Prostate Cancer Liver Metastasis: An Ominous Metastatic Site in Need of Distinct Management Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
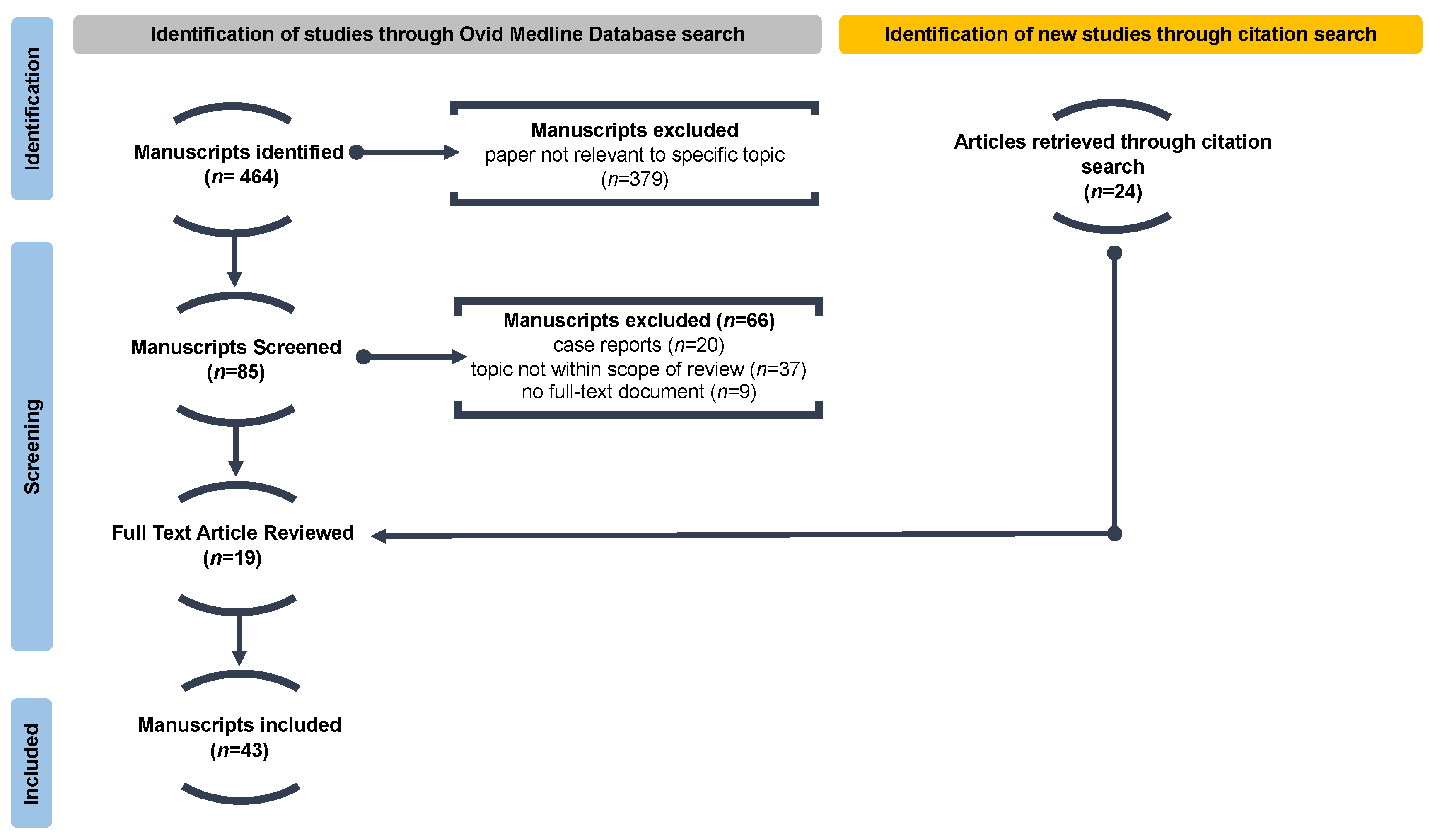
2.2. Article Screening and Selection
3. Results
3.1. Clinicopathological Characteristics Associated with Liver Metastasis
3.2. Treatment-Emergent Prostate Cancer Liver Metastasis
3.2.1. Androgen-Deprivation Therapy, including First Generation Anti-Androgens
3.2.2. Second-Generation Anti-Androgens
3.2.3. Taxane Chemotherapy
3.3. Treatment-Induced Liver Injury
3.4. Biomarkers
3.4.1. Serum Markers
3.4.2. Genetic Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Piombino, C.; Oltrecolli, M.; Tonni, E.; Pirola, M.; Matranga, R.; Baldessari, C.; Pipitone, S.; Dominici, M.; Sabbatini, R.; Vitale, M.G. De Novo Metastatic Prostate Cancer: Are We Moving toward a Personalized Treatment? Cancers 2023, 15, 4945. [Google Scholar] [CrossRef]
- Helgstrand, J.T.; Røder, M.A.; Klemann, N.; Toft, B.G.; Lichtensztajn, D.Y.; Brooks, J.D.; Brasso, K.; Vainer, B.; Iversen, P. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer—A population-based analysis of 2 national cohorts. Cancer 2018, 124, 2931–2938. [Google Scholar] [CrossRef]
- Vellky, J.E.; Ricke, W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef]
- Wallace, K.L.; Landsteiner, A.; Bunner, S.H.; Engel-Nitz, N.M.; Luckenbaugh, A.N. Increasing prevalence of metastatic castration-resistant prostate cancer in a managed care population in the United States. Cancer Causes Control. 2021, 32, 1365–1374. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wheeler, S.E.; Clark, A.M.; Whaley, D.L.; Yang, M.; Wells, A. Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology 2016, 64, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wells, A.; Wei, L.; Zheng, J. Prostate cancer liver metastasis: Dormancy and resistance to therapy. Semin. Cancer Biol. 2020, 71, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Fong, C.; Luthra, A.; Smith, S.A.; DiNatale, R.G.; Nandakumar, S.; Walch, H.; Chatila, W.K.; Madupuri, R.; Kundra, R.; et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 2022, 185, 563–575.e11. [Google Scholar] [CrossRef]
- Liu, K.; Jing, N.; Wang, D.; Xu, P.; Wang, J.; Chen, X.; Cheng, C.; Xin, Z.; He, Y.; Zhao, H.; et al. A novel mouse model for liver metastasis of prostate cancer reveals dynamic tumour-immune cell communication. Cell Prolif. 2021, 54, e13056. [Google Scholar] [CrossRef]
- Pezaro, C.J.; Omlin, A.; Lorente, D.; Rodrigues, D.N.; Ferraldeschi, R.; Bianchini, D.; Mukherji, D.; Riisnaes, R.; Altavilla, A.; Crespo, M.; et al. Visceral disease in castration-resistant prostate cancer. Eur. Urol. 2013, 65, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
- Shou, J.; Zhang, Q.; Wang, S.; Zhang, D. The prognosis of different distant metastases pattern in prostate cancer: A population based retrospective study. Prostate 2018, 78, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Pouessel, D.; Gallet, B.; Bibeau, F.; Avancès, C.; Iborra, F.; Sénesse, P.; Culine, S. Liver metastases in prostate carcinoma: Clinical characteristics and outcome. BJU Int. 2006, 99, 807–811. [Google Scholar] [CrossRef]
- Alshalalfa, M.; Seldon, C.; Franco, I.; Vince, R.; Carmona, R.; Punnen, S.; Kaochar, S.; Dess, R.; Kishan, A.; Spratt, D.E.; et al. Clinicogenomic characterization of prostate cancer liver metastases. Prostate Cancer Prostatic Dis. 2022, 25, 366–369. [Google Scholar] [CrossRef]
- van den Bergh, G.P.; Kuppen, M.C.; Westgeest, H.M.; Mehra, N.; Gerritsen, W.R.; Aben, K.K.; van Oort, I.M.; van Moorselaar, R.J.; Somford, D.M.; van den Eertwegh, A.J.; et al. Incidence and survival of castration-resistant prostate cancer patients with visceral metastases: Results from the Dutch CAPRI-registry. Prostate Cancer Prostatic Dis. 2022, 26, 162–169. [Google Scholar] [CrossRef]
- Singh, A.; Cheedella, N.K.S.; Shakil, S.A.; Gulmi, F.; Kim, D.-S.; Wang, J.C. Liver metastases in prostate carcinoma represent a relatively aggressive subtype refractory to hormonal therapy and short-duration response to docetaxel monotherapy. World J. Oncol. 2015, 6, 265–269. [Google Scholar] [CrossRef]
- Goodman, O.B.; Flaig, T.W.; Molina, A.; A Mulders, P.F.; Fizazi, K.; Suttmann, H.; Li, J.; Kheoh, T.; de Bono, J.S.; I Scher, H. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013, 17, 34–39. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Chowdhury, S.; Loriot, Y.; Sternberg, C.N.; de Bono, J.S.; Tombal, B.; Carles, J.; Flaig, T.W.; Dorff, T.B.; Phung, D.; et al. Effect of Visceral Disease Site on Outcomes in Patients with Metastatic Castration-resistant Prostate Cancer Treated With Enzalutamide in the PREVAIL Trial. Clin. Genitourin. Cancer 2017, 15, 610–617.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, B.; Zhang, P.; Yao, Y.; Chang, J. Clinical characteristics and prognostic factors of prostate cancer with liver metastases. Tumor Biol. 2013, 35, 595–601. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2013, 74, 210–216. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef]
- Kadeerhan, G.; Xue, B.; Wu, X.-L.; Chen, W.-N.; Wang, D.-W. Incidence trends and survival of metastatic prostate cancer with bone and visceral involvement: 2010-2019 surveillance, epidemiology, and end results. Front. Oncol. 2023, 13, 1201753. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Sartor, O.; Gauna, D.C.; Herrmann, K.; de Bono, J.; Shore, N.; Chi, K.; Crosby, M.; Rodriguez, J.P.; Flechon, A.; Wei, X.; et al. Phase 3 trial of [177 Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). Madrid 2023, 34 (Suppl. 2), S1254–S1335. [Google Scholar]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia. Available online: www.covidence.org (accessed on 13 February 2023).
- Sweeney, C.J.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Overall Survival of Men with Metachronous Metastatic Hormone-sensitive Prostate Cancer Treated with Enzalutamide and Androgen Deprivation Therapy. Eur. Urol. 2021, 80, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.G.; Pang, L.; Khalid, F.; Poon, R.; Huang, H.H.; Chen, K.; Tay, K.J.; Lau, W.; Cheng, C.; Ho, H.; et al. Local and systemic morbidities of de novo metastatic prostate cancer in Singapore: Insight from 685 consecutive patients from a large prospective Uro-oncology registry. BMJ Open 2020, 10, e034331. [Google Scholar] [CrossRef] [PubMed]
- Yekedüz, E.; McKay, R.R.; Gillessen, S.; Choueiri, T.K.; Ürün, Y. Visceral Metastasis Predicts Response to New Hormonal Agents in Metastatic Castration-Sensitive Prostate Cancer. Oncologist 2023, 28, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Shimada, T.; Kano, H.; Kadomoto, S.; Makino, T.; Naito, R.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Androgen receptor signaling-targeted therapy and taxane chemotherapy induce visceral metastasis in castration-resistant prostate cancer. Prostate 2020, 81, 72–80. [Google Scholar] [CrossRef]
- Ghedini, P.; Bossert, I.; Zanoni, L.; Ceci, F.; Graziani, T.; Castellucci, P.; Ambrosini, V.; Massari, F.; Nobili, E.; Melotti, B.; et al. Liver metastases from prostate cancer at 11C-Choline PET/CT: A multicenter, retrospective analysis. Eur. J. Nucl. Med. 2017, 45, 751–758. [Google Scholar] [CrossRef]
- Alshalalfa, M.; Goglia, A.G.; Swami, N.; Nguyen, B.; Hougen, H.Y.; Khan, A.; Kishan, A.U.; Punnen, S.; Nguyen, P.L.; A Mahal, B.; et al. Determinants of widespread metastases and of metastatic tropism in patients with prostate cancer: A genomic analysis of primary and metastatic tumors. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 253.e21–253.e26. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Sakhuja, S.; Waterbor, J.; Pisu, M.; Altekruse, S.F. Racial/ethnic disparities in de novo metastases sites and survival outcomes for patients with primary breast, colorectal, and prostate cancer. Cancer Med. 2018, 7, 1183–1193. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-generation antiandrogens: From discovery to standard of care in castration resistant prostate cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr. Oncol. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Boufaied, N.; Hallal, T.; Feit, A.; de Polo, A.; Luoma, A.M.; Alahmadi, W.; Larocque, J.; Zadra, G.; Xie, Y.; et al. MYC drives aggressive prostate cancer by disrupting transcriptional pause release at androgen receptor targets. Nat. Commun. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Buie, J.D.; Camacho, J.; Sharma, P.; de Riese, W.T. Evolution of Androgen Deprivation Therapy (ADT) and Its New Emerging Modalities in Prostate Cancer: An Update for Practicing Urologists, Clinicians and Medical Providers. Res. Rep. Urol. 2022, 14, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Clinton, T.N.; Woldu, S.L.; Raj, G.V. Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer. Expert Opin. Pharmacother. 2017, 18, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Student, S.; Hejmo, T.; Poterała-Hejmo, A.; Leśniak, A.; Bułdak, R. Anti-androgen hormonal therapy for cancer and other diseases. Eur. J. Pharmacol. 2019, 866, 172783. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Y.; Lu, Y.-H.; Chen, C.-Y. Comparative Effectiveness of Abiraterone and Enzalutamide in Patients With Metastatic Castration-Resistant Prostate Cancer in Taiwan. Front. Oncol. 2022, 12, 822375. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Thaper, D.; Vahid, S.; Davies, A.; Ketola, K.; Kuruma, H.; Jama, R.; Nip, K.M.; Angeles, A.; Johnson, F.; et al. The master neural transcription factor brn2 is an androgen receptor–suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017, 7, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, L.; Yang, G.; Geng, C.; Luo, Y.; Wu, W.; Manyam, G.C.; Korentzelos, D.; Park, S.; Tang, Z.; et al. PARP inhibition suppresses GR-MYCN-CDK5-RB1-E2F1 signaling and neuroendocrine differentiation in castration-resistant prostate cancer. Clin. Cancer Res. 2019, 25, 6839–6851. [Google Scholar] [CrossRef]
- Dhavale, M.; Abdelaal, M.K.; Alam, A.B.M.N.; Blazin, T.; Mohammed, L.M.; Prajapati, D.; Ballestas, N.P.; A Mostafa, J. Androgen Receptor Signaling and the Emergence of Lethal Neuroendocrine Prostate Cancer With the Treatment-Induced Suppression of the Androgen Receptor: A Literature Review. Cureus 2021, 13, e13402. [Google Scholar] [CrossRef]
- Hu, C.-D.; Choo, R.; Huang, J. Neuroendocrine differentiation in prostate cancer: A mechanism of radioresistance and treatment failure. Front. Oncol. 2015, 5, 90. [Google Scholar] [CrossRef]
- Grigore, A.D.; Ben-Jacob, E.; Farach-Carson, M.C. Prostate cancer and neuroendocrine differentiation: More neuronal, less endocrine? Front. Oncol. 2015, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.K.; Abbasova, S.; Bevan, C.L.; Leach, D.A. Liver Microenvironment Response to Prostate Cancer Metastasis and Hormonal Therapy. Cancers 2022, 14, 6189. [Google Scholar] [CrossRef]
- Teply, B.A.; Qiu, F.; Antonarakis, E.S.; Carducci, M.A.; Denmeade, S.R. Risk of development of visceral metastases subsequent to abiraterone vs placebo: An analysis of mode of radiographic progression in COU-AA-302. Prostate 2019, 79, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Machioka, K.; Izumi, K.; Kadono, Y.; Iwamoto, H.; Naito, R.; Makino, T.; Kadomoto, S.; Natsagdorj, A.; Keller, E.T.; Zhang, J.; et al. Establishment and characterization of two cabazitaxel-resistant prostate cancer cell lines. Oncotarget 2018, 9, 16185–16196. [Google Scholar] [CrossRef] [PubMed]
- Seruga, B.; Ocana, A.; Tannock, I.F. Drug resistance in metastatic castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 2010, 8, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Natsagdorj, A.; Izumi, K.; Hiratsuka, K.; Machioka, K.; Iwamoto, H.; Naito, R.; Makino, T.; Kadomoto, S.; Shigehara, K.; Kadono, Y.; et al. CCL2 induces resistance to the antiproliferative effect of cabazitaxel in prostate cancer cells. Cancer Sci. 2018, 110, 279–288. [Google Scholar] [CrossRef] [PubMed]
- van Soest, R.J.; van Royen, M.E.; de Morrée, E.S.; Moll, J.M.; Teubel, W.; Wiemer, E.A.C.; Mathijssen, R.H.; de Wit, R.; van Weerden, W.M. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2013, 49, 3821–3830. [Google Scholar] [CrossRef]
- Hu, X.; Marietta, A.; Dai, W.; Li, Y.; Ma, X.; Zhang, L.; Cai, S.; Peng, J. Prediction of hepatic metastasis and relapse in colorectal cancers based on concordance analyses with liver fibrosis scores. Clin. Transl. Med. 2020, 9, 13. [Google Scholar] [CrossRef]
- Lee, J.W.; Beatty, G.L. Inflammatory networks cultivate cancer cell metastasis to the liver. Cell Cycle 2020, 19, 642–651. [Google Scholar] [CrossRef]
- Lee, J.W.; Stone, M.L.; Porrett, P.M.; Thomas, S.K.; Komar, C.A.; Li, J.H.; Delman, D.; Graham, K.; Gladney, W.L.; Hua, X.; et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 2019, 567, 249–252. [Google Scholar] [CrossRef]
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Flutamide. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548908/ (accessed on 18 October 2023).
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Bicalutamide. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547970/ (accessed on 18 October 2023).
- Colomba, E.; Marret, G.; Baciarello, G.; Lavaud, P.; Massard, C.; Loriot, Y.; Albiges, L.; Carton, E.; Alexandre, J.; Huillard, O.; et al. Liver tests increase on abiraterone acetate in men with metastatic prostate cancer: Natural history, management and outcome. Eur. J. Cancer 2020, 129, 117–122. [Google Scholar] [CrossRef]
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Abiraterone. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548136/ (accessed on 18 October 2023).
- Fleshner, L.; Berlin, A.; Hersey, K.; Kenk, M.; Lajkosz, K.; Nguyen, S.; Wise, J.; O’Halloran, S. Time trends of drug-specific actionable adverse events among patients on androgen receptor antagonists: Implications for remote monitoring. Can. Urol. Assoc. J. 2021, 16, E146–E149. [Google Scholar] [CrossRef]
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Apalutamide. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547950/ (accessed on 29 October 2023).
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Enzalutamide. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548070/ (accessed on 29 October 2023).
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Darolutamuide. Na-tional Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK590050/ (accessed on 29 October 2023).
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Docetaxel. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548899/ (accessed on 29 October 2023).
- Bethesda (MD). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Cabazitaxel. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548533/ (accessed on 29 October 2023).
- Gild, P.; Cole, A.P.; Krasnova, A.; Dickerman, B.A.; von Landenberg, N.; Sun, M.; Mucci, L.A.; Lipsitz, S.R.; Chun, F.K.-H.; Nguyen, P.L.; et al. Liver Disease in Men Undergoing Androgen Deprivation Therapy for Prostate Cancer. J. Urol. 2018, 200, 573–581. [Google Scholar] [CrossRef]
- Markowski, M.C.; Chen, Y.; Feng, Z.; Cullen, J.; Trock, B.J.; Suzman, D.; Antonarakis, E.S.; Paller, C.J.; Rosner, I.; Han, M.; et al. PSA Doubling Time and Absolute PSA Predict Metastasis-free Survival in Men With Biochemically Recurrent Prostate Cancer After Radical Prostatectomy. Clin. Genitourin. Cancer 2019, 17, 470–475.e1. [Google Scholar] [CrossRef] [PubMed]
- Verburg, F.A.; Pfister, D.; Heidenreich, A.; Vogg, A.; Drude, N.I.; Vöö, S.; Mottaghy, F.M.; Behrendt, F.F. Extent of disease in recurrent prostate cancer determined by [68Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Cotogno, P.M.; Ranasinghe, L.K.; Ledet, E.M.; Lewis, B.E.; Sartor, O. Laboratory-Based Biomarkers and Liver Metastases in Metastatic Castration-Resistant Prostate Cancer. Oncologist 2018, 23, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, L.; Cotogno, P.; Ledet, E.; Bordlee, B.; Degeyter, K.; Nguyen, N.; Steinberger, A.; Manogue, C.; Barata, P.; Lewis, B.E.; et al. Relationship between serum markers and volume of liver metastases in castration-resistant prostate cancer. Cancer Treat. Res. Commun. 2019, 20, 100151. [Google Scholar] [CrossRef]
- Bray, A.W.; Duan, R.; Malalur, P.; Drusbosky, L.M.; Gourdin, T.S.; Hill, E.G.; Lilly, M.B. Elevated serum CEA is associated with liver metastasis and distinctive circulating tumor DNA alterations in patients with castration-resistant prostate cancer. Prostate 2022, 82, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A.M.; Harzstark, A.L.; Corn, P.G.; Wen, S.; Araujo, J.C.; Tu, S.-M.; Pagliaro, L.C.; Kim, J.; Millikan, R.E.; Ryan, C.; et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 3621–3630. [Google Scholar] [CrossRef]
- Ploussard, G.; Rozet, F.; Roubaud, G.; Stanbury, T.; Sargos, P.; Roupret, M. Chromogranin A: A useful biomarker in castration-resistant prostate cancer. World J. Urol. 2022, 41, 361–369. [Google Scholar] [CrossRef]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbé, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef]
- Quintanal-Villalonga, Á.; Chan, J.M.; Yu, H.A.; Pe’er, D.; Sawyers, C.L.; Sen, T.; Rudin, C.M. Lineage plasticity in cancer: A shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 2020, 17, 360–371. [Google Scholar] [CrossRef]
- Zhou, Z.; Flesken-Nikitin, A.; Corney, D.C.; Wang, W.; Goodrich, D.W.; Roy-Burman, P.; Nikitin, A.Y. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006, 66, 7889–7898. [Google Scholar] [CrossRef]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple functions in human malignant tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Pifano, M.; Garona, J.; Capobianco, C.S.; Gonzalez, N.; Alonso, D.F.; Ripoll, G.V. Peptide agonists of vasopressin v2 receptor reduce expression of neuroendocrine markers and tumor growth in human lung and prostate tumor cells. Front. Oncol. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Khemlina, G.; Ikeda, S.; Kurzrock, R. Molecular landscape of prostate cancer: Implications for current clinical trials. Cancer Treat. Rev. 2015, 41, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.H.; Zhang, L.; Graf, R.; Raskina, K.; Tukachinsky, H.; Huang, R.S.; McGregor, K.; Alshalalfa, M.; Hougen, H.Y.; Khan, A.; et al. The Molecular, Immunologic, and Clinicodemographic Landscape of MYC-Amplified Advanced Prostate Cancer. Clin. Genitourin. Cancer 2023. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Shepard, C.R.; Stolz, D.B.; Wells, A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer 2007, 96, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Abiko, K.; Ukita, M.; Murakami, R.; Yamanoi, K.; Yamaguchi, K.; Hamanishi, J.; Baba, T.; Matsumura, N.; Mandai, M. Tumor Immune Microenvironment during Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2021, 27, 4669–4679. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Rajwa, P.; Kawada, T.; Mori, K.; Fukuokaya, W.; Petrov, P.; Quhal, F.; Laukhtina, E.; von Deimling, M.; Bianchi, A.; et al. Efficacy of Systemic Treatment in Prostate Cancer Patients With Visceral Metastasis: A Systematic Review, Meta-analysis, and Network Meta-analysis. J. Urol. 2023, 210, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Findakly, D.; Duong, T.; Shimon, T.; Wang, J. Treatment-Refractory, Castration-Resistant Prostate Cancer With Liver Metastasis: A Promising Modality of Therapy. Cureus 2022, 14, e26881. [Google Scholar] [CrossRef] [PubMed]
- Hino, D.; Sugano, T.; Kino, M.; Nakata, T.; Kito, H.; Inoue, M.; Fujie, H.; Akakura, K. Successful radiofrequency ablation of liver metastases from prostate cancer. IJU Case Rep. 2022, 5, 455–458. [Google Scholar] [CrossRef]
- Yeo, A.-E.; Hendrix, A.; Confente, C.; Christian, N.; Mansvelt, B.; Pairet, G.; Seront, E. Highlighting the Place of Metastasis-Directed Therapy in Isolated Liver Metastases in Prostate Cancer: A Case Report. Front. Oncol. 2021, 11, 764758. [Google Scholar] [CrossRef]
| 1st line treatment | CRPC (i.e., resistant to ADT + 1st Generation AR Inhibitors) | Study | PREVAIL/ Enzalutamide [26] (Beer et al., 2014) | COU-AA-302/ Abiraterone [27] (Ryan et al., 2013) | |
| % VM % LM | 12% 4% | patients with VM excluded NR | |||
| 2nd line treatment | CRPC + Resistant to NHA | Study | PSMAfore/ 177Lu-PSMA-617 [28] (Sartor et al., ESMO 2023) | PROfound/ Olaparib [29,30] (de Bono et al. and Hussain et al., 2020) * | |
| % VM % LM | NR 4% | 32% NR | |||
| 2nd line treatment | CRPC + Chemoresistant (but no exposure to NHA) | Study | AFFIRM/ Enzalutamide [31] (Scher et al., 2012) | COU-AA-301/ Abiraterone [32] (de Bono et al., 2012) | |
| % VM % LM | 23% 10% | 17.5% 10% | |||
| 3rd line treatment | CRPC + Chemoresistant + Resistant to NHA | Study | CARD/ Cabazitaxel [33] (de Wit et al., 2019) | VISION/ 177Lu-PSMA-617 [8] (Sartor et al., 2021) | PROfound/ Olaparib [29,30] (de Bono et al. and Hussain et al., 2020) * |
| % VM % LM | 18% 12% | 21% NR | 32% NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiner, A.; Sperandio, R.C.; Naimi, M.; Emmenegger, U. Prostate Cancer Liver Metastasis: An Ominous Metastatic Site in Need of Distinct Management Strategies. J. Clin. Med. 2024, 13, 734. https://doi.org/10.3390/jcm13030734
Shiner A, Sperandio RC, Naimi M, Emmenegger U. Prostate Cancer Liver Metastasis: An Ominous Metastatic Site in Need of Distinct Management Strategies. Journal of Clinical Medicine. 2024; 13(3):734. https://doi.org/10.3390/jcm13030734
Chicago/Turabian StyleShiner, Audrey, Rubens Copia Sperandio, Mahdi Naimi, and Urban Emmenegger. 2024. "Prostate Cancer Liver Metastasis: An Ominous Metastatic Site in Need of Distinct Management Strategies" Journal of Clinical Medicine 13, no. 3: 734. https://doi.org/10.3390/jcm13030734
APA StyleShiner, A., Sperandio, R. C., Naimi, M., & Emmenegger, U. (2024). Prostate Cancer Liver Metastasis: An Ominous Metastatic Site in Need of Distinct Management Strategies. Journal of Clinical Medicine, 13(3), 734. https://doi.org/10.3390/jcm13030734







