Exploring the Link Between Interoception and Symptom Severity in Premature Ventricular Contractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition
2.2.1. Symptom Score
2.2.2. Questionnaires
2.2.3. Electrophysiological Data
2.2.4. Other Variables
2.3. Experimental Procedures
2.4. Data Preprocessing
2.4.1. Behavioral Interoceptive Accuracy (IA)
2.4.2. ECG, EMG Processing
2.4.3. EEG Processing
2.4.4. Heartbeat-Evoked Potentials (HEPs) Analysis
2.5. Statistical Analyses
2.5.1. Analysis of Behavioral Data
2.5.2. HEP Statistical Analyses
2.5.3. Correlation Between HEP Amplitude and IA
2.5.4. Exploratory Regression Analysis
- A1.
- Symptom score ~ IAHBD
- A2.
- Symptom score ~ IAMT
- A3.
- Symptom score ~ ΔHEPMT-REST in ROI ∈ {LF, CF, RF, LP, CO, RT}
- A4.
- Symptom score ~ ΔHEPHBD-REST in ROI ∈ {LF, CF, RF, LP, CO, RP}
- A5.
- Symptom score ~ ΔHEPHBD-EX in ROI ∈ {LF, CF, RF, LP, CO, RP}
3. Results
3.1. Sample Characteristics
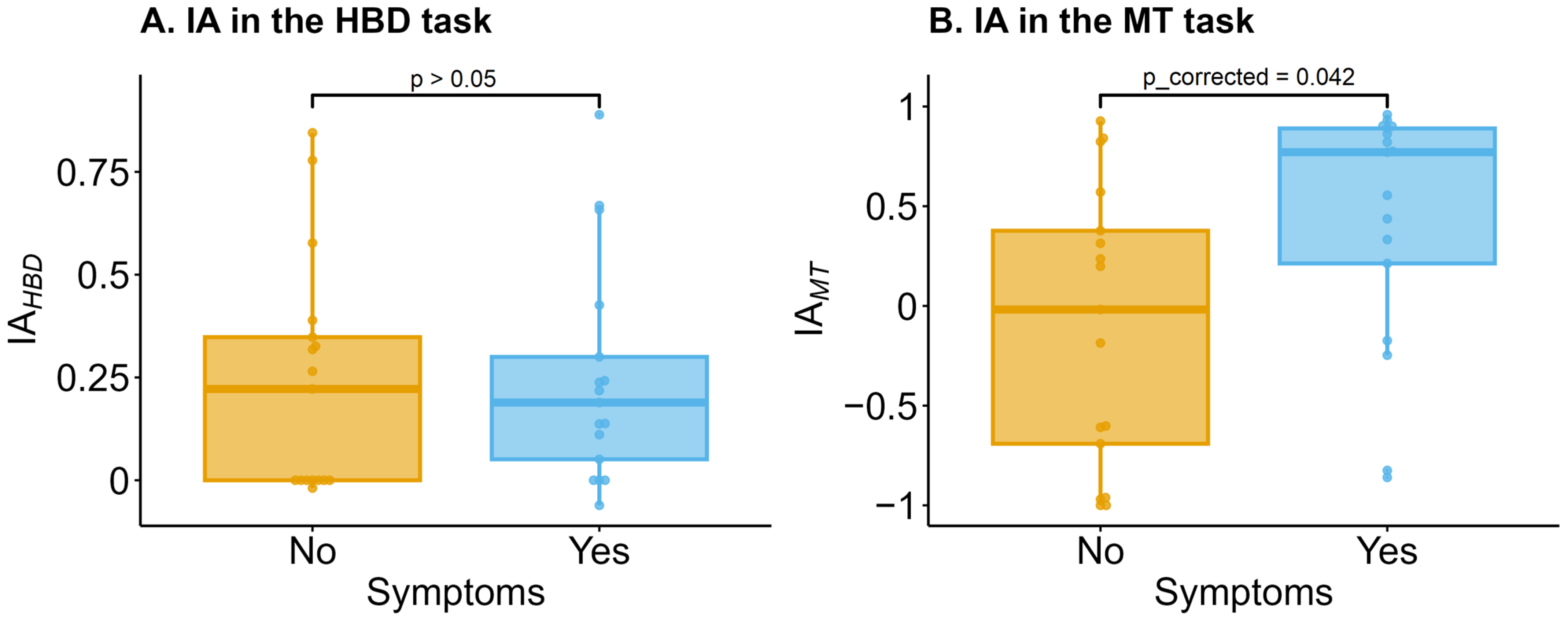
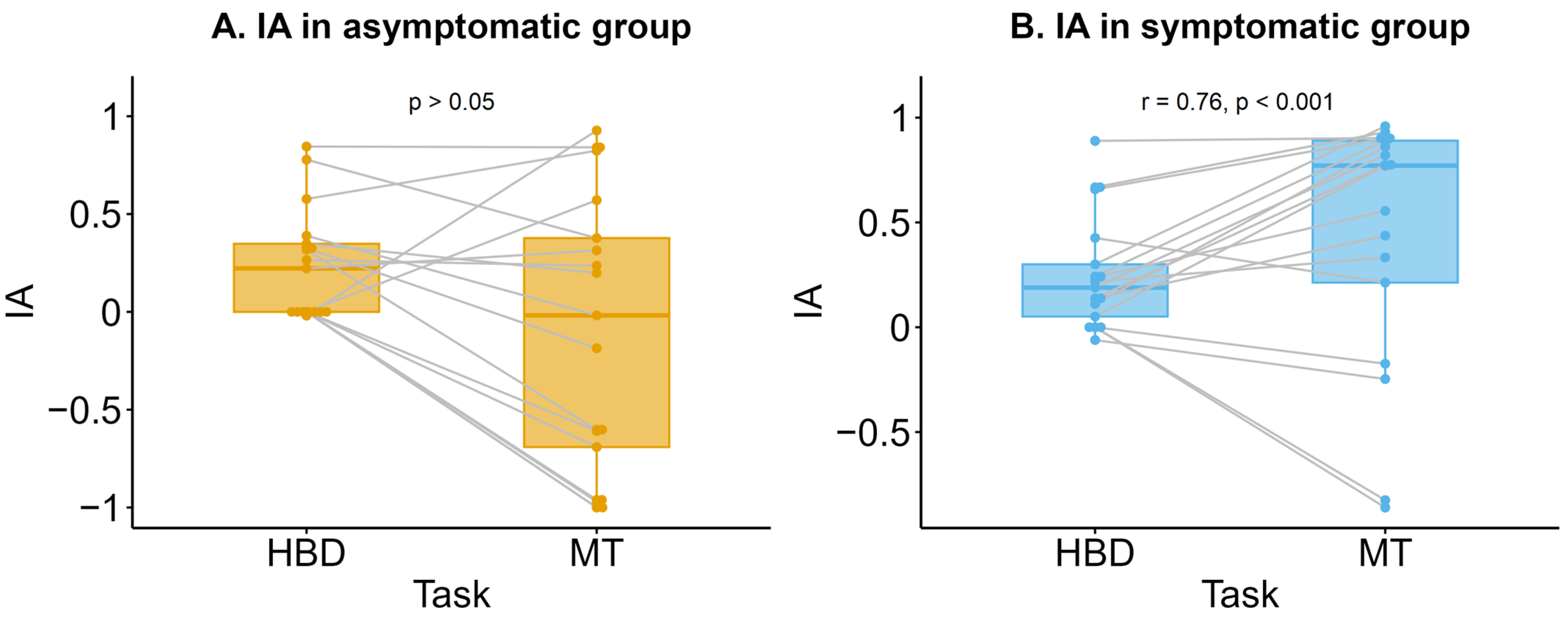
3.2. Interoceptive Accuracy (IA)
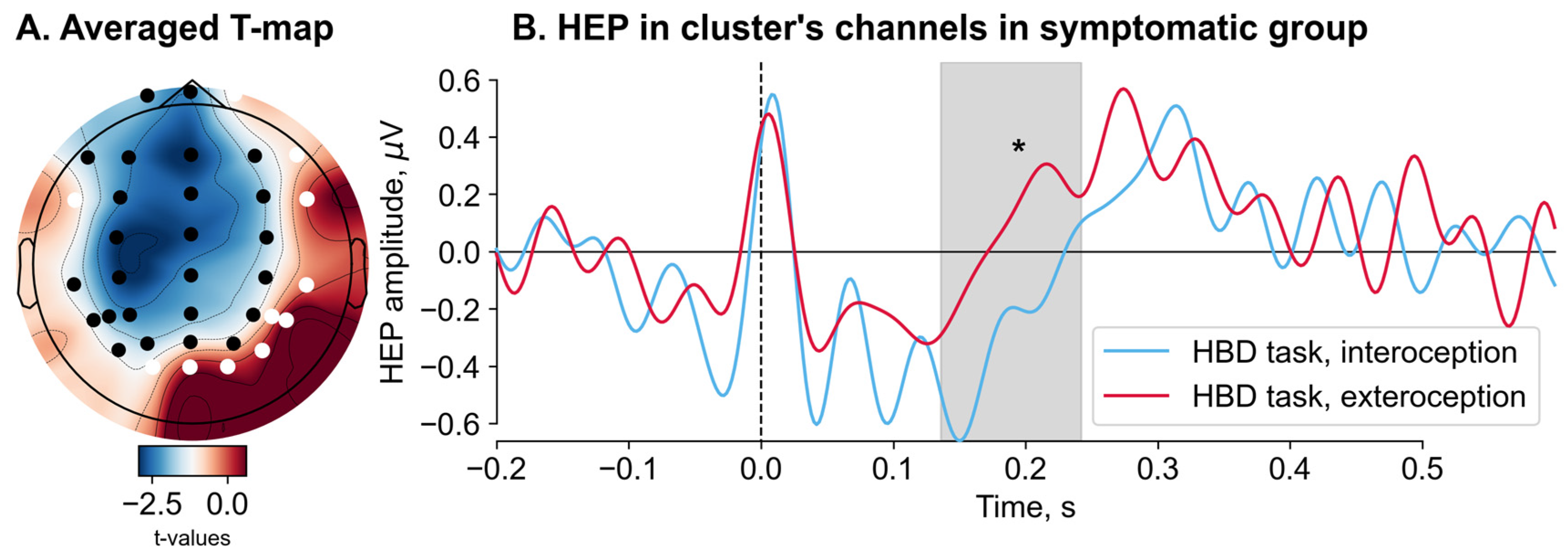
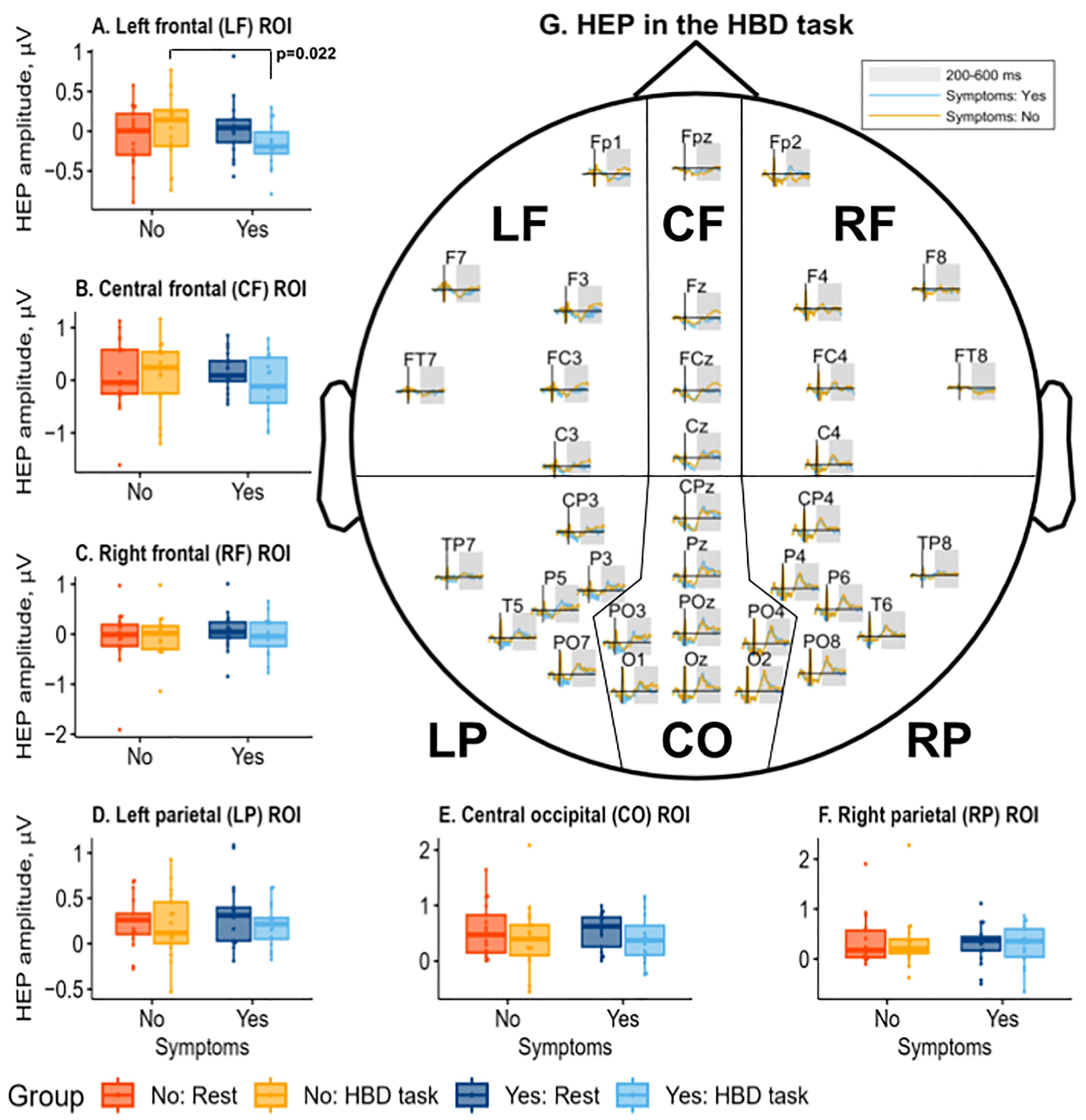
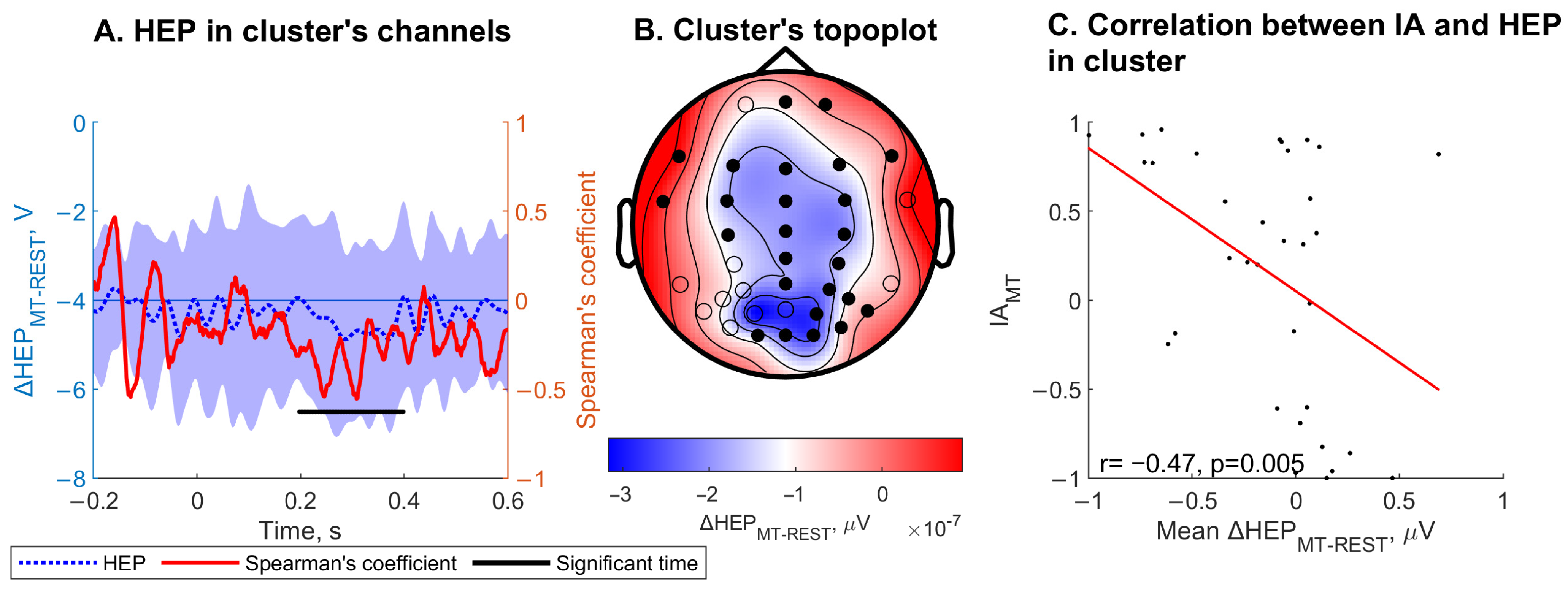
3.3. Heartbeat-Evoked Potentials (HEPs) Results
3.4. Regression Analyses
4. Discussion
4.1. Interoceptive Accuracy (IA) and Symptoms
4.2. Heartbeat-Evoked Potentials (HEPs) and Symptoms
4.3. Multimodality of Symptom Intensity
4.4. Interoceptive Metrics for Predicting Symptom Score
4.5. Clinical Applications of Interoception Modulation
5. Limitations and Avenues for Further Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, O.; Luminet, O.; Walentynowicz, M.; Corneille, O. The New Measures of Interoceptive Accuracy: A Systematic Review and Assessment. Neurosci. Biobehav. Rev. 2023, 153, 105388. [Google Scholar] [CrossRef]
- Coll, M.P.; Hobson, H.; Bird, G.; Murphy, J. Systematic Review and Meta-Analysis of the Relationship between the Heartbeat-Evoked Potential and Interoception. Neurosci. Biobehav. Rev. 2021, 122, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Lane, R.D.; Oshinsky, M.L.; Kenny, P.J.; Sinha, R.; Mayer, E.A.; Critchley, H.D. Diseases, Disorders, and Comorbidities of Interoception. Trends Neurosci. 2021, 44, 39–51. [Google Scholar] [CrossRef]
- Locatelli, G.; Matus, A.; James, R.; Salmoirago-Blotcher, E.; Ausili, D.; Vellone, E.; Riegel, B. What Is the Role of Interoception in the Symptom Experience of People with a Chronic Condition? A Systematic Review. Neurosci. Biobehav. Rev. 2023, 148, 105142. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Chu, S.H.; Dunne, J.; Spintzyk, E.; Locatelli, G.; Babicheva, V.; Lam, L.; Julio, K.; Chen, S.; Jurgens, C.Y. Body Listening in the Link between Symptoms and Self-Care Management in Cardiovascular Disease: A Cross-Sectional Correlational Descriptive Study. Int. J. Nurs. Stud. 2024, 156, 104809. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing Your Own Heart: Distinguishing Interoceptive Accuracy from Interoceptive Awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef]
- Kandiah, J.W.; Blumberger, D.M.; Rabkin, S.W. The Fundamental Basis of Palpitations: A Neurocardiology Approach. Curr. Cardiol. Rev. 2021, 18, 27–34. [Google Scholar] [CrossRef]
- Verma, A.; Champagne, J.; Sapp, J.; Essebag, V.; Novak, P.; Skanes, A.; Morillo, C.A.; Khaykin, Y.; Birnie, D. Discerning the Incidence of Symptomatic and Asymptomatic Episodes of Atrial Fibrillation before and after Catheter Ablation (DISCERN AF): A Prospective, Multicenter Study. JAMA Intern. Med. 2013, 173, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Barsky, A.J.; Cleary, P.D.; Barnett, M.C.; Christiansen, C.L.; Ruskin, J.N. The Accuracy of Symptom Reporting by Patients Complaining of Palpitations. Am. J. Med. 1994, 97, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Jonsbu, E.; Dammen, T.; Morken, G.; Lied, A.; Vik-Mo, H.; Martinsen, E.W. Cardiac and Psychiatric Diagnoses among Patients Referred for Chest Pain and Palpitations. Scand. Cardiovasc. J. 2009, 43, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Barsky, A.J.; Cleary, P.D.; Sarnie, M.K.; Ruskin, J.N. Panic Disorder, Palpitations, and the Awareness of Cardiac Activity. J. Nerv. Ment. Dis. 1994, 182, 63–71. [Google Scholar] [CrossRef]
- Barsky, A.J.; Delamater, B.A.; Clancy, S.A.; Antman, E.M.; Ahern, D.K. Somatized Psychiatric Disorder Presenting as Palpitations. Arch. Intern. Med. 1996, 156, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Klewer, J.; Springer, J.; Morshedzadeh, J. Premature Ventricular Contractions (PVCs): A Narrative Review. Am. J. Med. 2022, 135, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Barsky, A.; Cleary, P.; Brener, J.; Ruskin, J. The Perception of Cardiac Activity in Medical Outpatients. Cardiology 1993, 83, 304–315. [Google Scholar] [CrossRef]
- Ehlers, A.; Mayou, R.A.; Sprigings, D.C.; Birkhead, J. Psychological and Perceptual Factors Associated with Arrhythmias and Benign Palpitations. Psychosom. Med. 2000, 62, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Schandry, R. Heart Beat Perception and Emotional Experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef]
- McFarland, R.A. Heart Rate Perception and Heart Rate Control. Psychophysiology 1975, 12, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Blanke, O. Heartbeat-Evoked Cortical Responses: Underlying Mechanisms, Functional Roles, and Methodological Considerations. Neuroimage 2019, 197, 502–511. [Google Scholar] [CrossRef]
- Schulz, A.; Köster, S.; Beutel, M.E.; Schächinger, H.; Vögele, C.; Rost, S.; Rauh, M.; Michal, M. Altered Patterns of Heartbeat-Evoked Potentials in Depersonalization/Derealization Disorder: Neurophysiological Evidence for Impaired Cortical Representation of Bodily Signals. Psychosom. Med. 2015, 77, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.S.; Lapidus, R.C. Can Interoception Improve the Pragmatic Search for Biomarkers in Psychiatry? Front. Psychiatry 2016, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Katsunuma, R.; Terasawa, Y.; Sekiguchi, A. Interoceptive Training Impacts the Neural Circuit of the Anterior Insula Cortex. Transl. Psychiatry 2024, 14, 206. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural Systems Supporting Interoceptive Awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef]
- Zaki, J.; Davis, J.I.; Ochsner, K.N. Overlapping Activity in Anterior Insula during Interoception and Emotional Experience. Neuroimage 2012, 62, 493–499. [Google Scholar] [CrossRef]
- Shah, A.; Wittbrodt, M.; Bremner, J.; Vaccarino, V. Cardiovascular Pathophysiology from the Cardioneural Perspective and Its Clinical Applications. Trends Cardiovasc. Med. 2022, 32, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Kario, K. The Insular Cortex and Cardiovascular System: A New Insight into the Brain-Heart Axis. J. Am. Soc. Hypertens. 2010, 4, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Tungar, I.M.; Rama Krishna Reddy, M.M.; Flores, S.M.; Pokhrel, P.; Ibrahim, A.D. The Influence of Lifestyle Factors on the Occurrence and Severity of Premature Ventricular Contractions: A Comprehensive Review. Curr. Probl. Cardiol. 2024, 49, 102072. [Google Scholar] [CrossRef]
- Spielberger, C.; Gorsuch, R.; Lushene, R. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Bagby, R.M.; Parker, J.D.A.; Taylor, G.J. The Twenty-Item Toronto Alexithymia Scale—I. Item Selection and Cross-Validation of the Factor Structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Rost, S.; Flasinski, T.; Dierolf, A.M.; Lutz, A.P.C.; Münch, E.E.; Mertens, V.C.; Witthöft, M.; Vögele, C. Distinctive Body Perception Mechanisms in High versus Low Symptom Reporters: A Neurophysiological Model for Medically-Unexplained Symptoms. J. Psychosom. Res. 2020, 137, 110223. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Terhaar, J.; Viola, F.C.; Bär, K.J.; Debener, S. Heartbeat Evoked Potentials Mirror Altered Body Perception in Depressed Patients. Clin. Neurophysiol. 2012, 123, 1950–1957. [Google Scholar] [CrossRef]
- Jenkinson, P.M.; Fotopoulou, A.; Ibañez, A.; Rossell, S. Interoception in Anxiety, Depression, and Psychosis: A Review. eClinicalMedicine 2024, 73, 102673. [Google Scholar] [CrossRef] [PubMed]
- Harshaw, C. Interoceptive Dysfunction: Toward an Integrated Framework for Understanding Somatic and Affective Disturbance in Depression. Psychol. Bull. 2015, 141, 311–363. [Google Scholar] [CrossRef]
- Starostina, E.G.; Taylor, G.D.; Quilty, L.; Bobrov, A.E.; Moshnyaga, E.N.; Puzyreva, N.V.; Bobrova, M.A.; Ivashkina, M.G.; Krivchikova, M.N.; Shavrikova, E.P.; et al. A New 20-Item Version of the Toronto Alexithymia Scale: Validation of the Russian Language Translation in a Sample of Medical Patients. Soc. Clin. Psychiatry 2010, 20, 31–38. (In Russian) [Google Scholar]
- Hanin, Y. A Brief Guide for the Spielberger C.D State-Trait Anxiety Inventory; LNI-ITEK: Leningrad, Russia, 1976. (In Russian)
- Gray, M.A.; Taggart, P.; Sutton, P.M.; Groves, D.; Holdright, D.R.; Bradbury, D.; Brull, D.; Critchley, H.D. A Cortical Potential Reflecting Cardiac Function. Proc. Natl. Acad. Sci. USA 2007, 104, 6818–6823. [Google Scholar] [CrossRef] [PubMed]
- Yoris, A.; Abrevaya, S.; Esteves, S.; Salamone, P.; Lori, N.; Martorell, M.; Legaz, A.; Alifano, F.; Petroni, A.; Sánchez, R.; et al. Multilevel Convergence of Interoceptive Impairments in Hypertension: New Evidence of Disrupted Body–Brain Interactions. Hum. Brain Mapp. 2018, 39, 1563–1581. [Google Scholar] [CrossRef]
- Zaccaro, A.; della Penna, F.; Mussini, E.; Parrotta, E.; Perrucci, M.G.; Costantini, M.; Ferri, F. Attention to Cardiac Sensations Enhances the Heartbeat-Evoked Potential during Exhalation. iScience 2024, 27, 109586. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.C.; Gentsch, A.; Jelinčić, V.; Schütz-Bosbach, S. Exteroceptive Expectations Modulate Interoceptive Processing: Repetition-Suppression Effects for Visual and Heartbeat Evoked Potentials. Sci. Rep. 2017, 7, 16525. [Google Scholar] [CrossRef]
- Desmedt, O.; Luminet, O.; Corneille, O. The Heartbeat Counting Task Largely Involves Non-Interoceptive Processes: Evidence from Both the Original and an Adapted Counting Task. Biol. Psychol. 2018, 138, 185–188. [Google Scholar] [CrossRef]
- Couto, B.; Salles, A.; Sedeño, L.; Peradejordi, M.; Barttfeld, P.; Canales-Johnson, A.; Vidal, Y.; Santos, D.; Huepe, D.; Bekinschtein, T.; et al. The Man Who Feels Two Hearts: The Different Pathways of Interoception. Soc. Cogn. Affect. Neurosci. 2014, 9, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Schandry, R. Accuracy of Heartbeat Perception Is Reflected in the Amplitude of the Heartbeat-Evoked Brain Potential. Psychophysiology 2004, 41, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Abrevaya, S.; Fittipaldi, S.; García, A.M.; Dottori, M.; Santamaria-Garcia, H.; Birba, A.; Yoris, A.; Hildebrandt, M.K.; Salamone, P.; De la Fuente, A.; et al. At the Heart of Neurological Dimensionality: Cross-Nosological and Multimodal Cardiac Interoceptive Deficits. Psychosom. Med. 2020, 82, 850–861. [Google Scholar] [CrossRef]
- Yoris, A.; Legaz, A.; Abrevaya, S.; Alarco, S.; López Peláez, J.; Sánchez, R.; García, A.M.; Ibáñez, A.; Sedeño, L. Multicentric Evidence of Emotional Impairments in Hypertensive Heart Disease. Sci. Rep. 2020, 10, 14131. [Google Scholar] [CrossRef]
- Fittipaldi, S.; Abrevaya, S.; de la Fuente, A.; Pascariello, G.O.; Hesse, E.; Birba, A.; Salamone, P.; Hildebrandt, M.; Martí, S.A.; Pautassi, R.M.; et al. A Multidimensional and Multi-Feature Framework for Cardiac Interoception. Neuroimage 2020, 212, 116677. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A. MEG and EEG Data Analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef]
- Lutz, A.P.C.; Schulz, A.; Voderholzer, U.; Koch, S.; van Dyck, Z.; Vögele, C. Enhanced Cortical Processing of Cardio-Afferent Signals in Anorexia Nervosa. Clin. Neurophysiol. 2019, 130, 1620–1627. [Google Scholar] [CrossRef]
- Rief, W.; Barsky, A.J. Psychobiological Perspectives on Somatoform Disorders. Psychoneuroendocrinology 2005, 30, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.; Kraiuhin, C.; Meares, R.; Howson, A. Auditory Evoked Response Potentials in Somatization Disorder. J. Psychiatr. Res. 1986, 20, 237–248. [Google Scholar] [CrossRef]
- James, L.; Gordon, E.; Kraiuhin, C.; Howson, A.; Meares, R. Augmentation of Auditory Evoked Potentials in Somatization Disorder. J. Psychiatr. Res. 1990, 24, 155–163. [Google Scholar] [CrossRef]
- Wolters, C.; Gerlach, A.L.; Pohl, A. Interoceptive Accuracy and Bias in Somatic Symptom Disorder, Illness Anxiety Disorder, and Functional Syndromes: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0271717. [Google Scholar] [CrossRef] [PubMed]
- Körmendi, J.; Ferentzi, E.; Köteles, F. Expectation Predicts Performance in the Mental Heartbeat Tracking Task. Biol. Psychol. 2021, 164, 108170. [Google Scholar] [CrossRef] [PubMed]
- Körmendi, J.; Ferentzi, E.; Petzke, T.; Gál, V.; Köteles, F. Do We Need to Accurately Perceive Our Heartbeats? Cardioceptive Accuracy and Sensibility Are Independent from Indicators of Negative Affectivity, Body Awareness, Body Image Dissatisfaction, and Alexithymia. PLoS ONE 2023, 18, e0287898. [Google Scholar] [CrossRef]
- Petersen, S.; Van Staeyen, K.; Vögele, C.; von Leupoldt, A.; Van den Bergh, O. Interoception and Symptom Reporting: Disentangling Accuracy and Bias. Front. Psychol. 2015, 6, 732. [Google Scholar] [CrossRef] [PubMed]
- Petzschner, F.H.; Weber, L.A.; Wellstein, K.V.; Paolini, G.; Do, C.T.; Stephan, K.E. Focus of Attention Modulates the Heartbeat Evoked Potential. Neuroimage 2019, 186, 595–606. [Google Scholar] [CrossRef]
- Yuan, H.; Yan, H.-M.; Xu, X.-G.; Han, F.; Yan, Q. Effect of Heartbeat Perception on Heartbeat Evoked Potential Waves. Neurosci. Bull. 2007, 23, 357–362. [Google Scholar] [CrossRef]
- Montoya, P.; Schandry, R.; Müller, A. Heartbeat Evoked Potentials (HEP): Topography and Influence of Cardiac Awareness and Focus of Attention. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1993, 88, 163–172. [Google Scholar] [CrossRef]
- Rief, W.; Broadbent, E. Explaining Medically Unexplained Symptoms-Models and Mechanisms. Clin. Psychol. Rev. 2007, 27, 821–841. [Google Scholar] [CrossRef]
- Yoris, A.; García, A.M.; Traiber, L.; Santamaría-García, H.; Martorell, M.; Alifano, F.; Kichic, R.; Moser, J.S.; Cetkovich, M.; Manes, F.; et al. The Inner World of Overactive Monitoring: Neural Markers of Interoception in Obsessive–Compulsive Disorder. Psychol. Med. 2017, 47, 1957–1970. [Google Scholar] [CrossRef]
- García-Cordero, I.; Esteves, S.; Mikulan, E.P.; Hesse, E.; Baglivo, F.H.; Silva, W.; García, M.D.C.; Vaucheret, E.; Ciraolo, C.; García, H.S.; et al. Attention, in and Out: Scalp-Level and Intracranial EEG Correlates of Interoception and Exteroception. Front. Neurosci. 2017, 11, 411. [Google Scholar] [CrossRef]
- Leopold, C.; Schandry, R. The Heartbeat-Evoked Brain Potential in Patients Suffering from Diabetic Neuropathy and in Healthy Control Persons. Clin. Neurophysiol. 2001, 112, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Kirsch, W.; Schandry, R. Brain Structures Involved in Interoceptive Awareness and Cardioafferent Signal Processing: A Dipole Source Localization Study. Hum. Brain Mapp. 2005, 26, 54–64. [Google Scholar] [CrossRef]
- Park, H.D.; Bernasconi, F.; Salomon, R.; Tallon-Baudry, C.; Spinelli, L.; Seeck, M.; Schaller, K.; Blanke, O. Neural Sources and Underlying Mechanisms of Neural Responses to Heartbeats, and Their Role in Bodily Self-Consciousness: An Intracranial EEG Study. Cereb. Cortex 2018, 28, 2351–2364. [Google Scholar] [CrossRef]
- Kumral, D.; Al, E.; Cesnaite, E.; Kornej, J.; Sander, C.; Hensch, T.; Zeynalova, S.; Tautenhahn, S.; Hagendorf, A.; Laufs, U.; et al. Attenuation of the Heartbeat-Evoked Potential in Patients with Atrial Fibrillation. Clin. Electrophysiol. 2022, 8, 1219–1230. [Google Scholar] [CrossRef]
- Seth, A.K. Interoceptive Inference, Emotion, and the Embodied Self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef]
- Barrett, L.F.; Simmons, W.K. Interoceptive Predictions in the Brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Seth, A. The Cybernetic Bayesian Brain—From Interoceptive Inference to Sensorimotor Contingencies. Open MIND 2015, 35, 1–24. [Google Scholar] [CrossRef]
- Van den Bergh, O.; Witthöft, M.; Petersen, S.; Brown, R.J. Symptoms and the Body: Taking the Inferential Leap. Neurosci. Biobehav. Rev. 2017, 74, 185–203. [Google Scholar] [CrossRef]
- Ainley, V.; Apps, M.A.J.; Fotopoulou, A.; Tsakiris, M. ‘Bodily Precision’: A Predictive Coding Account of Individual Differences in Interoceptive Accuracy. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160003. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Tang, X.; Li, H.; Hu, Q.; Cui, H.; Zhang, L.; Li, W.; Zhu, Z.; Wang, J.; Li, C. Altered Interoceptive Processing in Generalized Anxiety Disorder—A Heartbeat-Evoked Potential Research. Front. Psychiatry 2019, 10, 616. [Google Scholar] [CrossRef]
- Desmedt, O.; Van Den Houte, M.; Walentynowicz, M.; Dekeyser, S.; Luminet, O.; Corneille, O. How Does Heartbeat Counting Task Performance Relate to Theoretically-Relevant Mental Health Outcomes? A Meta-Analysis. Collabra Psychol. 2022, 8, 33271. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Rudrauf, D.; Tranel, D. Interoceptive Awareness Declines with Age. Psychophysiology 2009, 46, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.; Boone, J.; Connolly, S.; Greene, M.; Klein, G.; Sheldon, R.; Talajic, M. Follow-up of Atrial Fibrillation: The Initial Experience of the Canadian Registry of Atrial Fibrillation. Eur. Heart J. 1996, 17, 48–51. [Google Scholar] [CrossRef]
- Paquette, M.; Roy, D.; Talajic, M.; Newman, D.; Couturier, A.; Yang, C.; Dorian, P. Role of Gender and Personality on Quality-of-Life Impairment in Intermittent Atrial Fibrillation. Am. J. Cardiol. 2000, 86, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Lavelle, T.; Essebag, V.; Cohen, D.J.; Zimetbaum, P. Influence of Age, Sex, and Atrial Fibrillation Recurrence on Quality of Life Outcomes in a Population of Patients with New-Onset Atrial Fibrillation: The Fibrillation Registry Assessing Costs, Therapies, Adverse Events and Lifestyle (FRACTAL) Study. Am. Heart J. 2006, 152, 1097–1103. [Google Scholar] [CrossRef]
- Brand, F.N. Characteristics and Prognosis of Lone Atrial Fibrillation. JAMA 1985, 254, 3449. [Google Scholar] [CrossRef] [PubMed]
- Flaker, G.C.; Belew, K.; Beckman, K.; Vidaillet, H.; Kron, J.; Safford, R.; Mickel, M.; Barrell, P. Asymptomatic Atrial Fibrillation: Demographic Features and Prognostic Information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Study. Am. Heart J. 2005, 149, 657–663. [Google Scholar] [CrossRef]
- Mavroudis, G.; Strid, H.; Jonefjäll, B.; Simrén, M. Visceral Hypersensitivity Is Together with Psychological Distress and Female Gender Associated with Severity of IBS-like Symptoms in Quiescent Ulcerative Colitis. Neurogastroenterol. Motil. 2021, 33, e13998. [Google Scholar] [CrossRef]
- Mayou, R.; Sprigings, D.; Birkhead, J.; Price, J. Characteristics of Patients Presenting to a Cardiac Clinic with Palpitation. QJM-Int. J. Med. 2003, 96, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Garimella, R.S.; Chung, E.H.; Mounsey, J.P.; Schwartz, J.D.; Pursell, I.; Gehi, A.K. Accuracy of Patient Perception of Their Prevailing Rhythm: A Comparative Analysis of Monitor Data and Questionnaire Responses in Patients with Atrial Fibrillation. Heart Rhythm 2015, 12, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Rouse, C.H.; Jones, G.E.; Jones, K.R. The effect of body composition and gender on cardiac awareness. Psychophysiology 1988, 25, 400–407. [Google Scholar] [CrossRef]
- Robinson, E.; Foote, G.; Smith, J.; Higgs, S.; Jones, A. Interoception and Obesity: A Systematic Review and Meta-Analysis of the Relationship between Interoception and BMI. Int. J. Obes. 2021, 45, 2515–2526. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; El Mansouri-Yachou, J.; Casas-Barragán, A.; Molina, F.; Rueda-Medina, B.; Aguilar-Ferrándiz, M.E. The Association of Body Mass Index and Body Composition with Pain, Disease Activity, Fatigue, Sleep and Anxiety in Women with Fibromyalgia. Nutrients 2019, 11, 1193. [Google Scholar] [CrossRef]
- Chalazan, B.; Dickerman, D.; Sridhar, A.; Farrell, M.; Gayle, K.; Samuels, D.C.; Shoemaker, B.; Darbar, D. Relation of Body Mass Index to Symptom Burden in Patients WithAtrial Fibrillation. Am. J. Cardiol. 2018, 122, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Guedes, E.P.; Madeira, E.; Mafort, T.T.; Madeira, M.; Moreira, R.O.; Mendonça, L.M.; Godoy-Matos, A.F.; Lopes, A.J.; Farias, M.L.F. Body Composition and Depressive/Anxiety Symptoms in Overweight and Obese Individuals with Metabolic Syndrome. Diabetol. Metab. Syndr. 2013, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Catmur, C.; Bird, G. Alexithymia Is Associated with a Multidomain, Multidimensional Failure of Interoception: Evidence from Novel Tests. J. Exp. Psychol. Gen. 2018, 147, 398–408. [Google Scholar] [CrossRef]
- Salamone, P.C.; Legaz, A.; Sedeño, L.; Moguilner, S.; Fraile-Vazquez, M.; Campo, C.G.; Fittipaldi, S.; Yoris, A.; Miranda, M.; Birba, A.; et al. Interoception Primes Emotional Processing: Multimodal Evidence from Neurodegeneration. J. Neurosci. 2021, 41, 4276–4292. [Google Scholar] [CrossRef]
- Rady, A.; Alamrawy, R.G.; Ramadan, I.; El Raouf, M.A. Prevalence of Alexithymia in Patients with Medically Unexplained Physical Symptoms: A Cross-Sectional Study in Egypt. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.M.; Herbert, C.; Pollatos, O. On the Relationship between Interoceptive Awareness and Alexithymia: Is Interoceptive Awareness Related to Emotional Awareness? J. Pers. 2011, 79, 1149–1175. [Google Scholar] [CrossRef] [PubMed]
- Flasbeck, V.; Popkirov, S.; Ebert, A.; Brüne, M. Altered Interoception in Patients with Borderline Personality Disorder: A Study Using Heartbeat-Evoked Potentials. Borderline Personal. Disord. Emot. Dysregul. 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Palser, E.R.; Palmer, C.E.; Galvez-Pol, A.; Hannah, R.; Fotopoulou, A.; Kilner, J.M. Alexithymia Mediates the Relationship between Interoceptive Sensibility and Anxiety. PLoS ONE 2018, 13, e0203212. [Google Scholar] [CrossRef]
- Frommeyer, G.; Eckardt, L.; Breithardt, G. Panic Attacks and Supraventricular Tachycardias: The Chicken or the Egg? Neth. Heart J. 2013, 21, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Carnlöf, C.; Iwarzon, M.; Jensen-Urstad, M.; Gadler, F.; Insulander, P. Women with PSVT Are Often Misdiagnosed, Referred Later than Men, and Have More Symptoms after Ablation. Scand. Cardiovasc. J. 2017, 51, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lessmeier, T.J.; Gamperling, D.; Johnson-Liddon, V.; Fromm, B.S.; Steinman, R.T.; Meissner, M.D.; Lehmann, M.H. Unrecognized Paroxysmal Supraventricular Tachycardia. Potential for Misdiagnosis as Panic Disorder. Arch. Intern. Med. 1997, 157, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, T.; Kenkre, T.S.; Bittner, V.; Krantz, D.S.; Thompson, D.V.; Linke, S.E.; Eastwood, J.-A.; Eteiba, W.; Cornell, C.E.; Vaccarino, V.; et al. Anxiety Associations with Cardiac Symptoms, Angiographic Disease Severity, and Healthcare Utilization: The NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation. Int. J. Cardiol. 2013, 168, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y.; Li, L.; Wu, X.; Liu, J.; Tang, J.; Wang, W.; Lu, L. Relationship Between Severity of Gastrointestinal Symptoms and Anxiety Symptoms in Patients with Chronic Gastrointestinal Disease: The Mediating Role of Illness Perception. Psychol. Res. Behav. Manag. 2023, 16, 4921–4933. [Google Scholar] [CrossRef]
- Reigada, L.C.; Hoogendoorn, C.J.; Walsh, L.C.; Lai, J.; Szigethy, E.; Cohen, B.H.; Bao, R.; Isola, K.; Benkov, K.J. Anxiety Symptoms and Disease Severity in Children and Adolescents with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, P.; Zimmermann, T.; Sattel, H. Medically Unexplained Physical Symptoms, Anxiety, and Depression. Psychosom. Med. 2003, 65, 528–533. [Google Scholar] [CrossRef]
- Chen, E.; Hermann, C.; Rodgers, D.; Oliver-Welker, T.; Strunk, R.C. Symptom Perception in Childhood Asthma: The Role of Anxiety and Asthma Severity. Health Psychol. 2006, 25, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Feldman, J.L.; Leggio, L.; Napadow, V.; Park, J.; Price, C.J. Interventions and Manipulations of Interoception. Trends Neurosci. 2021, 44, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Villani, V.; Tsakiris, M.; Azevedo, R.T. Transcutaneous Vagus Nerve Stimulation Improves Interoceptive Accuracy. Neuropsychologia 2019, 134, 107201. [Google Scholar] [CrossRef] [PubMed]
- Poppa, T.; Benschop, L.; Horczak, P.; Vanderhasselt, M.A.; Carrette, E.; Bechara, A.; Baeken, C.; Vonck, K. Auricular transcutaneous vagus nerve stimulation modulates the heart-evoked potential. Brain Stimul. 2022, 15, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Sagliano, L.; Magliacano, A.; Parazzini, M.; Fiocchi, S.; Trojano, L.; Grossi, D. Modulating Interoception by Insula Stimulation: A Double-Blinded TDCS Study. Neurosci. Lett. 2019, 696, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.; Hajian, S.A.; Mai-Lippold, S.; Pollatos, O. Enhancing Interoceptive Abilities and Emotional Processing: Effects of HD-TDCS. J. Integr. Neurosci. 2024, 23, 8. [Google Scholar] [CrossRef]
- Pollatos, O.; Herbert, B.M.; Mai, S.; Kammer, T. Changes in Interoceptive Processes Following Brain Stimulation. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160016. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, B.; Singer, T. Taking Time to Feel Our Body: Steady Increases in Heartbeat Perception Accuracy and Decreases in Alexithymia over 9 Months of Contemplative Mental Training. Psychophysiology 2017, 54, 469–482. [Google Scholar] [CrossRef]
- Quadt, L.; Garfinkel, S.N.; Mulcahy, J.S.; Larsson, D.E.; Silva, M.; Jones, A.-M.; Strauss, C.; Critchley, H.D. Interoceptive Training to Target Anxiety in Autistic Adults (ADIE): A Single-Center, Superiority Randomized Controlled Trial. eClinicalMedicine 2021, 39, 101042. [Google Scholar] [CrossRef]
- Schaefer, M.; Egloff, B.; Gerlach, A.L.; Witthöft, M. Improving Heartbeat Perception in Patients with Medically Unexplained Symptoms Reduces Symptom Distress. Biol. Psychol. 2014, 101, 69–76. [Google Scholar] [CrossRef]
- Meyerholz, L.; Irzinger, J.; Witthöft, M.; Gerlach, A.L.; Pohl, A. Contingent Biofeedback Outperforms Other Methods to Enhance the Accuracy of Cardiac Interoception: A Comparison of Short Interventions. J. Behav. Ther. Exp. Psychiatry 2019, 63, 12–20. [Google Scholar] [CrossRef]
- Sugawara, A.; Terasawa, Y.; Katsunuma, R.; Sekiguchi, A. Effects of Interoceptive Training on Decision Making, Anxiety, and Somatic Symptoms. Biopsychosoc. Med. 2020, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.M.; Lutz, J.; Schuman-Olivier, Z. Interoception in Psychiatric Disorders: A Review of Randomized, Controlled Trials with Interoception-Based Interventions. Harv. Rev. Psychiatry 2018, 26, 250–263. [Google Scholar] [CrossRef]
- Di Lernia, D.; Lacerenza, M.; Ainley, V.; Riva, G. Altered Interoceptive Perception and the Effects of Interoceptive Analgesia in Musculoskeletal, Primary, and Neuropathic Chronic Pain Conditions. J. Pers. Med. 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Craske, M.G.; Wolitzky-Taylor, K.B.; Labus, J.; Wu, S.; Frese, M.; Mayer, E.A.; Naliboff, B.D. A Cognitive-Behavioral Treatment for Irritable Bowel Syndrome Using Interoceptive Exposure to Visceral Sensations. Behav. Res. Ther. 2011, 49, 413–421. [Google Scholar] [CrossRef]
- Schulz, A.; Strelzyk, F.; Ferreira de Sá, D.S.; Naumann, E.; Vögele, C.; Schächinger, H. Cortisol Rapidly Affects Amplitudes of Heartbeat-Evoked Brain Potentials—Implications for the Contribution of Stress to an Altered Perception of Physical Sensations? Psychoneuroendocrinology 2013, 38, 2686–2693. [Google Scholar] [CrossRef]
- De la Fuente, A.; Sedeño, L.; Vignaga, S.S.; Ellmann, C.; Sonzogni, S.; Belluscio, L.; García-Cordero, I.; Castagnaro, E.; Boano, M.; Cetkovich, M.; et al. Multimodal Neurocognitive Markers of Interoceptive Tuning in Smoked Cocaine. Neuropsychopharmacology 2019, 44, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Canales-Johnson, A.; Silva, C.; Huepe, D.; Rivera-Rei, Á.; Noreika, V.; Garcia, M.D.C.; Silva, W.; Ciraolo, C.; Vaucheret, E.; Sedeño, L.; et al. Auditory Feedback Differentially Modulates Behavioral and Neural Markers of Objective and Subjective Performance When Tapping to Your Heartbeat. Cereb. Cortex 2015, 25, 4490–4503. [Google Scholar] [CrossRef]
- Perogamvros, L.; Park, H.-D.; Bayer, L.; Perrault, A.A.; Blanke, O.; Schwartz, S. Increased Heartbeat-Evoked Potential during REM Sleep in Nightmare Disorder. NeuroImage Clin. 2019, 22, 101701. [Google Scholar] [CrossRef]
- Gentsch, A.; Sel, A.; Marshall, A.C.; Schütz-Bosbach, S. Affective Interoceptive Inference: Evidence from Heart-beat Evoked Brain Potentials. Hum. Brain Mapp. 2019, 40, 20–33. [Google Scholar] [CrossRef]
- Luft, C.D.B.; Bhattacharya, J. Aroused with Heart: Modulation of Heartbeat Evoked Potential by Arousal Induction and Its Oscillatory Correlates. Sci. Rep. 2015, 5, 15717. [Google Scholar] [CrossRef]
- Park, H.-D.; Bernasconi, F.; Bello-Ruiz, J.; Pfeiffer, C.; Salomon, R.; Blanke, O. Transient Modulations of Neural Responses to Heartbeats Covary with Bodily Self-Consciousness. J. Neurosci. 2016, 36, 8453–8460. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Ferreira de Sá, D.S.; Dierolf, A.M.; Lutz, A.; van Dyck, Z.; Vögele, C.; Schächinger, H. Short-term Food Deprivation Increases Amplitudes of Heartbeat-evoked Potentials. Psychophysiology 2015, 52, 695–703. [Google Scholar] [CrossRef]
- Santarnecchi, E.; D’Arista, S.; Egiziano, E.; Gardi, C.; Petrosino, R.; Vatti, G.; Reda, M.; Rossi, A. Correction: Interaction between Neuroanatomical and Psychological Changes after Mindfulness-Based Training. PLoS ONE 2015, 10, e0129754. [Google Scholar] [CrossRef] [PubMed]
- Subic-Wrana, C.; Bruder, S.; Thomas, W.; Lane, R.D.; Köhle, K. Emotional Awareness Deficits in Inpatients of a Psychosomatic Ward: A Comparison of Two Different Measures of Alexithymia. Psychosom. Med. 2005, 67, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Preece, D.A.; Petrova, K.; Mehta, A.; Sikka, P.; Gross, J.J. Alexithymia or General Psychological Distress? Discriminant Validity of the Toronto Alexithymia Scale and the Perth Alexithymia Questionnaire. J. Affect. Disord. 2024, 352, 140–145. [Google Scholar] [CrossRef]
- Adams, K.L.; Edwards, A.; Peart, C.; Ellett, L.; Mendes, I.; Bird, G.; Murphy, J. The Association between Anxiety and Cardiac Interoceptive Accuracy: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2022, 140, 104754. [Google Scholar] [CrossRef]
- Reinfeld, P.; Steinfath, T.P.; Ku, P.-H.; Nikulin, V.; Neumann, J.; Villringer, A. Neural Representations of Extrasystoles: A Predictive Coding Perspective. bioRxiv 2024. [Google Scholar] [CrossRef]
| Condition | Description |
|---|---|
| 1 | at rest (HEPREST) |
| 2 | during the HBD task (HEPHBD) |
| 3 | during the MT task (HEPMT) |
| 4 | HEP modulation (ΔHEP) in the HBD task compared to rest (ΔHEPHBD-REST) |
| 5 | ΔHEP in the MT task compared to rest (ΔHEPMT-REST) |
| 6 | ΔHEP between the HEPHBD and exteroceptive conditions in the HBD task (ΔHEPHBD-EX) |
| Symptomatic Group, n = 17 1 | Asymptomatic Group, n = 17 1 | W/t/χ2 | p | |
|---|---|---|---|---|
| Age, years old | 43.0 [39.0; 46.0] | 41.0 [38.0; 42.0] | 119 | 0.39 a |
| Sex, males | 3 (18%) | 11 (65%) | 5.95 | 0.015 b |
| BMI, kg/m2 | 22.5 [20.2; 25.5] | 24.6 [23.9; 27] | 2.36 | 0.025 c |
| Percent of body fat (%) | 26 (24; 31) | 25 (19; 32) | −1.02 | 0.31 c |
| Smoking | 1 (5.9%) | 4 (23.5%) | 0.94 | 0.33 b |
| Pharmacological treatment: | 5 (29.4%) | 3 (17.7%) | 0.16 | 0.69 b |
| Beta-blockers | 3 | 0 | ||
| Class 1C antiarrhythmics | 0 | 1 | ||
| Beta-blockers + Angiotensin II receptor blockers + Calcium channel blockers | 0 | 1 | ||
| Beta-blockers + Angiotensin II receptor blockers + Class 1C antiarrhythmics | 0 | 1 | ||
| Beta-blockers + Class 1C antiarrhythmics | 1 | 0 | ||
| Angiotensin II receptor blockers + Class 1C antiarrhythmics | 1 | 0 | ||
| DBP, mmHg. | 74 [70; 80] | 75.3 [72; 82] | 175.5 | 0.29 a |
| SBP, mmHg. | 106 [102; 116] | 110 [105; 118] | 0.82 | 0.42 c |
| HR, bpm: | ||||
| Rest | 68.4 [61.8, 74.04] | 65.56 [63.3; 72.76] | −0.39 | 0.6 c |
| HBD task, interoceptive condition | 69.3 [57.5; 73.6] | 67.2 [62.1; 73.1] | 0.2 | 0.84 c |
| HBD task, exteroceptive condition | 69.5 [62.4; 75.5] | 67.4 [61.8; 72.2] | −0.55 | 0.59 c |
| Number of PVCs during experiment: | ||||
| Rest | 0 | 10 [0; 20] | 202 | 0.029 a |
| HBD task, interoceptive condition | 0 | 3 [0; 7] | 196 | 0.047 a |
| HBD task, exteroceptive condition | 0 | 1 [0; 7] | 191.5 | 0.059 a |
| MT task | 0 | 6 [0; 11] | 203.5 | 0.015 a |
| 24 h Holter PVCs | 3672 [920; 5485] | 10,173 [1902; 14,536] | 213 | 0.018 a |
| STAI-T (trait scale score) | 45 [39; 50] | 37 [31; 48] | −1.87 | 0.07 c |
| TAS-20-R (total alexithymia index) | 43 [36; 48] | 35 [29; 39] | −1.57 | 0.14 c |
| Model | ΔHEPCONDITION | ROI | |||||
|---|---|---|---|---|---|---|---|
| In Predictors | LF | CF | RF | LP | CO | RP | |
| A3 | ΔHEPMT-REST | 0.503 | 0.766 | 0.919 | 0.209 | 0.124 | 0.095 |
| B3 | ΔHEPMT-REST | 0.271 | 0.340 | 0.904 | 0.221 | 0.115 | 0.124 |
| A4 | ΔHEPHBD-REST | 0.017 ** β = −0.84, SE = 0.35 AIC = 93.92 | 0.016 ** β = −0.84, SE = 0.35 AIC = 93.13 | 0.082 | 0.037 | 0.095 | 0.103 |
| B4 | ΔHEPHBD-REST | 0.035 | 0.019 * β = −0.98, SE = 0.42 AIC = 87.63 | 0.094 | 0.019 * β= −1.05, SE = 0.45 AIC = 88.12 | 0.018 * β = −0.99 SE = 0.42 AIC = 87.41 | 0.033 |
| A5 | ΔHEPHBD-EX | 0.025 ** β = −0.78, SE = 0.35 AIC = 93.78 | 0.022 ** β = −0.62, SE = 0.27 AIC = 93.87 | 0.234 | 0.153 | 0.182 | 0.206 |
| B5 | ΔHEPHBD-EX | 0.131 | 0.181 | 0.305 | 0.466 | 0.537 | 0.741 |
| Model | ΔHEPCONDITION in Predictors | ROI | Predictors | β | SE | p | AIC |
|---|---|---|---|---|---|---|---|
| A6 | ΔHEPHBD-REST | LF | IAMT ΔHEPCONDITION | 0.93 −1.03 | 0.32 0.38 | 0.004 0.006 | 86.05 |
| B6 | IAMT ΔHEPCONDITION clinical parameters (male sex) | 0.9 −1.12 −2.04 | 0.37 0.47 0.77 | 0.015 0.016 0.008 | 83.72 | ||
| A6 | CF | IAMT ΔHEPCONDITION | 0.92 −0.99 | 0.32 0.36 | 0.004 0.005 | 85.14 | |
| B6 | IAMT ΔHEPCONDITION clinical parameters (male sex) | 0.77 −1.04 −2.16 | 0.34 0.45 0.79 | 0.025 0.022 0.006 | 83.79 | ||
| A6 | LP | IAMT ΔHEPCONDITION | 1.28 −1.69 | 0.39 0.52 | 0.001 0.001 | 82.49 | |
| B6 | IAMT ΔHEPCONDITION clinical parameters (male sex) | 1.11 −1.61 −1.97 | 0.42 0.56 0.81 | 0.008 0.004 0.016 | 80.86 | ||
| A7 | ΔHEPHBD-EX | LF | IAMT ΔHEPCONDITION | 0.77 −0.67 | 0.32 0.33 | 0.015 0.044 | 88.71 |
| B7 | IAMT ΔHEPCONDITION clinical parameters (male sex) | 0.71 −0.50 −1.78 | 0.35 0.44 0.72 | 0.039 0.265 0.014 | 87.95 | ||
| A7 | CF | IAMT ΔHEPCONDITION | 0.80 −0.55 | 0.32 0.25 | 0.012 0.029 | 88.28 | |
| B7 | IAMT ΔHEPCONDITION clinical parameters (male sex) | 0.74 −0.40 −1.80 | 0.34 0.36 0.73 | 0.031 0.267 0.014 | 87.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limonova, A.S.; Minenko, I.A.; Sukmanova, A.A.; Kutsenko, V.A.; Kulikova, S.P.; Nazarova, M.A.; Davtyan, K.V.; Drapkina, O.M.; Ershova, A.I. Exploring the Link Between Interoception and Symptom Severity in Premature Ventricular Contractions. J. Clin. Med. 2024, 13, 7756. https://doi.org/10.3390/jcm13247756
Limonova AS, Minenko IA, Sukmanova AA, Kutsenko VA, Kulikova SP, Nazarova MA, Davtyan KV, Drapkina OM, Ershova AI. Exploring the Link Between Interoception and Symptom Severity in Premature Ventricular Contractions. Journal of Clinical Medicine. 2024; 13(24):7756. https://doi.org/10.3390/jcm13247756
Chicago/Turabian StyleLimonova, Alena S., Irina A. Minenko, Anastasia A. Sukmanova, Vladimir A. Kutsenko, Sofya P. Kulikova, Maria A. Nazarova, Karapet V. Davtyan, Oxana M. Drapkina, and Alexandra I. Ershova. 2024. "Exploring the Link Between Interoception and Symptom Severity in Premature Ventricular Contractions" Journal of Clinical Medicine 13, no. 24: 7756. https://doi.org/10.3390/jcm13247756
APA StyleLimonova, A. S., Minenko, I. A., Sukmanova, A. A., Kutsenko, V. A., Kulikova, S. P., Nazarova, M. A., Davtyan, K. V., Drapkina, O. M., & Ershova, A. I. (2024). Exploring the Link Between Interoception and Symptom Severity in Premature Ventricular Contractions. Journal of Clinical Medicine, 13(24), 7756. https://doi.org/10.3390/jcm13247756







