Rhythm. 2016, 13, 1402–1409. [Google Scholar] [CrossRef]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the Left Atrial Appendage Morphology Correlate with the Risk of Stroke in Patients with Atrial Fibrillation? Results from a Multicenter Study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef]
- Romero, J.; Husain, S.A.; Kelesidis, I.; Sanz, J.; Medina, H.M.; Garcia, M.J. Detection of Left Atrial Appendage Thrombus by Cardiac Computed Tomography in Patients with Atrial Fibrillation: A Meta-Analysis. Circ. Cardiovasc. Imaging 2013, 6, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS Expert Consensus Statement on Transcatheter Left Atrial Appendage Closure. Heart Rhythm. 2023, 20, e1–e16. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, M.; Fuchs, A.; Bieliauskas, G.; Wong, I.; Kofoed, K.F.; Søndergaard, L.; De Backer, O. Computed Tomography-Based Selection of Transseptal Puncture Site for Percutaneous Left Atrial Appendage Closure. EuroIntervention 2022, 17, E1435–E1444. [Google Scholar] [CrossRef] [PubMed]
- Nelles, D.; Amli, H.; Sugiura, A.; Vij, V.; Beiert, T.; Nickenig, G.; Kütting, D.; Schrickel, J.W.; Sedaghat, A. The CT Derived Angle between the Transseptal Puncture Site and the Left Atrial Appendage as a Predictor for Complex Interventional Occlusion Procedures. Echocardiography 2023, 40, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Hozman, M.; Herman, D.; Zemanek, D.; Fiser, O.; Vrba, D.; Poloczek, M.; Varvarovsky, I.; Obona, P.; Pokorny, T.; Osmancik, P. Transseptal Puncture in Left Atrial Appendage Closure Guided by 3D Printing and Multiplanar CT Reconstruction. Catheter. Cardiovasc. Interv. 2023, 102, 1331–1340. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Yan, B.P.; Lam, Y.Y. Left Atrial Appendage Exclusion: State-of-the-Art. Catheter. Cardiovasc. Interv. 2010, 75, 806–813. [Google Scholar] [CrossRef]
- Sun, A.; Ren, S.; Xiao, Y.; Chen, Y.; Wang, N.; Li, C.; Tan, X.; Pan, Y.; Sun, F.; Ren, W. Real-Time 3D Echocardiographic Transilluminated Imaging Combined with Artificially Intelligent Left Atrial Appendage Measurement for Atrial Fibrillation Interventional Procedures. Front. Physiol. 2022, 13, 1043551. [Google Scholar] [CrossRef]
- Okafor, J.; Rana, B.S. Importance of Echocardiography in Navigating Left Atrial Appendage Function, Thrombus Exclusion and Percutaneous Closure. Clin. Res. Cardiol. 2024, 1–3. [Google Scholar] [CrossRef]
- Ramchand, J.; Harb, S.C.; Miyasaka, R.; Kanj, M.; Saliba, W.; Jaber, W.A. Imaging for Percutaneous Left Atrial Appendage Closure: A Contemporary Review. Struct. Heart 2019, 3, 364–382. [Google Scholar] [CrossRef]
- Wang, D.D.; Eng, M.; Kupsky, D.; Myers, E.; Forbes, M.; Rahman, M.; Zaidan, M.; Parikh, S.; Wyman, J.; Pantelic, M.; et al. Application of 3-Dimensional Computed Tomographic Image Guidance to WATCHMAN Implantation and Impact on Early Operator Learning Curve: Single-Center Experience. JACC Cardiovasc. Interv. 2016, 9, 2329–2340. [Google Scholar] [CrossRef]
- Nucifora, G.; Faletra, F.F.; Regoli, F.; Pasotti, E.; Pedrazzini, G.; Moccetti, T.; Auricchio, A. Evaluation of the Left Atrial Appendage with Real-Time 3-Dimensional Transesophageal Echocardiography Implications for Catheter-Based Left Atrial Appendage Closure. Circ. Cardiovasc. Imaging 2011, 4, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yosefy, C.; Laish-Farkash, A.; Azhibekov, Y.; Khalameizer, V.; Brodkin, B.; Katz, A. A New Method for Direct Three-Dimensional Measurement of Left Atrial Appendage Dimensions during Transesophageal Echocardiography. Echocardiography 2016, 33, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.; Young, W.; Saric, M.; Holmes, D.; Fowler, S.; Chinitz, L. Three-Dimensional Transesophageal Echocardiography to Facilitate Transseptal Puncture and Left Atrial Appendage Occlusion via Upper Extremity Venous Access. Circ. Arrhythm. Electrophysiol. 2015, 8, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Vainrib, A.F.; Harb, S.C.; Jaber, W.; Benenstein, R.J.; Aizer, A.; Chinitz, L.A.; Saric, M. Left Atrial Appendage Occlusion/Exclusion: Procedural Image Guidance with Transesophageal Echocardiography. J. Am. Soc. Echocardiogr. 2018, 31, 454–474. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Hijazi, Z.M.; Holmes, D.R.; Rihal, C.S.; Wiegers, S.E. Intracardiac Echocardiography in Structural Heart Disease Interventions. JACC Cardiovasc. Interv. 2018, 11, 2133–2147. [Google Scholar] [CrossRef]
- Alkhouli, M.; Chaker, Z.; Alqahtani, F.; Raslan, S.; Raybuck, B. Outcomes of Routine Intracardiac Echocardiography to Guide Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2020, 6, 393–400. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Magnocavallo, M.; Gianni, C.; Mohanty, S.; Al-Ahmad, A.; Bassiouny, M.; Denora, M.; La Fazia, V.M.; Lavalle, C.; Gallinghouse, G.J.; et al. Three-Dimensional Intracardiac Echocardiography for Left Atrial Appendage Sizing and Percutaneous Occlusion Guidance. EP Eur. 2023, 26, euae010. [Google Scholar] [CrossRef]
- Ho, E.C.; Assafin, M.; Sugiura, T.; Granada, J.F.; Chau, M.; Latib, A. 3-Dimensional Intracardiac Echocardiography for Structural Heart Interventions. Front. Cardiovasc. Med. 2023, 10, 1180299. [Google Scholar] [CrossRef]
- Gidney, B.; Della Rocca, D.G.; Horton, R.; Hoffman, J.; Valderrábano, M.; Natale, A.; Garg, J.; Bhardwaj, R.; Doshi, S. Step-by-Step Recommendations Utilizing Four-Dimensional Intracardiac Echocardiography in Left Atrial Appendage Procedures. J. Cardiovasc. Electrophysiol. 2024, 35, 1601–1613. [Google Scholar] [CrossRef]
- Ferro, E.G.; Alkhouli, M.; Nair, D.G.; Kapadia, S.R.; Hsu, J.C.; Gibson, D.N.; Freeman, J.V.; Price, M.J.; Roy, K.; Allocco, D.J.; et al. Intracardiac vs Transesophageal Echocardiography for Left Atrial Appendage Occlusion With Watchman FLX in the U.S. JACC Clin. Electrophysiol. 2023, 9, 2587–2599. [Google Scholar] [CrossRef]
- Alkhouli, M.; Sievert, H.; Rihal, C.S. Device Embolization in Structural Heart Interventions: Incidence, Outcomes, and Retrieval Techniques. JACC Cardiovasc. Interv. 2019, 12, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left Atrial Appendage Occlusion for Stroke Prevention in Atrial Fibrillation: Multicentre Experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Lalmand, J.; Tzikas, A.; Budts, W.; Benit, E.; Kefer, J. Embolization of Left Atrial Appendage Closure Devices: A Systematic Review of Cases Reported with the Watchman Device and the Amplatzer Cardiac Plug. Catheter. Cardiovasc. Interv. 2015, 86, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Papazoglou, A.S.; Balomenakis, C.; Bekiaridou, A.; Moysidis, D.V.; Patsiou, V.; Orfanidis, A.; Giannakoulas, G.; Kassimis, G.; Fragakis, N.; et al. Residual Leaks Following Percutaneous Left Atrial Appendage Occlusion and Outcomes: A Meta-Analysis. Eur. Heart J. 2024, 45, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Sahore, A.; Della Rocca, D.G.; Anannab, A.; Mohanty, S.; Akella, K.; Murtaza, G.; Trivedi, C.; Gianni, C.; Chen, Q.; Bassiouny, M.; et al. Clinical Implications and Management Strategies for Left Atrial Appendage Leaks. Card. Electrophysiol. Clin. 2020, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; De Backer, O.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Sievert, H.; Natale, A.; Lakkireddy, D.; Holmes, D.R. Peridevice Leak After Left Atrial Appendage Occlusion: Incidence, Mechanisms, Clinical Impact, and Management. JACC Cardiovasc. Interv. 2023, 16, 627–642. [Google Scholar] [CrossRef]
- Korsholm, K.; Kramer, A.; Andersen, A.; Saw, J.; Nørgaard, B.L.; Jensen, J.M.; Nielsen-Kudsk, J.E. Left Atrial Appendage Sealing Performance of the Amplatzer Amulet and Watchman FLX Device. J. Interv. Card. Electrophysiol. 2023, 66, 391–401. [Google Scholar] [CrossRef]
- Banga, S.; Osman, M.; Sengupta, P.P.; Benjamin, M.M.; Shrestha, S.; Challa, A.; Zeb, I.; Kadiyala, M.; Mills, J.; Balla, S.; et al. CT Assessment of the Left Atrial Appendage Post-Transcatheter Occlusion—A Systematic Review and Meta Analysis. J. Cardiovasc. Comput. Tomogr. 2021, 15, 348–355. [Google Scholar] [CrossRef]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion: A Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alarouri, H.; Kramer, A.; Korsholm, K.; Collins, J.; De Backer, O.; Hatoum, H.; Nielsen-Kudsk, J.E. Device-Related Thrombus After Left Atrial Appendage Occlusion: Clinical Impact, Predictors, Classification, and Management. JACC Cardiovasc. Interv. 2023, 16, 2695–2707. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kar, S.; Holmes, D.R.; Doshi, S.K.; Swarup, V.; Gibson, D.N.; Maini, B.; Gordon, N.T.; Main, M.L.; Reddy, V.Y. Device-Related Thrombus After Left Atrial Appendage Closure: Incidence, Predictors, and Outcomes. Circulation 2018, 138, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.; Vij, V.; Al-Kassou, B.; Gloekler, S.; Galea, R.; Fürholz, M.; Meier, B.; Valgimigli, M.; O’Hara, G.; Arzamendi, D.; et al. Device-Related Thrombus After Left Atrial Appendage Closure: Data on Thrombus Characteristics, Treatment Strategies, and Clinical Outcomes From the EUROC-DRT-Registry. Circ. Cardiovasc. Interv. 2021, 14, E010195. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.; Nickenig, G.; Schrickel, J.W.; Ince, H.; Schmidt, B.; Protopopov, A.V.; Betts, T.R.; Gori, T.; Sievert, H.; Mazzone, P.; et al. Incidence, Predictors and Outcomes of Device-Related Thrombus after Left Atrial Appendage Closure with the WATCHMAN Device—Insights from the EWOLUTION Real World Registry. Catheter. Cardiovasc. Interv. 2021, 97, E1019–E1024. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Nielsen-Kudsk, J.E.; Ellis, C.R.; Thaler, D.; Sabir, S.A.; Gambhir, A.; Landmesser, U.; Shah, N.; Gray, W.; Swarup, V.; et al. Incidence, Predictors, and Clinical Outcomes of Device-Related Thrombus in the Amulet IDE Trial. JACC Clin. Electrophysiol. 2023, 9, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Nielsen-Kudsk, J.E. Detection of Device-Related Thrombosis Following Left Atrial Appendage Occlusion: A Comparison Between Cardiac Computed Tomography and Transesophageal Echocardiography. Circ. Cardiovasc. Interv. 2019, 12, e008112. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S.; Holmes, D.R.; Van Tassel, R.A.; Hauser, R.; Henry, T.D.; Mooney, M.; Matthews, R.; Doshi, S.; Jones, R.M.; Virmani, R. Left Atrial Appendage Obliteration: Mechanisms of Healing and Intracardiac Integration. JACC Cardiovasc. Interv. 2010, 3, 870–877. [Google Scholar] [CrossRef]
- Kar, S.; Hou, D.; Jones, R.; Werner, D.; Swanson, L.; Tischler, B.; Stein, K.; Huibregtse, B.; Ladich, E.; Kutys, R.; et al. Impact of Watchman and Amplatzer Devices on Left Atrial Appendage Adjacent Structures and Healing Response in a Canine Model. JACC Cardiovasc. Interv. 2014, 7, 801–809. [Google Scholar] [CrossRef]
- Ellis, C.R.; Alkhouli, M.; Anderson, J.A.; Swarup, V. Comparative Endothelialization of Amulet LAA Occluder and Watchman 2.5 LAA Device: Observations from Explanted Hearts. JACC Clin. Electrophysiol. 2022, 8, 828–829. [Google Scholar] [CrossRef]
- Main, M.L.; Fan, D.; Reddy, V.Y.; Holmes, D.R.; Gordon, N.T.; Coggins, T.R.; House, J.A.; Liao, L.; Rabineau, D.; Latus, G.G.; et al. Assessment of Device-Related Thrombus and Associated Clinical Outcomes with the WATCHMAN Left Atrial Appendage Closure Device for Embolic Protection in Patients With Atrial Fibrillation (from the PROTECT-AF Trial). Am. J. Cardiol. 2016, 117, 1127–1134. [Google Scholar] [CrossRef]
- Price, M.J.; Valderrabano, M.; Zimmerman, S.; Friedman, D.J.; Kar, S.; Curtis, J.P.; Masoudi, F.A.; Freeman, J.V. Periprocedural Pericardial Effusion Complicating Transcatheter Left Atrial Appendage Occlusion: A Report from the NCDR LAAO Registry. Circ. Cardiovasc. Interv. 2022, 15, E011718. [Google Scholar] [CrossRef]
- Wilkins, B.; Fukutomi, M.; De Backer, O.; Søndergaard, L. Left Atrial Appendage Closure: Prevention and Management of Periprocedural and Postprocedural Complications. Card. Electrophysiol. Clin. 2020, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Bini, T.; Krsnik, J.P.; Touray, M.; Temperli, F.G.; Kassar, M.; Papadis, A.; Gloeckler, S.; Brugger, N.; Madhkour, R.; et al. Pericardial Effusion After Left Atrial Appendage Closure: Timing, Predictors, and Clinical Impact. JACC Cardiovasc. Interv. 2024, 17, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, A.; Ng, M.K.C.; Storey, P.; McGuire, M.A. Death from Pulmonary Artery Erosion Complicating Implantation of Percutaneous Left Atrial Appendage Occlusion Device. Heart Rhythm. 2013, 10, 1810–1811. [Google Scholar] [CrossRef] [PubMed]
- van Laar, C.; Verberkmoes, N.J.; van Es, H.W.; Lewalter, T.; Dunnington, G.; Stark, S.; Longoria, J.; Hofman, F.H.; Pierce, C.M.; Kotecha, D.; et al. Thoracoscopic Left Atrial Appendage Clipping: A Multicenter Cohort Analysis. JACC Clin. Electrophysiol. 2018, 4, 893–901. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajhosseiny, R.; Ariff, B.; Cole, G.; Koa-Wing, M.; Pabari, P.; Sutaria, N.; Qureshi, N.; Kanagaratnam, P.; Rana, B. Advancements in 3D Transoesophageal Echocardiography (TOE) and Computed Tomography (CT) for Stroke Prevention in Left Atrial Appendage Occlusion Interventions. J. Clin. Med. 2024, 13, 6899. https://doi.org/10.3390/jcm13226899
Hajhosseiny R, Ariff B, Cole G, Koa-Wing M, Pabari P, Sutaria N, Qureshi N, Kanagaratnam P, Rana B. Advancements in 3D Transoesophageal Echocardiography (TOE) and Computed Tomography (CT) for Stroke Prevention in Left Atrial Appendage Occlusion Interventions. Journal of Clinical Medicine. 2024; 13(22):6899. https://doi.org/10.3390/jcm13226899
Chicago/Turabian StyleHajhosseiny, Reza, Ben Ariff, Graham Cole, Michael Koa-Wing, Punam Pabari, Nilesh Sutaria, Norman Qureshi, Prapa Kanagaratnam, and Bushra Rana. 2024. "Advancements in 3D Transoesophageal Echocardiography (TOE) and Computed Tomography (CT) for Stroke Prevention in Left Atrial Appendage Occlusion Interventions" Journal of Clinical Medicine 13, no. 22: 6899. https://doi.org/10.3390/jcm13226899
APA StyleHajhosseiny, R., Ariff, B., Cole, G., Koa-Wing, M., Pabari, P., Sutaria, N., Qureshi, N., Kanagaratnam, P., & Rana, B. (2024). Advancements in 3D Transoesophageal Echocardiography (TOE) and Computed Tomography (CT) for Stroke Prevention in Left Atrial Appendage Occlusion Interventions. Journal of Clinical Medicine, 13(22), 6899. https://doi.org/10.3390/jcm13226899




