Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet’s Disease †
Abstract
:1. Introduction
2. Patients and Methods
2.1. Design, Enrolment Criteria and Definitions
2.2. Outcome Variables
2.3. Statistical Analysis
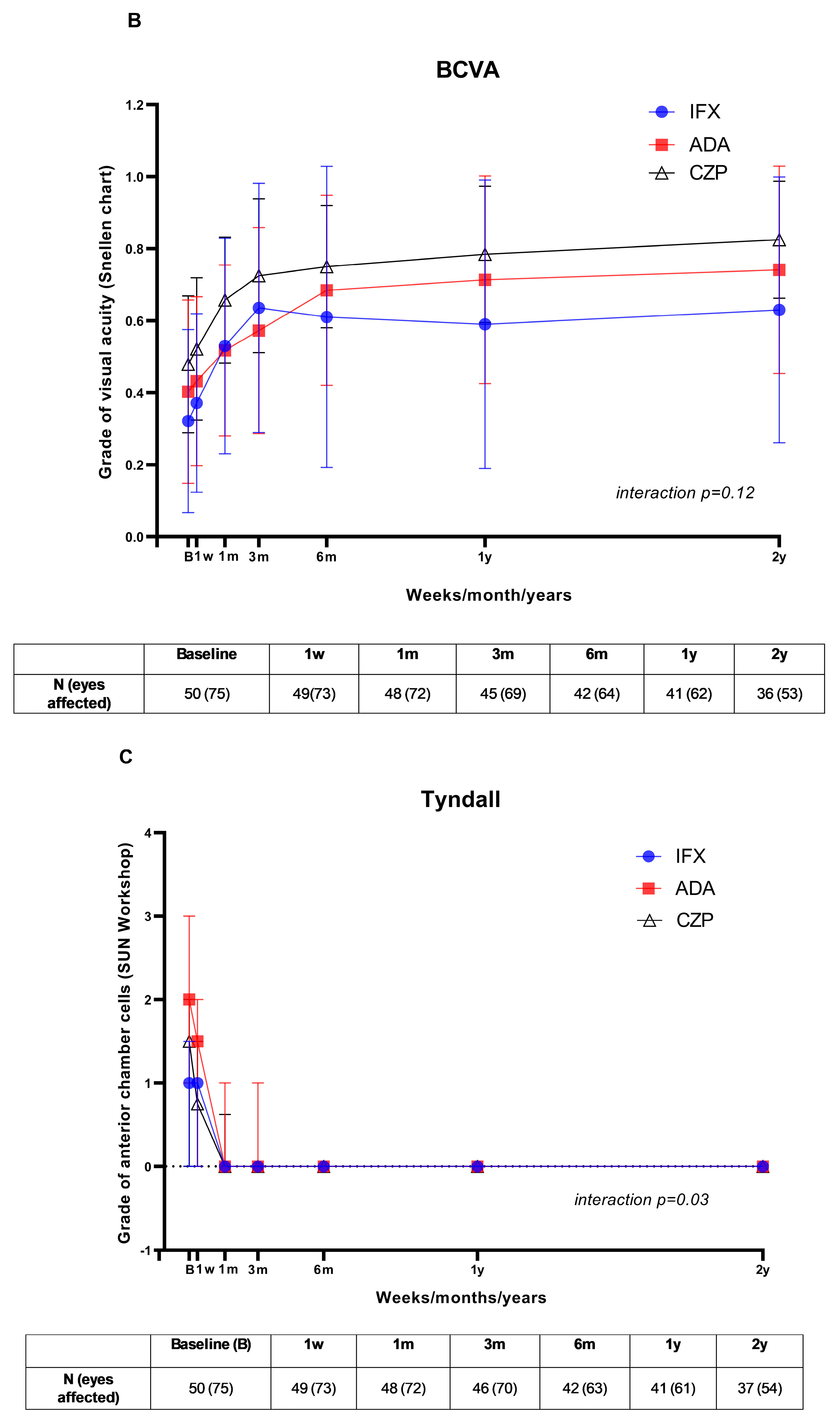
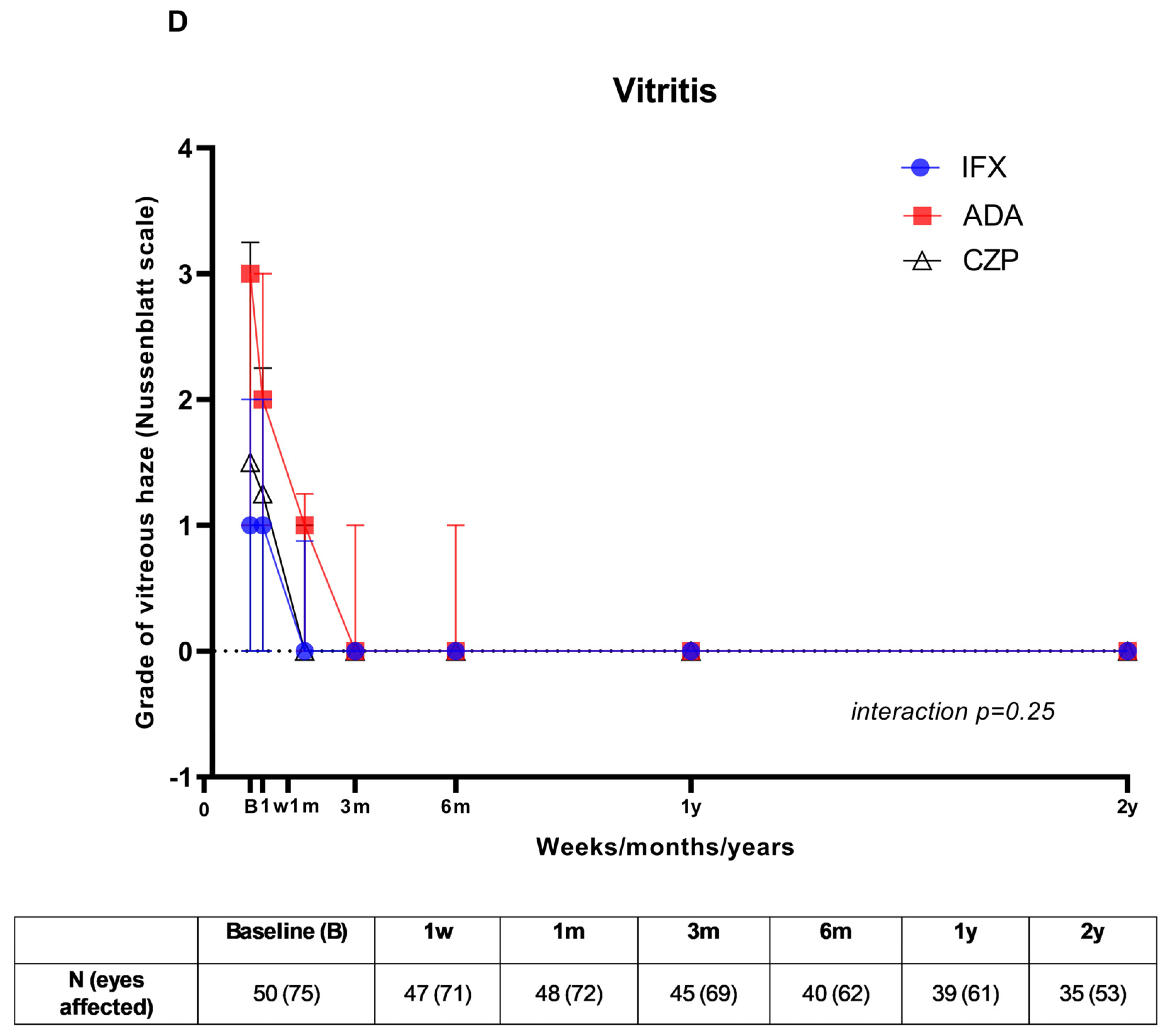
3. Results
3.1. Baseline Demographic and Clinical Features of the Study Sample
3.2. Visual Outcome and Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yazici, Y.; Hatemi, G.; Bodaghi, B.; Cheon, J.H.; Suzuki, N.; Ambrose, N.; Yazici, H. Behçet syndrome. Nat. Rev. Dis. Primers 2021, 7, 67. [Google Scholar] [CrossRef]
- Davatchi, F.; Chams-Davatchi, C.; Shams, H.; Shahram, F.; Nadji, A.; Akhlaghi, M.; Faezi, T.; Ghodsi, Z.; Abdollahi, B.S.; Ashofteh, F.; et al. Behcet’s disease: Epidemiology, clinical manifestations, and diagnosis. Expert Rev. Clin. Immunol. 2017, 13, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kitaichi, N.; Miyazaki, A.; Iwata, D.; Ohno, S.; Stanford, M.R.; Chams, H. Ocular features of Behçet’s disease: An international collaborative study. Br. J. Ophtalmol. 2007, 91, 1579–1582. [Google Scholar] [CrossRef]
- Taylor, S.R.; Singh, J.; Menezo, V.; Wakefield, D.; McCluskey, P.; Lightman, S. Behçet disease: Visual prognosis and factors influencing the development of visual loss. Am. J. Ophthalmol. 2011, 152, 1059–1066. [Google Scholar] [CrossRef]
- Tugal-Tutkun, I.; Onal, S.; Altan-Yaycioglu, R.; Huseyin Altunbas, H.; Urgancioglu, M. Uveitis in Behçet disease: An analysis of 880 patients. Am. J. Ophthalmol. 2004, 138, 373–380. [Google Scholar] [CrossRef]
- Ozyazgan, Y.; Ucar, D.; Hatemi, G.; Yazici, Y. Ocular involvement of Behcet’s syndrome: A comprehensive review. Clin. Rev. Allergy Immunol. 2015, 49, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Fardeau, C.; Champion, E.; Massamba, N.; LeHoang, P. Uveitic macular edema. Eye 2016, 30, 1277–1292. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004, 36, 241–249. [Google Scholar] [CrossRef]
- Lardenoye, C.W.; van Kooij, B.; Rothova, A. Impact of macular edema on visual acuity in uveitis. Ophthalmology 2006, 113, 1446–1449. [Google Scholar] [CrossRef]
- Rotsos, T.G.; Moschos, M.M. Cystoid macular edema. Clin. Ophthalmol. 2008, 2, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R. A look at autoimmunity and inflammation in the eye. J. Clin. Investig. 2010, 120, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Valentincic, N.V.; de Groot-Mijnes, J.D.; Kraut, A.; Korosec, P.; Hawlina, M.; Rothova, A. Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol. Vis. 2011, 17, 2003–2010. [Google Scholar] [PubMed]
- Alibaz-Oner, F.; Direskeneli, H. Advances in the Treatment of Behcet’s Disease. Curr. Rheumatol. Rep. 2021, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Non-infectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Merrill, P.T.; Jaffe, G.J.; Dick, A.D.; Kurup, S.K.; Sheppard, J.; Schlaen, A.; Pavesio, C.; Cimino, L.; Van Calster, J.; et al. Adalimumab for prevention of uveitis flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): A multicentre, double-masked, randomized, placebo-controlled phase 3 trial. Lancet 2016, 338, 1183–1192. [Google Scholar] [CrossRef]
- Suhler, E.B.; Adán, A.; Brézin, A.P.; Fortin, E.; Goto, H.; Jaffe, G.J.; Kaburaki, T.; Kramer, M.; Lim, L.L.; Muccioli, C.; et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology 2018, 125, 1075–1087. [Google Scholar] [CrossRef]
- Ohno, S.; Umebayashi, I.; Matsukawa, M.; Goto, T.; Yano, T. Safety and efficacy of infliximab in the treatment of refractory uveoretinitis in Behçet’s disease: A large-scale, long-term postmarketing surveillance in Japan. Arthritis Res. Ther. 2019, 21, 2. [Google Scholar] [CrossRef]
- Ohno, S.; Nakamura, S.; Hori, S.; Shimakawa, M.; Kawashima, H.; Mochizuki, M. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behçet’s disease with refractory uveoretinitis. J. Rheumatol. 2004, 31, 1362–1368. [Google Scholar]
- Calvo-Río, V.; Blanco, R.; Beltrán, E.; Sánchez-Bursón, J.; Mesquida, M.; Adán, A.; Hernandez, M.V.; Garfella, M.H.; Pascual, E.V.; Martínez-Costa, L.; et al. Anti-TNF-alfa therapy in patients with refractory uveitis due to Behçet’s disease: A 1-year-follow-up study of 124 patients. Rheumatology 2014, 53, 2223–2231. [Google Scholar] [CrossRef]
- Santos-Gómez, M.; Calvo-Río, V.; Blanco, R.; Beltrán, E.; Mesquida, M.; Adán, A.; Cordero-Coma, M.; García-Aparicio, M.; Pascual, E.V.; Martínez-Costa, L.; et al. The effect of biologic therapy different from infliximab or adalimumab in patients with refractory uveitis due to Behçet’s disease: Results of a multicentre open-label study. Clin. Exp. Rheumatol. 2016, 34, 34–40. [Google Scholar]
- Tugal-Tutkun, I.; Mudun, A.; Urgancioglu, M.; Kamali, S.; Kasapoglu, E.; Inanc, M.; Gül, A. Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behçet’s disease: An open-label trial. Arthritis Rheum 2005, 52, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, L.; Nannini, C.; Benucci, M.; Chindamo, D.; Cassarà, E.; Salvarani, C.; Cimino, L.; Gini, G.; Lenzetti, I.; Cantini, F.; et al. Long-term efficacy of infliximab in refractory posterior uveitis of Behçet’s disease: A 24-month follow-up study. Rheumatology 2007, 46, 1161–1164. [Google Scholar] [CrossRef]
- Martín-Varillas, J.L.; Calvo-Río, V.; Beltrán, E.; Sánchez-Bursón, J.; Mesquida, M.; Adán, A.; Hernandez, M.V.; Garfella, M.H.; Pascual, E.V.; Martínez-Costa, L.; et al. Successful Optimization of Adalimumab Therapy in Refractory Uveitis Due to Behçet’s Disease. Ophthalmology 2018, 125, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Martín-Varillas, J.L.; Atienza-Mateo, B.; Calvo-Rio, V.; Beltrán, E.; Sánchez-Bursón, J.; Adán, A.; Hernández-Garfella, M.; Valls-Pascual, E.; Sellas-Fernández, A.; Ortego, N.; et al. Long-term Follow-up and Optimization of Infliximab in Refractory Uveitis Due to Behçet Disease: National Study of 103 White Patients. J. Rheumatol. 2021, 48, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Atienza-Mateo, B.; Martín-Varillas, J.L.; Calvo-Río, V.; Demetrio-Pablo, R.; Beltrán, E.; Sánchez-Bursón, J.; Mesquida, M.; Adan, A.; Hernandez, V.; Hernández-Garfella, M.; et al. Comparative Study of Infliximab Versus Adalimumab in Refractory Uveitis due to Behçet’s Disease: National Multicenter Study of 177 Cases. Arthritis Rheumatol. 2019, 71, 2081–2089a. [Google Scholar] [CrossRef]
- Lopalco, G.; Emmi, G.; Gentileschi, S.; Guerriero, S.; Vitale, A.; Silvestri, E.; Becatti, M.; Cavallo, I.; Fabiani, C.; Frediani, B.; et al. Certolizumab Pegol treatment in Behcet’s disease with different organ involvement: A multicenter retrospective observational study. Mod. Rheumatol. 2017, 27, 1031–1035. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Calderón-Goercke, M.; Adán, A.; Chamorro-López, L.; Maíz-Alonso, O.; De Dios-Jiménez Aberásturi, J.R.; Veroz, R.; Blanco, S.; Martín-Santos, J.M.; Navarro, F.; et al. Efficacy and safety of certolizumab pegol in pregnant women with uveitis. Recommendations on the management with immunosuppressive and biologic therapies in uveitis during pregnancy. Clin. Exp. Rheumatol. 2021, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Martín-Varillas, J.L.; Sanchez-Bilbao, L.; Calvo-Río, V.; Adán, A.; Hernanz, I.; Gallego-Flores, A.; Beltran-Catalan, E.; Castro-Oreiro, S.; Fanlo, P.; Martos, A.G.; et al. Long-term follow-up of certolizumab pegol in uveitis due to immune-mediated inflammatory diseases: Multicentre study of 80 patients. RMD Open 2022, 8, e002693. [Google Scholar] [CrossRef]
- van der Horst-Bruinsma, I.; van Bentum, R.; Verbraak, F.D.; Rath, T.; Rosenbaum, J.T.; Misterska-Skora, M.; Hoepken, B.; Irvin-Sellers, O.; VanLunen, B.; Bauer, L.; et al. The impact of certolizumab pegol treatment on the incidence of anterior uveitis flares in patients with axial spondyloarthritis: 48-week interim results from C-VIEW. RMD Open 2020, 6, e001161. [Google Scholar] [CrossRef]
- Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet 1990, 335, 1078–1080. [Google Scholar]
- International Team for the Revision of the Inter-national Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): A collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 338–347. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar]
- Nussenblatt, R.B.; Palestine, A.G.; Chan, C.C.; Roberge, F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985, 92, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Levenson, J.H.; Kozarsky, A. Visual Acuity. In Clinical Methods: The History, Physical, and Laboratory Examinations, Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; 3rd ed.; Butter-Worths: Boston, MA, USA, 1990; Chapter 115. [Google Scholar]
- Barroso-García, N.; Atienza-Mateo, B.; Ferraz-Amaro, I.; Prieto-Peña, D.; Beltrán, E.; Adán, A.; Hernández-Garfella, M.; Martínez-Costa, L.; Cordero-Coma, M.; Díaz-Llopis, M.; et al. Anti-TNF vs. tocilizumab in refractory uveitic cystoid macular edema due to Behcet’s disease. Multicenter study of 49 patients. Semin Arthritis Rheum. 2023, 58, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Suhler, E.B.; Rosenbaum, J.T. The future of uveitis treatment. Ophthalmology 2014, 121, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Tao, T.; Liu, X.; Su, W. JAK-STAT signaling pathway in non-infectious uveitis. Biochem. Pharmacol. 2022, 204, 115236. [Google Scholar] [CrossRef]
- Hatemi, G.; Christensen, R.; Bang, D.; Bodaghi, B.; Celik, A.F.; Fortune, F.; Gaudric, J.; Gul, A.; Kötter, I.; Leccese, P.; et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018, 77, 808–818. [Google Scholar] [CrossRef]
- Silvestri, E.; Bitossi, A.; Bettiol, A.; Emmi, G.; Urban, M.L.; Mattioli, I.; Di Scala, G.; Bacherini, D.; Lopalco, G.; Venerito, V.; et al. Adalimumab effectively controls both anterior and posterior noninfectious uveitis associated with systemic inflammatory diseases: Focus on Behçet’s syndrome. Inflammopharmacology 2020, 28, 711–718. [Google Scholar] [CrossRef]
- Fabiani, C.; Vitale, A.; Rigante, D.; Emmi, G.; Bitossi, A.; Lopalco, G.; Sota, J.; Guerriero, S.; Orlando, I.; Gentileschi, S.; et al. Comparative efficacy between adalimumab and infliximab in the treatment of non-infectious intermediate uveitis, posterior uveitis, and panuveitis: A retrospective observational study of 107 patients. Clin. Rheumatol. 2019, 38, 407–415. [Google Scholar] [CrossRef]
- Park, S.E.; Jun, J.W.; Lee, D.H.; Lee, S.C.; Kim, M. The effect of adalimumab in Korean patients with refractory noninfectious uveitis. Yonsei Med. J. 2021, 62, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Sota, J.; Vitale, A.; Rigante, D.; Emmi, G.; Lopalco, G.; Guerriero, S.; Orlando, I.; Iannone, F.; Frediani, B.; et al. Efficacy and safety of certolizumab pegol and golimumab in the treatment of non-infectious uveitis. Clin. Exp. Rheumatol. 2019, 37, 680–683. [Google Scholar] [PubMed]
- Llorenç, V.; Mesquida, M.; Sainz de la Maza, M.; Blanco, R.; Calvo, V.; Maíz, O.; Blanco, A.; de Dios-Jiménez de Aberásturi, J.R.; Adán, A. Certolizumab Pegol, a New Anti-TNF-α in the Armamentarium against Ocular Inflammation. Ocul. Immunol. Inflamm. 2016, 24, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J.; Sanchez, H.N.; Schoenbrunner, N. Defining response to TNF-inhibitors in rheumatoid arthritis: The negative impact of anti-TNF cycling and the need for a personalized medicine approach to identify primary non-responders. Clin. Rheumatol. 2019, 38, 2967–2976. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Brocq, O.; Perdriger, A.; Lassoued, S.; Berthelot, J.-M.; Wendling, D.; Euller-Ziegler, L.; Soubrier, M.; Richez, C.; Fautrel, B.; et al. Non-TNF-Targeted Biologic vs a Second Anti-TNF Drug to Treat Rheumatoid Arthritis in Patients with Insufficient Response to a First Anti-TNF Drug: A Randomized Clinical Trial. JAMA 2016, 316, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Rubbert-Roth, A.; Szabó, M.Z.; Kedves, M.; Nagy, G.; Atzeni, F.; Sarzi-Puttini, P. Failure of anti-TNF treatment in patients with rheumatoid arthritis: The pros and cons of the early use of alternative biological agents. Autoimmun. Rev. 2019, 18, 102398. [Google Scholar] [CrossRef]
- Taylor, P.C.; Cerinic, M.M.; Alten, R.; Avouac, J.; Westhovens, R. Managing inadequate response to initial anti-TNF therapy in rheumatoid arthritis: Optimising treatment outcomes. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221114101. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Wiley, R.W.; Rapp, B. Statistical analysis in Small-N Designs: Using linear mixed-effects modeling for evaluating intervention effectiveness. Aphasiology 2019, 33, 1–30. [Google Scholar] [CrossRef]

| ADA Group n = 24 | IFX Group n = 15 | CZP Group n = 10 | p | |
|---|---|---|---|---|
| Age, mean (SD) years | 41 (11) | 38 (9) | 36 (8) | 0.42 |
| Sex, men/women, n/n | 12/12 | 7/8 | 3/7 | 0.65 |
| HLA-B51-positive, n (%) | 19 (79) | 10 (67) | 4 (40) | 0.07 |
| Duration of uveitis before anti-TNF, median (IQR) months | 27 (12–60) | 15 (8–60) | 72 (36–120) | 0.03 |
| Number of eyes with CME, n (%) | ||||
| 10 (42) | 9 (60) | 3 (30) | 0.33 |
| 14 (58) | 6 (40) | 7 (70) | |
| Ocular features at the time of anti-TNF/anti-IL6 therapy onset | ||||
| 2 (1–3) | 1 (0–1.5) | 1 (0–2) | 0.099 |
| 2.75 (1.75–3) | 1 (0–2) | 1 (0–2) | 0.025 |
| 0.4 (0.2–0.6) | 0.3 (0.2–0.5) | 0.20 (0.25) | 0.19 |
| 437 (117) | 483 (126) | 381 (96) | 0.084 |
| Pattern of uveitis, n (%) | ||||
| 24 (100) | 15 (100) | 8 (80) | 0.038 |
| 4 (83) | 5 (33) | 3 (33) | 0.43 |
| 20 (83) | 10 (67) | 4 (40) | 0.051 |
| Previous treatment before anti-TNF therapy onset, n (%) | ||||
| 13 (54) | 9 (60) | 5 (50) | 0.87 |
| 22 (92) | 11 (73) | 6 (60) | 0.07 |
| 14 (58) | 8 (53) | 4 (40) | 0.70 |
| 12 (50) | 8 (53) | 2(20) | 0.24 |
| 0 (0) | 0 (0) | 7 (70) | <0.001 |
| 0 (0) | 0 (0) | 7 (70) | <0.001 |
| 0 (0) | 0 (0) | 1 (10) | <0.001 |
| 0 (0) | 0 (0) | 2 (20) | <0.001 |
| Prednisone dose at anti-TNF onset, median (IQR), mg/d | 45 (25–60) | 30 (20–60) | 9 (6–20) | 0.051 |
| Combined treatment at anti-TNF/anti-IL6 onset, n (%) | ||||
| 2 (13) | 3 (20) | 2 (20) | 0.68 |
| 10 (42) | 5 (33) | 1 (10) | 0.25 |
| 2 (8) | 2 (13) | 4 (40) | 0.11 |
| Follow-up on anti-TNF/anti-IL6 therapy, median (IQR), months | 24 (18–45) | 24 (3–36) | 30 (24–60) | 0.12 |
| 18 (75) | 9 (60) | 7 (70) | 0.66 |
| 8 (32) | 8 (53) | 2 (20) | 0.22 |
| 1 (4) | 1 (7) | 0 (0) | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso-García, N.; Martín-Varillas, J.L.; Ferraz-Amaro, I.; Sánchez-Bilbao, L.; Martín-Gutiérrez, A.; Adán, A.; Hernanz-Rodríguez, I.; Beltrán-Catalán, E.; Cordero-Coma, M.; Díaz-Valle, D.; et al. Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet’s Disease. J. Clin. Med. 2024, 13, 7388. https://doi.org/10.3390/jcm13237388
Barroso-García N, Martín-Varillas JL, Ferraz-Amaro I, Sánchez-Bilbao L, Martín-Gutiérrez A, Adán A, Hernanz-Rodríguez I, Beltrán-Catalán E, Cordero-Coma M, Díaz-Valle D, et al. Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet’s Disease. Journal of Clinical Medicine. 2024; 13(23):7388. https://doi.org/10.3390/jcm13237388
Chicago/Turabian StyleBarroso-García, Nuria, José Luis Martín-Varillas, Iván Ferraz-Amaro, Lara Sánchez-Bilbao, Adrián Martín-Gutiérrez, Alfredo Adán, Inés Hernanz-Rodríguez, Emma Beltrán-Catalán, Miguel Cordero-Coma, David Díaz-Valle, and et al. 2024. "Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet’s Disease" Journal of Clinical Medicine 13, no. 23: 7388. https://doi.org/10.3390/jcm13237388
APA StyleBarroso-García, N., Martín-Varillas, J. L., Ferraz-Amaro, I., Sánchez-Bilbao, L., Martín-Gutiérrez, A., Adán, A., Hernanz-Rodríguez, I., Beltrán-Catalán, E., Cordero-Coma, M., Díaz-Valle, D., Hernández-Garfella, M., Martínez-Costa, L., Díaz-Llopis, M., Herreras, J. M., Maíz-Alonso, O., Torre-Salaberri, I., Atanes-Sandoval, A., Insúa, S., Almodóvar-González, R., ... Blanco, R. (2024). Comparative Study of Adalimumab, Infliximab and Certolizumab Pegol in the Treatment of Cystoid Macular Edema Due to Behçet’s Disease. Journal of Clinical Medicine, 13(23), 7388. https://doi.org/10.3390/jcm13237388






