A Multicenter, Retrospective Comparison Study of Pregnancy Outcomes According to Placental Location in Placenta Previa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Ethical Considerations
2.2. Eligibility Criteria and Group Definition
2.3. Statistical Analysis
3. Results
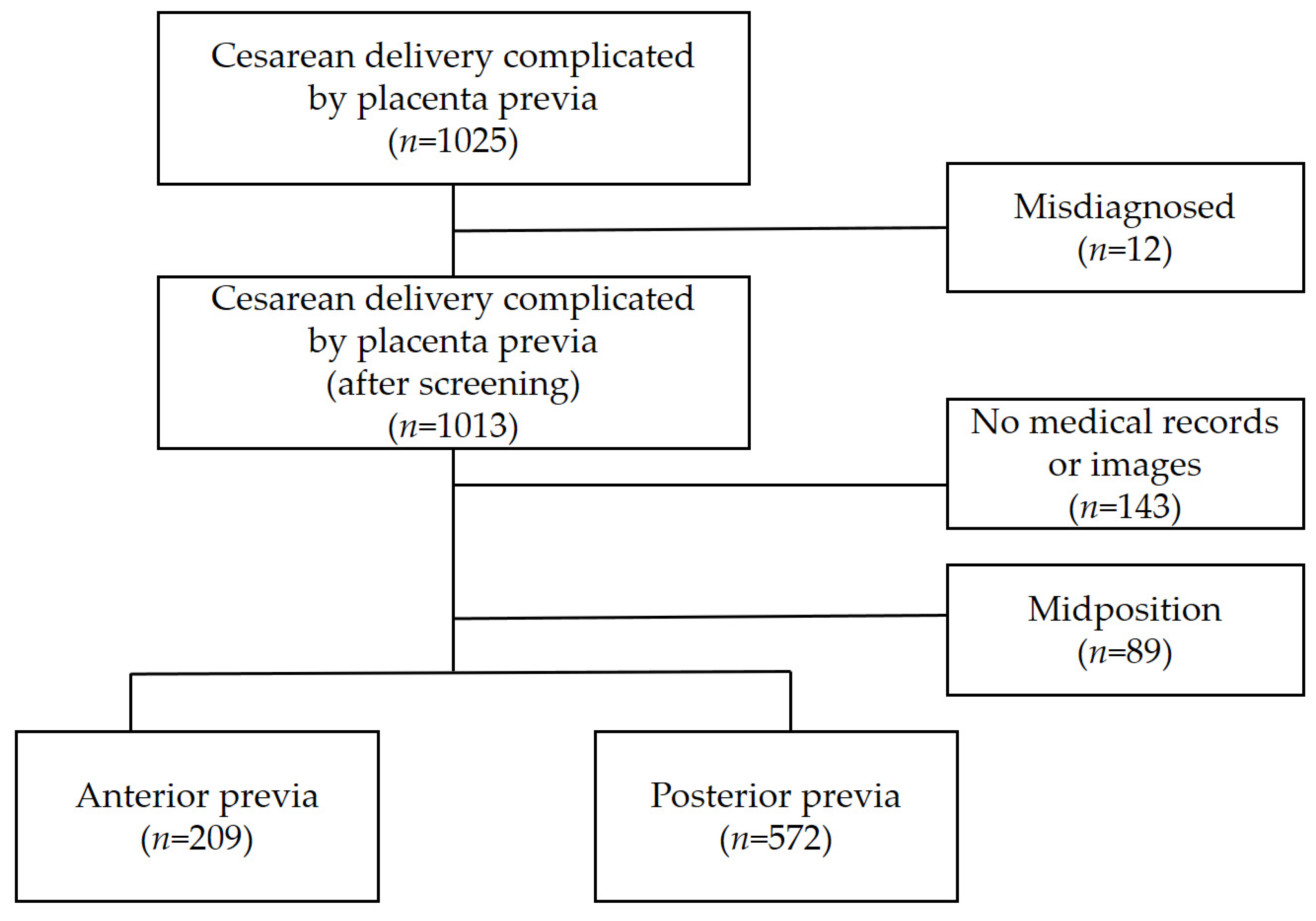
3.1. Study Population
3.2. Baseline Characteristics
3.3. Pregnancy Outcomes
3.4. Neonatal Outcomes
3.5. Multivariable Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silver, R.M. Abnormal placentation: Placenta Previa, Vasa Previa, and Placenta Accreta. Obstet. Gynecol. 2015, 126, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Post, R.J.; Chang, J.; Ziogas, A.; Crosland, B.A.; Silver, R.M.; Haas, D.M.; Grobman, W.A.; Saade, G.R.; Uma, M. Risk factors and perinatal outcomes for persistent placenta previa in nulliparas. Am. J. Obstet. Gynecol. MFM. 2023, 5, 101136. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, D.; Xu, J.; Liu, X.; You, Y.; Peng, B. Parallel transverse uterine incisions, a novel approach for managing heavy hemorrhage and preserving the uterus: A retrospective cohort study for patients with anterior placenta previa and accreta. Medicine 2019, 98, e17742. [Google Scholar] [CrossRef]
- Salim, N.A.; Satti, I. Risk factors of placenta previa with maternal and neonatal outcome at Dongola/Sudan. J. Fam. Med. Prim. Care 2021, 10, 1215–1217. [Google Scholar]
- Rosenberg, T.; Pariente, G.; Sergienko, R.; Wiznitzer, A.; Sheiner, E. Critical analysis of risk factors and outcome of placenta previa. Arch. Gynecol. Obstet. 2011, 284, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gronvall, M.; Stefanovic, V.; Paavonen, J.; Loukovaara, M.; Tikkanen, M. Major or minor placenta previa: Does it make a differences? Placenta 2019, 85, 9–14. [Google Scholar] [CrossRef]
- Ananth, C.V.; Demissie, K.; Smulian, J.C.; Vintzileos, A.M. Placenta previa in singleton and twin births in the United States,1989 through 1998: A comparison of risk factor profiles and associated conditions. Am. J. Obstet. Gynecol. 2003, 188, 275–281. [Google Scholar] [CrossRef]
- Ananth, C.V.; Smulian, J.C.; Vintzileos, A.M. The effect of placenta previa on neonatal mortality: A population-based study in the United States, 1989 through 1997. Am. J. Obstet. Gynecol. 2003, 188, 1299–1304. [Google Scholar] [CrossRef]
- Levin, G.; Rottenstreich, A.; Ilan, H.; Cahan, T.; Tsur, A.; Meyer, R. Predictors of adverse neonatal outcome in pregnancies complicated by placenta previa. Placenta 2021, 104, 119–123. [Google Scholar] [CrossRef]
- Cahill, A.G.; Beigi, R.; Heine, R.P.; Silver, R.M.; Wax, J.R. Placenta Accreta Spectrum. Am. J. Obstet. Gynecol. 2018, 219, B2–B16. [Google Scholar] [CrossRef]
- Jauniaux, E.; Ayres-de-Campos, D.; Langhoff-Roos, J.; Fox, K.A.; Collins, S. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynaecol. Obstet. 2019, 146, 20–24. [Google Scholar] [CrossRef]
- Cunningham, F.G.; Leveno, K.J.; Dashe, J.S.; Hoffman, B.L.; Sponge, C.Y.; Casey, B.M. Hemorrhagic Placental Disorders. In Williams Obstetrics; McGraw Hill: New York, NY, USA, 2022; p. 26e. [Google Scholar]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Baergen, R.N. The placenta as witness. Clin. Perionatol. 2007, 34, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Warland, J.; McCutcheon, H.; Baghurst, P. Placental position and late stillbirth: A case-control study. J. Clin. Nurs. 2009, 18, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Gonser, M.; Tillack, N.; Pfeiffer, K.H.; Mielke, G. Placental location and incidence of preeclampsia. Ultraschall Med. 1996, 17, 236–238. [Google Scholar] [CrossRef]
- Vaillant, P.; Best, M.C.; Cynober, E.; Devulder, G. Pathological uterine readings when the placenta is laterally situated. J. Gynecol. Obstet. Biol. Reprod. 1993, 22, 301–307. [Google Scholar]
- Zia, S. Placental location and pregnancy outcome. J. Turk. Ger. Gynecol. Assoc. 2013, 1, 190–193. [Google Scholar] [CrossRef]
- Magnn, E.F.; Doherty, D.A.; Turner, K.; Lanneau, G.S., Jr.; Morrison, J.C.; Newnham, J.P. Second trimester placental location as a predictor of an adverse pregnancy outcome. J. Perinatol. 2007, 27, 9–14. [Google Scholar] [CrossRef]
- Gibbins, K.J.; Einerson, B.D.; Varner, M.W.; Silver, R.M. Placenta previa and maternal hemorrhagic morbidity. J. Matern. Fetal Neonatal Med. 2018, 31, 494–499. [Google Scholar] [CrossRef]
- Lal, A.K.; Nyholm, J.; Wax, J.; Rose, C.H.; Watson, W.J. Resolution of complete placenta previa: Does prior cesarean delivery matter? J. Ultrasound Med. 2021, 31, 577–580. [Google Scholar] [CrossRef]
- Kramer, M.S.; Berg, C.; Abenhaim, H.; Dahhou, M.; Rouleau, J.; Mehrabadi, A.; Joseph, K.S. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am. J. Obstet. Gynecol. 2013, 209, 499.e1–499.e7. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, M.; Vannuccini, S.; Monici, I.; Cannoni, A.; Voltolini, C.; Conti, N.; Di Tommaso, M.; Severi, F.M.; Petraglia, F. Anterior placental location influences onset and progress the labor and postpartum outcome. Placenta 2015, 36, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.M.; Landon, M.B.; Rouse, D.J.; Leveno, K.J.; Spong, C.Y.; Thom, E.A.; Moawad, A.H.; Caritis, S.N.; Harper, M.; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network; et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 2006, 107, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; You, J.Y.; Choi, S.J.; Oh, S.Y.; Kim, J.H.; Roh, C.R. A combined ultrasound and clinical scoring model for the prediction of peripartum complications in pregnancies complicated by placenta previa. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 180, 111–115. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Aitallah, A.; Abdelghafar, H.M.; Alsammani, M.A. Major Placenta Previa: Rate, Maternal and Neonatal Outcomes Experience at a Tertiary Maternity Hospital, Sohag, Egypt: A Prospective study. J. Clin. Diagn. Res. 2015, 9, Qc17–Qc19. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Wang, W.; Li, H.; Haung, X.; Wang, J.; Han, J.; Zhu, H. Effect of placental location detected by ultrasound on the severity of placenta accreta spectrum in patients with placenta previa and placenta accreta spectrum. BMC Pregnancy Childbirth 2023, 23, 406. [Google Scholar] [CrossRef]
- Keita, H.; Satoru, I.; Yuya, T.; Maki, O.; Toyohide, E.; Yu, S.; Ryota, I.; Yoshifumi, K.; Daigo, O.; Mamoru, T. Ultrasonographic prediction of placental invasion in placenta previa by placenta accreta index. J. Clin. Med. 2023, 12, 1090. [Google Scholar]
- Jing, L.; Wei, G.; Mengfan, S.; Yanyan, H. Effect of site of placentation on pregnancy outcomes in patients with placenta previa. PLoS ONE 2018, 13, e0200252. [Google Scholar] [CrossRef]
- Jauniaux, E.; Hussein, A.M.; Fox, K.A.; Collins, S.L. New evidence-based diagnostic and management strategies for placenta accrete spectrum disorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 61, 75–88. [Google Scholar] [CrossRef]
- Do, Q.N.; Lewis, M.A.; Xi, Y.; Madhuranthakam, A.J.; Happe, S.K.; Dashe, J.S.; Lenkinski, R.E.; Khan, A.; Twickler, D.M. MRI of the Placenta Accreta Spectrum (PAS) Disorder: Radiomics Analysis Correlates with Surgical and Pathological Outcome. J. Magn. Reson. Imaging 2020, 51, 936–946. [Google Scholar] [CrossRef]
- Palacios-Jaraquemada, J.M.; Bruno, C.H. Utility of MRI in Placenta Accreta Spectrum for the Surgical Team. AJR Am. J. Roentgenol. 2021, 217, 1257. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Lacovella, C.; Bhide, A. Prenatal identification of invasive placentation using ultrasound: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013, 42, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Kingdom, J.C.; Silver, R.M. A comparison of recent guidelines in the diagnosis and management of placenta accrete spectrum disdorders. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 72, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Alfirevic, Z.; Bhide, A.G.; Belfort, M.A.; Burton, G.J.; Collins, S.L.; Dornan, S.; Jurkovic, D.; Kayem, G.; Royal College of Obstetricians and Gynecologists; et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-Top Guideline No.27a; Royal College of Obstetricians and Gynaecologists: London, UK, 2019; Volume 126, pp. e1–e48. [Google Scholar]
- Jain, V.; Bos, H.; Bujold, E. Guideline No. 402: Diagnosis and Management of Placenta Previa. J. Obstet. Gynaecol. Can. 2020, 42, 906–917.e901. [Google Scholar] [CrossRef]
| Characteristic | Location of the Placenta | p-Value | |
|---|---|---|---|
| Anterior, n = 209 | Posterior, n = 572 | ||
| Maternal age (years) | 33.2 ± 4.2 | 33.5 ± 3.9 | 0.49 |
| Abortion (%) | 100 (47.8%) | 228 (39.9%) | 0.045 |
| History of delivery 1 (%) | 124 (59.3%) | 275 (48.1%) | 0.005 |
| Parity (%) | <0.001 | ||
| 0 | 85 (40.7%) | 297 (51.9%) | |
| 1 | 87 (41.6%) | 231 (40.4%) | |
| ≥2 | 37 (17.7%) | 44 (7.7%) | |
| IUP 2 (%) | 157 (75.1%) | 363 (63.5%) | 0.002 |
| Number of IUPs (%) | <0.001 | ||
| 0 | 52 (24.9%) | 209 (36.5%) | |
| 1 | 60 (28.7%) | 182 (31.8%) | |
| ≥2 | 97 (46.4%) | 181 (31.6%) | |
| Gestational age at delivery (day) | 253.0 ± 21.6 | 257.6 ± 19.1 | 0.008 |
| Pre-pregnancy BMI (kg/m2) | 21.3 ± 2.8 | 21.1 ± 2.9 | 0.43 |
| CS Indication (%) | 0.015 | ||
| Bleeding | 72 (34.4%) | 150 (26.2%) | |
| Labor | 20 (9.6%) | 45 (7.9%) | |
| Fetal distress | 11 (5.3%) | 17 (3.0%) | |
| Elective | 89 (42.6%) | 322 (56.3%) | |
| Others | 17 (8.1%) | 38 (6.6%) | |
| Previous CS (%) | 73 (34.9%) | 114 (19.9%) | <0.001 |
| Admission for bleeding (%) | 95 (45.5%) | 210 (36.7%) | 0.027 |
| Previa type (%) | 0.218 | ||
| Low lying | 78 (37.3%) | 188 (32.9%) | |
| Marginal | 27 (12.9%) | 111 (19.4%) | |
| Partialis | 22 (10.5%) | 66 (11.5%) | |
| Complete | 81 (38.8%) | 205 (35.8%) | |
| Vasa previa | 1 (0.5%) | 2 (0.3%) | |
| Previous placenta previa (%) | 3 (1.4%) | 6 (1.0%) | 0.707 |
| Previous uterine surgery except CS (%) | 4 (1.9%) | 12 (2.1%) | >0.999 |
| Previous PPH (%) | 1 (0.5%) | 1 (0.2%) | 0.464 |
| Characteristic | Location of the Placenta | p-Value | |
|---|---|---|---|
| Anterior, n = 209 | Posterior, n = 572 | ||
| Preop Hgb (mg/dL) | 11.3 ± 1.5 | 11.4 ± 1.4 | 0.47 |
| POD#1 Hgb (mg/dL) | 10.1 ± 1.6 | 10.4 ± 1.6 | 0.056 |
| POD#3 Hgb (mg/dL) | 9.3 ± 1.4 | 9.5 ± 1.4 | 0.17 |
| Transfusion (%) | 120 (57.4%) | 210 (36.7%) | <0.001 |
| >3 units of packed RBCs (%) | 38 (34.9%) | 32 (17.8%) | 0.001 |
| >5 units of packed RBCs (%) | 25 (22.9%) | 17 (9.4%) | 0.002 |
| >10 units of packed RBCs (%) | 11 (10.1%) | 1 (0.6%) | <0.001 |
| EBL (cc) | 974.9 ± 1287.2 | 639.4 ± 450.4 | <0.001 |
| PAS 1 (%) | 40 (19.1%) | 50 (8.8%) | <0.001 |
| Placental abruption (%) | 6 (2.9%) | 12 (2.1%) | 0.59 |
| PPH (%) | 14 (14.7%) | 31 (8.8%) | 0.085 |
| DIC (%) | 3 (1.4%) | 2 (0.3%) | 0.122 |
| ICU admission (%) | 0 (0.0%) | 2 (0.3%) | >0.999 |
| Characteristic | Location of the Placenta | p-Value | |
|---|---|---|---|
| Anterior, n = 209 | Posterior, n = 572 | ||
| Birth weight (g) | 2689.3 ± 704.9 | 2815.7 ± 623.5 | 0.038 |
| Neonatal sex (%) | 0.795 | ||
| Male | 50 (52.6%) | 181 (51.1%) | |
| Female | 45 (47.4%) | 173 (48.9%) | |
| Apgar score < 7 (1 min) (%) | 58 (27.8%) | 138 (24.1%) | 0.301 |
| Apgar score < 7 (5 min) (%) | 21 (10.0%) | 40 (7.0%) | 0.159 |
| Fetal presentation (%) | <0.001 | ||
| Vertex | 74 (79.6%) | 327 (92.4%) | |
| Non-vertex (Breech, transverse position) | 19 (20.4%) | 27 (7.6%) | |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Transfusion | 2.23 | 1.50–3.33 | <0.001 |
| >3 units packed RBCs | 1.67 | 0.85–3.39 | 0.14 |
| >5 units packed RBCs | 1.82 | 0.81–4.4 | 0.15 |
| PAS | 2.16 | 1.21–3.97 | 0.009 |
| Admission for bleeding | 1.3 | 0.88–1.94 | 0.19 |
| Non-vertex fetal presentation | 2.47 | 1.09–5.88 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.U.; Jo, J.H.; Lee, H.; Na, Y.; Park, I.Y. A Multicenter, Retrospective Comparison Study of Pregnancy Outcomes According to Placental Location in Placenta Previa. J. Clin. Med. 2024, 13, 675. https://doi.org/10.3390/jcm13030675
Lee SU, Jo JH, Lee H, Na Y, Park IY. A Multicenter, Retrospective Comparison Study of Pregnancy Outcomes According to Placental Location in Placenta Previa. Journal of Clinical Medicine. 2024; 13(3):675. https://doi.org/10.3390/jcm13030675
Chicago/Turabian StyleLee, Seon Ui, Ji Hye Jo, Haein Lee, Yoojin Na, and In Yang Park. 2024. "A Multicenter, Retrospective Comparison Study of Pregnancy Outcomes According to Placental Location in Placenta Previa" Journal of Clinical Medicine 13, no. 3: 675. https://doi.org/10.3390/jcm13030675
APA StyleLee, S. U., Jo, J. H., Lee, H., Na, Y., & Park, I. Y. (2024). A Multicenter, Retrospective Comparison Study of Pregnancy Outcomes According to Placental Location in Placenta Previa. Journal of Clinical Medicine, 13(3), 675. https://doi.org/10.3390/jcm13030675





