An Appraisal of the Evidence behind the Use of the CHRODIS Plus Initiative for Chronic Pain: A Scoping Review
Abstract
1. Introduction
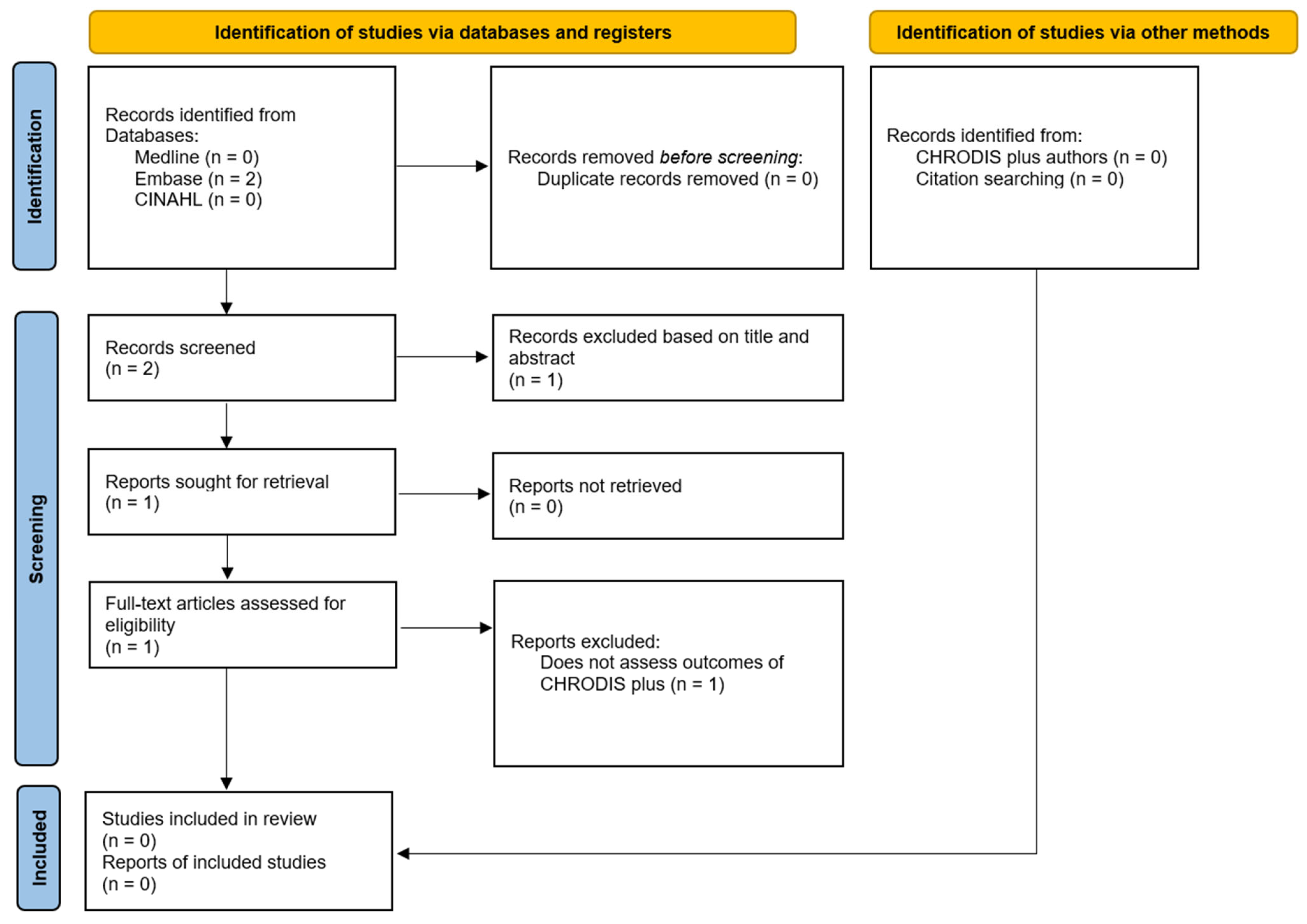
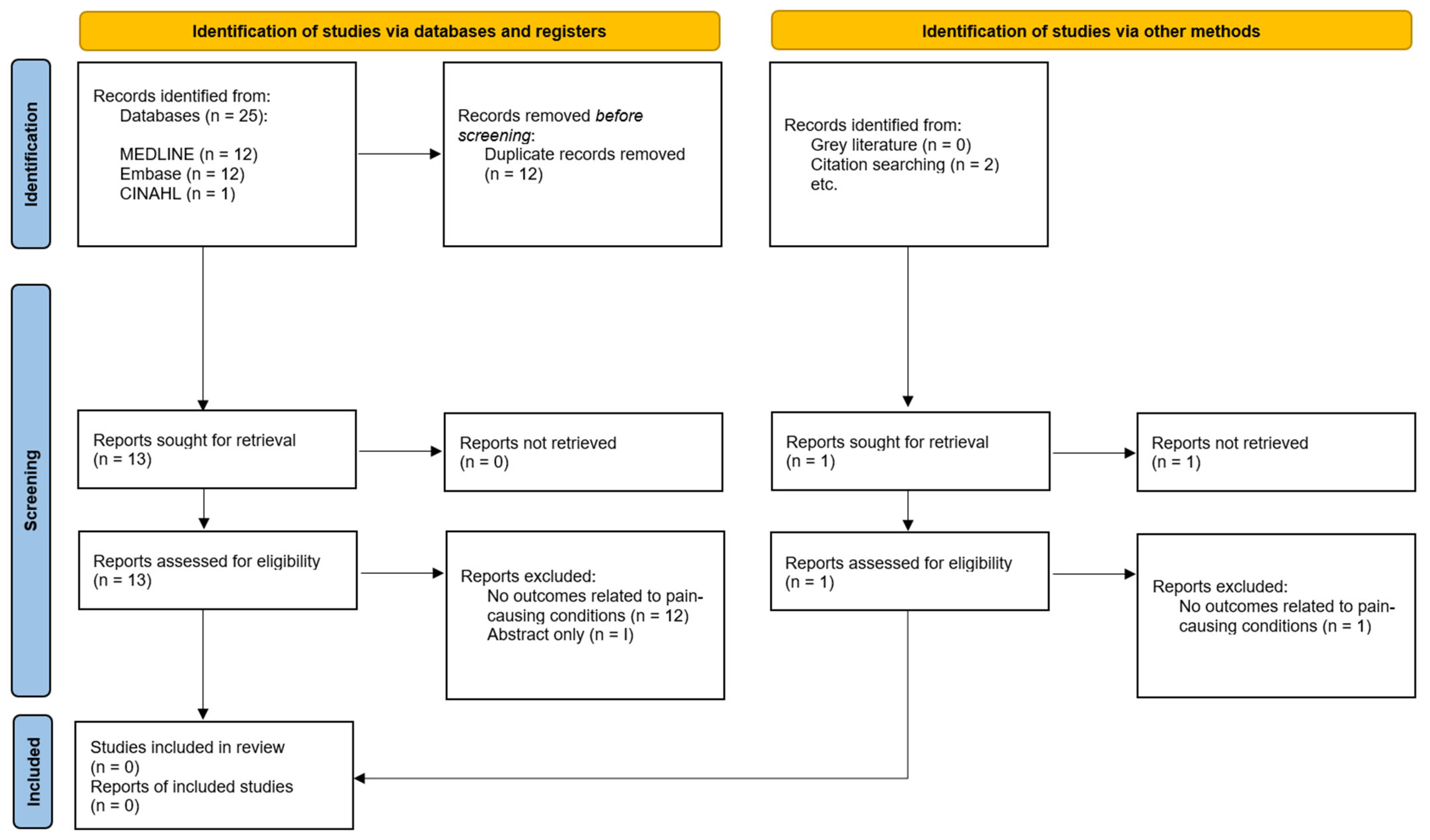
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Divo, M.J.; Martinez, C.H.; Mannino, D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Shibuya, K.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Thommasen, H.V.; Zhang, W. Impact of chronic disease on quality of life in the Bella Coola Valley. Rural Remote Health 2006, 6, 528. [Google Scholar] [CrossRef]
- Fouad, A.M.; Waheed, A.; Gamal, A.; Amer, S.A.; Abdellah, R.F.; Shebl, F.M. Effect of Chronic Diseases on Work Productivity: A Propensity Score Analysis. J. Occup. Environ. Med. 2017, 59, 480–485. [Google Scholar] [CrossRef]
- OECD/EU. Health at a Glance: Europe 2016; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Yang, H.; Haldeman, S.; Lu, M.L.; Baker, D. Low Back Pain Prevalence and Related Workplace Psychosocial Risk Factors: A Study Using Data From the 2010 National Health Interview Survey. J. Manip. Physiol. Ther. 2016, 39, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Joint Action CHRODIS. JA-CHRODIS Final Conference successfully ends the three-year Joint Action. In JA-CHRODIS Final Conference; Joint Action CHRODIS: Brussels, Belgium, 2017; Available online: http://chrodis.eu/wp-content/uploads/2015/02/ja-chrodis_the-last-update.pdf (accessed on 1 June 2022).
- Rantala, E.; Lindström, J.; Valve, P.; Leonardi, M.; Silvaggi, F.; Scaratti, C. The Chrodis Plus Workbox on Employment and Chronic Conditions. Helsinki. 2020. Available online: https://workbox.chrodis.eu/staging/ (accessed on 1 June 2022).
- Murray, C.J.L.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Abraham, J.; Alvarado, M.; Baddour, L.M.; Bartels, D.H.; et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Farquharson, R.; Carroll, D.; Moore, A.; Phillips, C.J.; Taylor, R.S.; Barden, J. The Impact and Burden of Chronic Pain in the Workplace: A Qualitative Systematic Review. Pain Pract. 2012, 12, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Wegrzynek, P.A.; Wainwright, E.; Ravalier, J. Return to work interventions for chronic pain: A systematic review. Occup. Med. 2020, 70, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Treweek, S.; Bevan, S.; Bower, P.; Briel, M.; Campbell, M.; Christie, J.; Collett, C.; Cotton, S.; Devane, D.; El Feky, A.; et al. Trial Forge Guidance 2: How to decide if a further Study within a Trial (SWAT) is needed. Trials 2020, 21, 33. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Rafael, B.; Milton, C.; Stefan, E.; Nanna, B.F.; Michael, B.F.; et al. A classification of chronic pain for ICD-11. Pain 2015, 156, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, S.; Manuwald, U.; Leonardi, M.; Silvaggi, F.; Foucaud, J.; Lamore, K.; Guastafierro, E.; Scaratti, C.; Lindström, J.; Rothe, U. Chronic Diseases and Employment: Which Interventions Support the Maintenance of Work and Return to Work among Workers with Chronic Illnesses? A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1864. [Google Scholar] [CrossRef] [PubMed]
- Lamore, K.; Dubois, T.; Rothe, U.; Leonardi, M.; Girard, I.; Manuwald, U.; Nazarov, S.; Silvaggi, F.; Guastafierro, E.; Scaratti, C.; et al. Return to Work Interventions for Cancer Survivors: A Systematic Review and a Methodological Critique. Int. J. Environ. Res. Public Health 2019, 16, 1343. [Google Scholar] [CrossRef] [PubMed]
- Proper, K.I.; van Oostrom, S.H. The effectiveness of workplace health promotion interventions on physical and mental health outcomes—A systematic review of reviews. Scand. J. Work Env. Health 2019, 45, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R.; et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Oprešnik, D.; Somekh, D.; Ninov, L.; Zaletel, J. D7.2 Guide for the Implementation of JA CHRODIS Recommendations and Criteria (QCR) to Improve the Quality of Care for People with Chronic Diseases. 2020. Available online: http://chrodis.eu/wp-content/uploads/2021/03/d7.2_guide-for-implementation-of-qcr_chrodis-plus_final.pdf (accessed on 1 June 2022).
- Scascighini, L.; Toma, V.; Dober-Spielmann, S.; Sprott, H. Multidisciplinary treatment for chronic pain: A systematic review of interventions and outcomes. Rheumatology 2008, 47, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Alamo, M.M.; Moral, R.R.; Pérula de Torres, L.A. Evaluation of a patient-centred approach in generalized musculoskeletal chronic pain/fibromyalgia patients in primary care. Patient Educ. Couns. 2002, 48, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blazquez, C.; João Forjaz, M.; Gimeno-Miguel, A.; Bliek-Bueno, K.; Poblador-Plou, B.; Pilar Luengo-Broto, S.; de Alba, I.G.-F.; Carriazo, A.M.; Lama, C.; Rodríguez-Acuña, R.; et al. Assessing the Pilot Implementation of the Integrated Multimorbidity Care Model in Five European Settings: Results from the Joint Action CHRODIS-PLUS. Int. J. Environ. Res. Public Health 2020, 17, 5268. [Google Scholar] [CrossRef] [PubMed]
- Luquini, A.; Zheng, Y.; Xie, H.; Backman, C.; Rogers, P.; Kwok, A.; Knight, A.; Gignac, M.; Mosher, D.; Li, L.; et al. OP0010 Effectiveness of the making it worktm program at improving presenteeism and work cessation in workers with inflammatory arthritis—Results of a randomized controlled trial. Ann. Rheum. Dis. 2020, 79 (Suppl. 1), 7. [Google Scholar] [CrossRef]
- Tran, K.; Xiang, X.; Seah, X.; van As, B.; Rogers, P.; Backman, C.; Gignac, M.; Li, L.; Esdaile, J.; Lacaille, D. Process Evaluation of Employment and Arthritis: Making it Work, an Online Program to Help People with Inflammatory Arthritis Remain Employed. J. Rheumatol. 2018, 45, 964–1069. [Google Scholar] [CrossRef]
- Blake, H.; Somerset, S.; Greaves, S. The Pain at Work Toolkit for Employees with Chronic or Persistent Pain: A Collaborative-Participatory Study. Healthcare 2021, 10, 56. [Google Scholar] [CrossRef]
- Pain Alliance Europe. PAE SURVEY 2018 Impact of Chronic Pain on Work Life and Employment. In CHRODIS+ Conference; CHRODIS Plus: Budapest, Hungary, 2018; Available online: https://www.sip-platform.eu/resources/details/pain-alliance-europe-pae-at-chrodis-plus-conference-in-budapest (accessed on 1 June 2022).
| Certainty of Evidence | Using Grading of Recommendations, Assessment, Development, and Evaluations (GRADE), Certainty of Evidence is Lower than ‘High’ [16]. |
| Cumulated Evidence | The effect estimates for each outcome needed to make informed decision have not converged. |
| Study Contexts Do Not Translate to Local Clinical Context | Population in the local context is so different from those described that the evidence does not provide sufficient certainty. |
| Interventions in the local context differ sufficiently from those described so as to not provide sufficient certainty. | |
| Comparator in the local context is so different from those described that it does not provide sufficient certainty. | |
| Outcome is so different to those considered relevant in the local context that evidence does not provide sufficient certainty. | |
| Time since the existing evaluations were conducted means that evaluations are less relevant. | |
| Balance for the Patients | Balance of benefits and disadvantages (i.e., risk–benefit analysis) at the patient level in the local context is not clear. |
| Balance for the System | Balance of benefits and disadvantages (i.e., cost–benefit analysis) at the system level in the local community/organisation is not clear. |
| Research Question and Inclusion Criteria Include PICO Components | A Priori Design | Justification of Included Study Designs | Comprehensive Literature Search Strategy | Study Selection Performed in Duplicate | Data Extraction Performed in Duplicate | List of Excluded Studies with Justifications | Included Studies Described in Adequate Detail | Satisfactory Technique to Assess Risk of Bias | Report on Funding Sources in Studies | Appropriate Method for Statistical Combination | Impact of RoB on Meta-Analysis Results | Account for RoB in Individual Studies When Interpreting Results | Explanation of Heterogeneity in Results | Assessed Publication Bias | Reported Conflicts of Interest | Overall Quality | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nazarov et al., 2019 [18] | Yes | Partial yes | Yes | Yes | Yes | No | Yes | Partial yes | Partial yes | No | No meta-analysis | No meta-analysis | No | No | No meta- analysis | Yes | Low |
| Lamore et al., 2019 [19] | No | No | Yes | Yes | No | No | Yes | Partial yes | Partial yes | No | No meta-analysis | No meta-analysis | No | Yes | No meta-analysis | Yes | Critically low |
| Proper et al., 2019 [20] | No | Partial yes | Yes | Yes | Yes | No | Yes | No | Partial yes | Yes | No meta-analysis | No meta-analysis | Yes | Yes | No meta-analysis | Yes | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilley, R.; Wainwright, E.; Forget, P. An Appraisal of the Evidence behind the Use of the CHRODIS Plus Initiative for Chronic Pain: A Scoping Review. J. Clin. Med. 2024, 13, 686. https://doi.org/10.3390/jcm13030686
Lilley R, Wainwright E, Forget P. An Appraisal of the Evidence behind the Use of the CHRODIS Plus Initiative for Chronic Pain: A Scoping Review. Journal of Clinical Medicine. 2024; 13(3):686. https://doi.org/10.3390/jcm13030686
Chicago/Turabian StyleLilley, Ross, Elaine Wainwright, and Patrice Forget. 2024. "An Appraisal of the Evidence behind the Use of the CHRODIS Plus Initiative for Chronic Pain: A Scoping Review" Journal of Clinical Medicine 13, no. 3: 686. https://doi.org/10.3390/jcm13030686
APA StyleLilley, R., Wainwright, E., & Forget, P. (2024). An Appraisal of the Evidence behind the Use of the CHRODIS Plus Initiative for Chronic Pain: A Scoping Review. Journal of Clinical Medicine, 13(3), 686. https://doi.org/10.3390/jcm13030686






