Multiple Cardiac Diseases Involving the Aortic Arch: Beating Heart Debranching, and Normothermic Arch Replacement: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
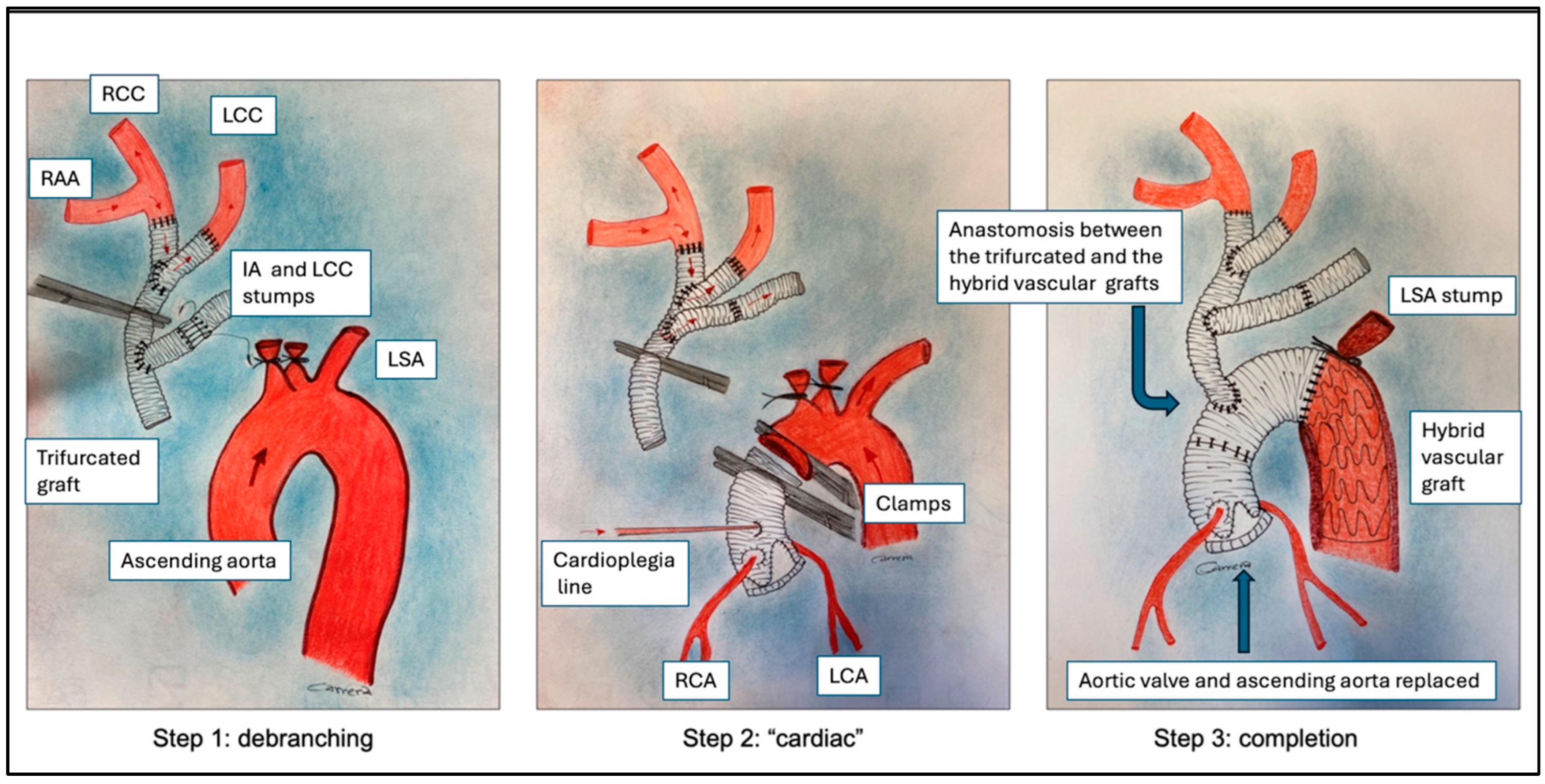
2.2. Surgical Technique
2.3. Definition of Events
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishimura, M.; Ohtake, S.; Sawa, Y.; Takahashi, T.; Matsumiya, G.; Kagisaki, K.; Miyamoto, Y.; Matsuda, H. Arch-first technique for aortic arch aneurysm repair through median sternotomy. Ann. Thorac. Surg. 2002, 74, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.J.; Kanagarajah, A.R.; Seevanayagam, S.; Kim, M.; Matalanis, G. Branch-first Continuous Perfusion Aortic Arch Replacement: Midterm Results. Ann. Thorac. Surg. 2023, 116, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Matalanis, G.; Perera, N.K.; Galvin, S.D. Aortic arch replacement without circulatory arrest or deep hypothermia: The “branch-first” technique. J. Thorac. Cardiovasc. Surg. 2015, 149, S76–S82. [Google Scholar] [CrossRef] [PubMed]
- Gallingani, A.; Venturini, A.; Scarpanti, M.; Mangino, D.; Formica, F. Frozen Elephant Trunk: Technical Overview and Our Experience with a Patient-Tailored Approach. J. Clin. Med. 2022, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease. J. Thorac. Cardiovasc. Surg. 2023, 166, e182–e331. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Fittipaldi, A.; Giambuzzi, I.; Redaelli, P.; Verzini, A.; Cambiaghi, T.; Bargagna, M.; Alfieri, O.; Chiesa, R.; Castiglioni, A. Preliminary Results of Debranch-First Technique in Frozen Elephant Trunk Procedures. Ann. Thorac. Surg. 2019, 108, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y. Neuro-protection in open arch surgery. Ann. Cardiothorac. Surg. 2018, 7, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tsagakis, K.; Pacini, D.; Grabenwöger, M.; Borger, M.A.; Goebel, N.; Hemmer, W.; Santos, A.L.; Sioris, T.; Widenka, K.; Risteski, P.; et al. Results of frozen elephant trunk from the international E-vita Open registry. Ann. Cardiothorac. Surg. 2020, 9, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Jakob, H.; Tsagakis, K. International E-vita open registry. Ann. Cardiothorac. Surg. 2013, 2, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.H.; Weller, J.; Hasmat, S.; Preventza, O.; Forrest, P.; Kiat, H.; Yan, T.D. Temperature Selection in Antegrade Cerebral Perfusion for Aortic Arch Surgery: A Meta-Analysis. Ann. Thorac. Surg. 2019, 108, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Matalanis, G. Illustrated Technique of “Branch-First” Total Aortic Arch Replacement. Oper. Tech. Thorac. Cardiovasc. Surg. 2022, 27, 23–38. [Google Scholar] [CrossRef]
- Rylski, B.; Szeto, W.Y.; Bavaria, J.E.; Branchetti, E.; Moser, W.; Milewski, R.K. Development of a Single Endovascular Device for Aortic Valve Replacement and Ascending Aortic Repair. J. Card. Surg. 2014, 29, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Juraszek, A.; Czerny, M.; Rylski, B. Update in aortic dissection. Trends Cardiovasc. Med. 2021, 32, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Marco, L.; Murana, G.; Lovato, L.; Gliozzi, G.; Buia, F.; Attinà, D.; Pacini, D. Endovascular Solutions for Aortic Arch Diseases: Total and Hybrid. Surg. Technol. Online 2021, 38, 331–338. [Google Scholar] [CrossRef] [PubMed]
| Variables | Patients (n = 12) |
|---|---|
| Age (years), mean (SD) | 61 (6.7) |
| Male (%) | 6 (50) |
| LVEF, mean (SD) | 56.6 (6.8) |
| Obesity (%) | 1 (8.3) |
| Hypertension (%) | 7 (66) |
| CAD (%) | 3 (25) |
| Malperfusion (%) | 1 (8.3) |
| Stroke (%) | 1 (8.3) |
| Malperfusion syndrome (%) | 2 (8.3) |
| Euroscore II, mean (SD) | 10.1 (7.4) |
| Aortic disease | |
| PAU | 4 (33.3) |
| Type A acute aortic dissection (%) | 4 (33.3) |
| Chronic aortic dissection | 2 (16.6) |
| Aortic aneurysm with arch involvement | 2 (16.6) |
| Variables | Patients (n = 12) |
|---|---|
| Emergency (%) | 8 (66.6) |
| Reintervention (%) | 4 (33.3) |
| Bentall (%) | 4 (33.3) |
| David (%) | 3 (25%) |
| CABG (%) | 3 (25) |
| Ascending Aorta replacement (%) | 3 (25 |
| Operation time, minutes (SD) | 467 (33) |
| CPB time, minutes (SD) | 206 (30.7) |
| Aortic-Clamp time, minutes (SD) | 93 (41.7) |
| CA time, minutes (SD) | 14.3 (9.6) |
| Nasopharyngeal Temperature, °C (SD) | 34 (0.6) |
| Variables | Patients (n = 12) |
|---|---|
| Early mortality (%) | 1 (8.3) |
| Stroke (%) | 0 |
| TND (%) | 1 (8.3) |
| Reoperation for postoperative bleeding (%) | 0 |
| AKI (%) | 0 |
| Ventilation time, days (SD) | 2.8 (2.1) |
| Transfusion with RBC (%) | 11 (91.6) |
| ICU stay, days (SD) | 7.5 (4.2) |
| Hospital stay, days (SD) | 13.7 (7.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, A.; Scarpari, C.; Borrelli, E.; Formica, F. Multiple Cardiac Diseases Involving the Aortic Arch: Beating Heart Debranching, and Normothermic Arch Replacement: A Case Series. J. Clin. Med. 2024, 13, 732. https://doi.org/10.3390/jcm13030732
Motta A, Scarpari C, Borrelli E, Formica F. Multiple Cardiac Diseases Involving the Aortic Arch: Beating Heart Debranching, and Normothermic Arch Replacement: A Case Series. Journal of Clinical Medicine. 2024; 13(3):732. https://doi.org/10.3390/jcm13030732
Chicago/Turabian StyleMotta, Alessandro, Cristian Scarpari, Ermelinda Borrelli, and Francesco Formica. 2024. "Multiple Cardiac Diseases Involving the Aortic Arch: Beating Heart Debranching, and Normothermic Arch Replacement: A Case Series" Journal of Clinical Medicine 13, no. 3: 732. https://doi.org/10.3390/jcm13030732





