Skeletal Muscle Mass Loss Leads to Prolonged Mechanical Ventilation and Higher Tracheotomy Rates in Critically Ill Patients
Abstract
1. Introduction
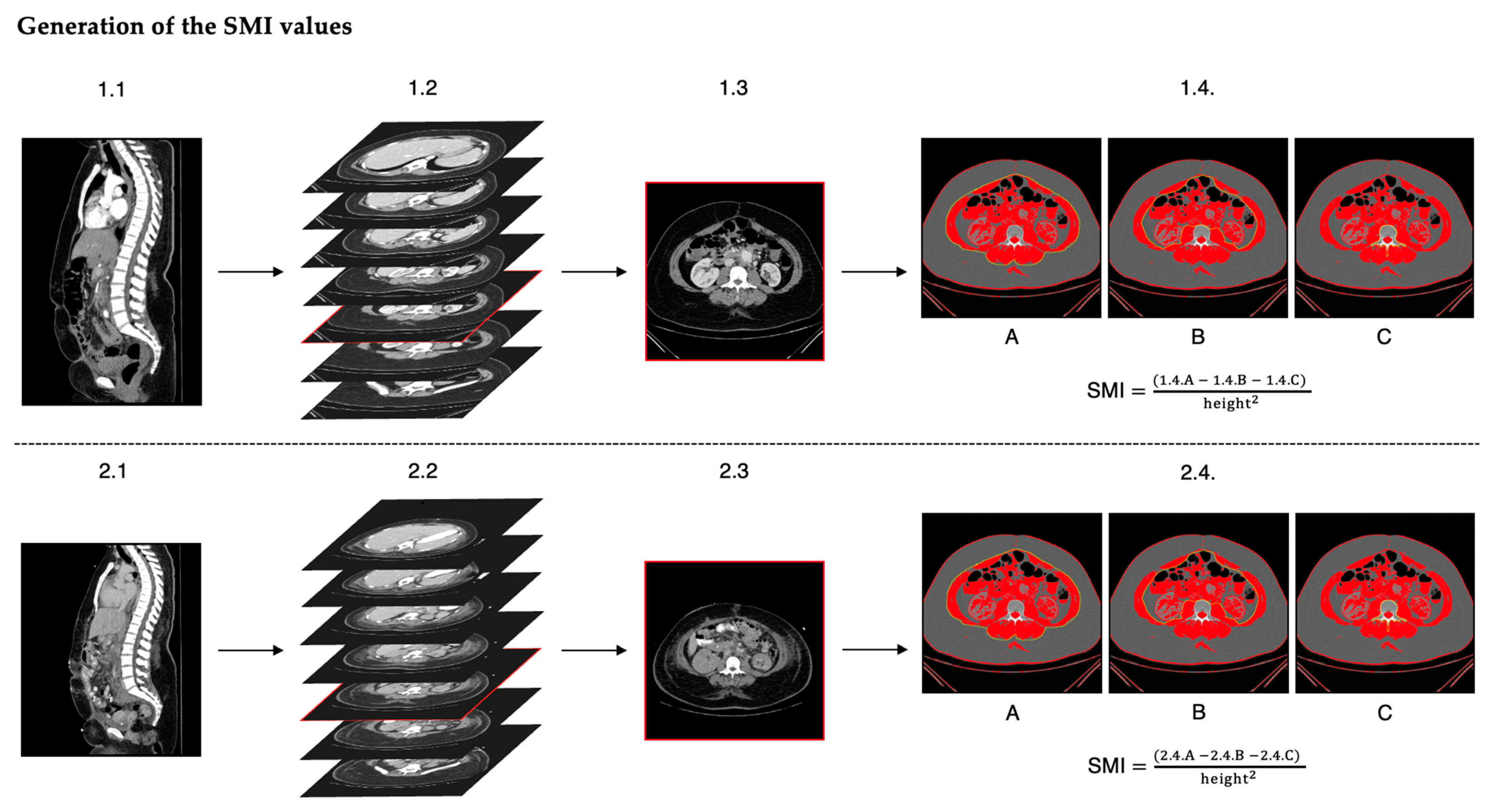
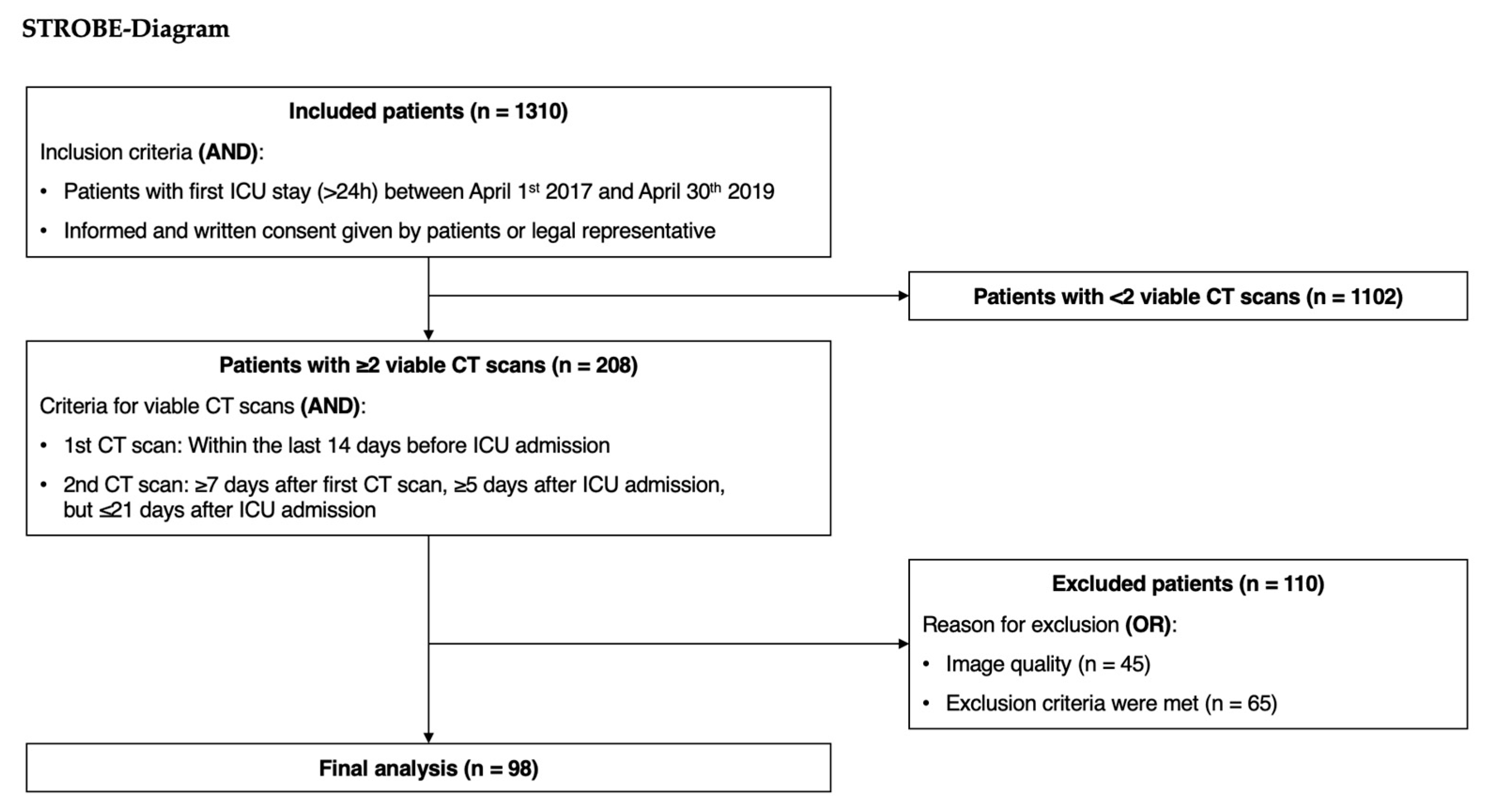
2. Materials and Methods
3. Results
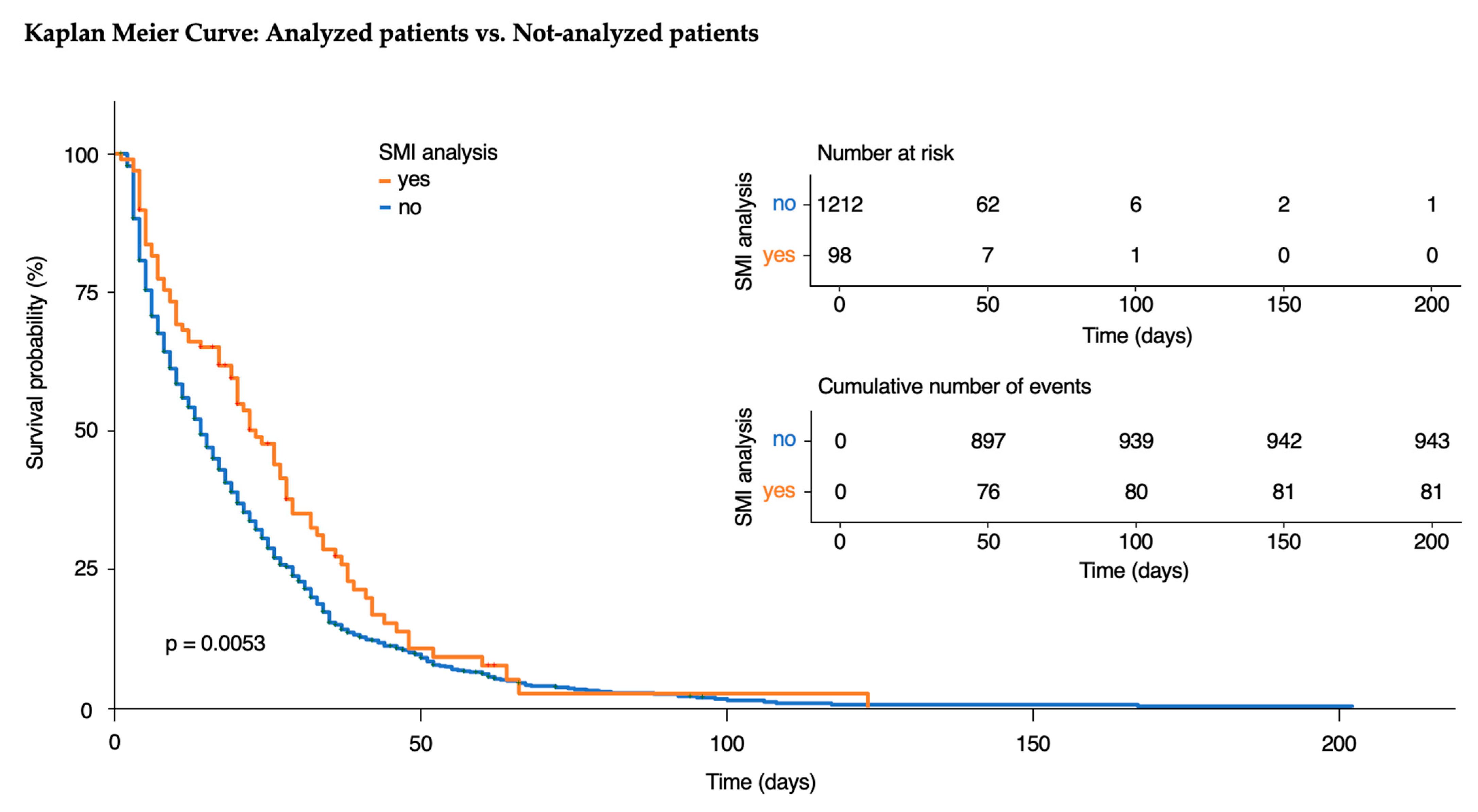
3.1. Analyzed Patients
3.2. Demographic Characteristics
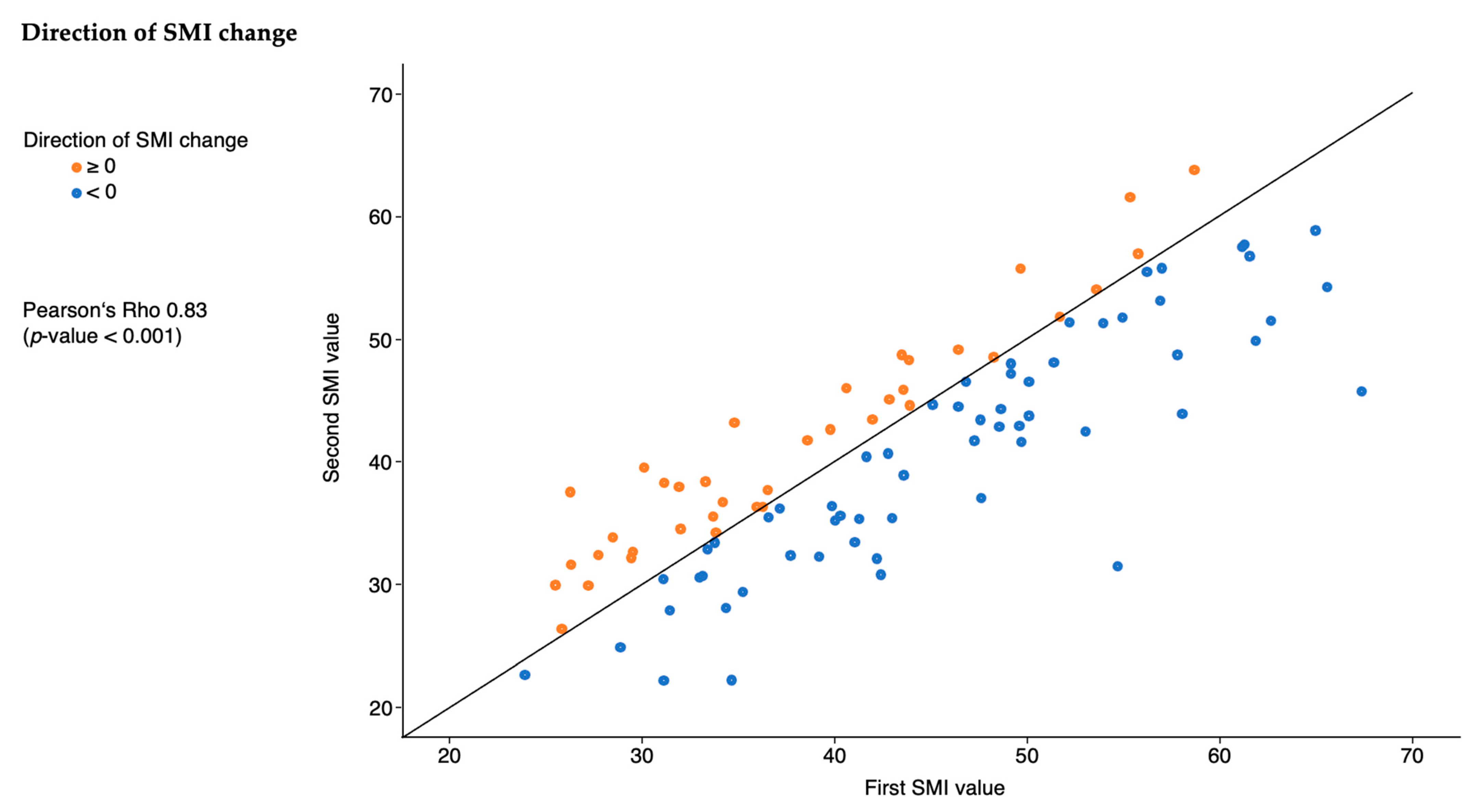
3.3. SMI Characteristics
3.4. Outcomes
3.5. Exploratory Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A.; for the Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef] [PubMed]
- de Hoogt, P.A.; Reisinger, K.W.; Tegels, J.J.W.; Bosmans, J.; Tijssen, F.; Stoot, J. Functional Compromise Cohort Study (FCCS): Sarcopenia is a Strong Predictor of Mortality in the Intensive Care Unit. World J. Surg. 2018, 42, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Fazzini, B.; Märkl, T.; Costas, C.; Blobner, M.; Schaller, S.J.; Prowle, J.; Puthucheary, Z.; Wackerhage, H. The rate and assessment of muscle wasting during critical illness: A systematic review and meta-analysis. Crit. Care 2023, 27, 2. [Google Scholar] [CrossRef]
- Lorenz, M.; Fuest, K.; Ulm, B.; Grunow, J.J.; Warner, L.; Bald, A.; Arsene, V.; Verfuß, M.; Daum, N.; Blobner, M.; et al. The optimal dose of mobilisation therapy in the ICU: A prospective cohort study. J. Intensive Care 2023, 11, 56. [Google Scholar] [CrossRef]
- Fuest, K.E.; Lorenz, M.; Grunow, J.J.; Weiss, B.; Mörgeli, R.; Finkenzeller, S.; Bogdanski, R.; Heim, M.; Kapfer, B.; Kriescher, S.; et al. The Functional Trajectory in Frail Compared With Non-frail Critically Ill Patients During the Hospital Stay. Front. Med. 2021, 8, 748812. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St.-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Heo, M.; Thomas, D.; Pietrobelli, A. Scaling of body composition to height: Relevance to height-normalized indexes. Am. J. Clin. Nutr. 2011, 93, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Teigen, L.M.; Kuchnia, A.J.; Nagel, E.; Deuth, C.; Vock, D.M.; Mulasi, U.; Earthman, C.P. Impact of Software Selection and ImageJ Tutorial Corrigendum on Skeletal Muscle Measures at the Third Lumbar Vertebra on Computed Tomography Scans in Clinical Populations. JPEN J. Parenter. Enteral Nutr. 2018, 42, 933–941. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, J.L.; Levolger, S.; Gharbharan, A.; Koek, M.; Niessen, W.J.; Burger, J.W.; Willemsen, S.P.; de Bruin, R.W.; Ijzermans, J.N. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Perez, S.; McKeever, L.; Sheean, P. Tutorial: A Step-by-Step Guide (Version 2.0) for Measuring Abdominal Circumference and Skeletal Muscle From a Single Cross-Sectional Computed-Tomography Image Using the National Institutes of Health ImageJ. JPEN J. Parenter. Enteral Nutr. 2020, 44, 419–424. [Google Scholar] [CrossRef]
- Gomez-Perez, S.L.; Haus, J.M.; Sheean, P.; Patel, B.; Mar, W.; Chaudhry, V.; McKeever, L.; Braunschweig, C. Measuring Abdominal Circumference and Skeletal Muscle From a Single Cross-Sectional Computed Tomography Image: A Step-by-Step Guide for Clinicians Using National Institutes of Health ImageJ. JPEN J. Parenter. Enteral Nutr. 2016, 40, 308–318. [Google Scholar] [CrossRef]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Flaatten, H.; De Lange, D.W.; Morandi, A.; Andersen, F.H.; Artigas, A.; Bertolini, G.; Boumendil, A.; Cecconi, M.; Christensen, S.; Faraldi, L.; et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years). Intensive Care Med. 2017, 43, 1820–1828. [Google Scholar] [CrossRef]
- Mehta, R.; Chinthapalli, K. Glasgow coma scale explained. BMJ 2019, 365, l1296. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Giraudo, C.; Librizzi, G.; Fichera, G.; Motta, R.; Balestro, E.; Calabrese, F.; Carretta, G.; Cattelan, A.M.; Navalesi, P.; Pelloso, M.; et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID-19 patients. PLoS ONE 2021, 16, e0253433. [Google Scholar] [CrossRef]
- Damanti, S.; Cristel, G.; Ramirez, G.A.; Bozzolo, E.P.; Da Prat, V.; Gobbi, A.; Centurioni, C.; Di Gaeta, E.; Del Prete, A.; Calabrò, M.G.; et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2022, 41, 2965–2972. [Google Scholar] [CrossRef]
- Giani, M.; Rezoagli, E.; Grassi, A.; Porta, M.; Riva, L.; Famularo, S.; Barbaro, A.; Bernasconi, D.; Ippolito, D.; Bellani, G.; et al. Low skeletal muscle index and myosteatosis as predictors of mortality in critically ill surgical patients. Nutrition 2022, 101, 111687. [Google Scholar] [CrossRef]
- Jaitovich, A.; Dumas, C.L.; Itty, R.; Chieng, H.C.; Khan, M.; Naqvi, A.; Fantauzzi, J.; Hall, J.B.; Feustel, P.J.; Judson, M.A. ICU admission body composition: Skeletal muscle, bone, and fat effects on mortality and disability at hospital discharge-a prospective, cohort study. Crit. Care 2020, 24, 566. [Google Scholar] [CrossRef]
- Dusseaux, M.M.; Antoun, S.; Grigioni, S.; Béduneau, G.; Carpentier, D.; Girault, C.; Grange, S.; Tamion, F. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS ONE 2019, 14, e0216991. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef] [PubMed]
- Fuest, K.E.; Lanz, H.; Schulz, J.; Ulm, B.; Bennett, V.A.; Grunow, J.J.; Weiss, B.; Blobner, M.; Schaller, S.J. Comparison of Different Ultrasound Methods to Assess Changes in Muscle Mass in Critically ill Patients. J. Intensive Care Med. 2023, 38, 431–439. [Google Scholar] [CrossRef] [PubMed]
- de Andrade-Junior, M.C.; de Salles, I.C.D.; de Brito, C.M.M.; Pastore-Junior, L.; Righetti, R.F.; Yamaguti, W.P. Skeletal Muscle Wasting and Function Impairment in Intensive Care Patients With Severe COVID-19. Front. Physiol. 2021, 12, 640973. [Google Scholar] [CrossRef]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef]
| n = 98 | |
|---|---|
| Female gender | 69 (70.4%) |
| BMI (kg/m2) | 25.0 (23.4,28.0) |
| Underweight | 1 (1.0%) |
| Normal | 48 (49.0%) |
| Overweight | 32 (32.7%) |
| Obese | 17 (17.3%) |
| Age (years) | 68 (59,76) |
| ≤50 | 14 (14.3%) |
| 51–65 | 30 (30.6%) |
| 66–80 | 48 (49.0%) |
| >80 | 6 (6.1%) |
| Department | |
| Neurosurgery | 14 (14.3%) |
| Thoracic Surgery | 1 (1.0%) |
| Abdominal Surgery | 55 (56.1%) |
| Vascular Surgery | 8 (8.2%) |
| Trauma Surgery | 3 (3.1%) |
| Neurology | 5 (5.1%) |
| Enterology | 3 (3.1%) |
| Gynecology | 1 (1.0%) |
| Urology | 2 (2.0%) |
| Internal Medicine | 4 (4.1%) |
| Other | 2 (2.0%) |
| Admission | |
| From home | 67 (68.4%) |
| From hospital | 30 (30.6%) |
| From nursing home | 1 (1.0%) |
| ICU admission category | |
| Sepsis | 20 (20.4%) |
| Polytrauma | 5 (5.1%) |
| Traumatic brain injury | 2 (2.0%) |
| Non traumatic brain injury | 4 (4.1%) |
| Postoperative monitoring | 27 (27.6%) |
| Cardiac failure | 8 (8.2%) |
| Respiratory failure | 35 (35.7%) |
| Other | 22 (22.4%) |
| Frail (CFS 5–9) | 14 (14.3%) |
| GCS | 15 (11,15) |
| APACHE II | 14 (10,19) |
| SOFA | 7 (5,9) |
| CCI | 2 (0,3) |
| Barthel Score at hospital admission | 30.0 (30.0, 30.0) |
| n = 98 | |
|---|---|
| Days between CT scans (days) | 14 (10,20) |
| <0 | 16 (12,20) |
| ≥0 | 12 (9,18) |
| Direction of change in SMI | |
| <0 | 60 (61.2%) |
| ≥0 | 38 (38.8%) |
| Absolute change in SMI (cm2/m2) | −1.2 (−5.5, 2.3) |
| Percentage change in SMI (%) | −3 (−12, 5) |
| Change in SMI per day ((cm2/m2)/days) | −0.12 (−0.33, 0.14) |
| Variables | Atrophy (n = 60) | Swelling (n = 38) | Univariate p Value | Multivariate | |
|---|---|---|---|---|---|
| Estimate/OR [95% CI] | p Value | ||||
| Hours of ventilation | 415 (116,594) | 42 (0, 491) | 0.003 | 155 (−17 to 328) a | 0.076 |
| Ventilated during ICU stay | 53 (88.3%) | 23 (60.5%) | 0.002 | 5.85 (1.90 to 20.40) b | 0.002 |
| If ventilated, hours of ventilation | 440 (186, 668) | 340 (72, 673) | 0.3 | ||
| Tracheotomy | 30 (50.0%) | 9 (23.7%) | 0.011 | 3.96 (1.43 to 10.40) b | 0.006 |
| ICU mortality | 12 (20.0%) | 4 (10.5%) | 0.3 | ||
| Hospital mortality | 17 (28.3%) | 8 (21.1%) | 0.5 | ||
| ICU length of stay (days) | 22 (14,35) | 13 (5,28) | 0.045 | 5.2 (−3.0 to 13.0) a | 0.2 |
| Hospital length of stay (days) | 48 (31,61) | 44 (31,64) | 0.8 | ||
| ECMO | 3 (5.0%) | 0 (0.0%) | 0.3 | ||
| Dialysis | 28 (46.7%) | 15 (39.5%) | 0.5 | ||
| Resuscitation | 12 (20.0%) | 2 (5.3%) | 0.072 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allgayer, G.M.; Ulm, B.; Sauter, A.P.; Schaller, S.J.; Blobner, M.; Fuest, K.E. Skeletal Muscle Mass Loss Leads to Prolonged Mechanical Ventilation and Higher Tracheotomy Rates in Critically Ill Patients. J. Clin. Med. 2024, 13, 7772. https://doi.org/10.3390/jcm13247772
Allgayer GM, Ulm B, Sauter AP, Schaller SJ, Blobner M, Fuest KE. Skeletal Muscle Mass Loss Leads to Prolonged Mechanical Ventilation and Higher Tracheotomy Rates in Critically Ill Patients. Journal of Clinical Medicine. 2024; 13(24):7772. https://doi.org/10.3390/jcm13247772
Chicago/Turabian StyleAllgayer, Gabriel M., Bernhard Ulm, Andreas P. Sauter, Stefan J. Schaller, Manfred Blobner, and Kristina E. Fuest. 2024. "Skeletal Muscle Mass Loss Leads to Prolonged Mechanical Ventilation and Higher Tracheotomy Rates in Critically Ill Patients" Journal of Clinical Medicine 13, no. 24: 7772. https://doi.org/10.3390/jcm13247772
APA StyleAllgayer, G. M., Ulm, B., Sauter, A. P., Schaller, S. J., Blobner, M., & Fuest, K. E. (2024). Skeletal Muscle Mass Loss Leads to Prolonged Mechanical Ventilation and Higher Tracheotomy Rates in Critically Ill Patients. Journal of Clinical Medicine, 13(24), 7772. https://doi.org/10.3390/jcm13247772






