Heart Rate Variability as a Tool for Seizure Prediction: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
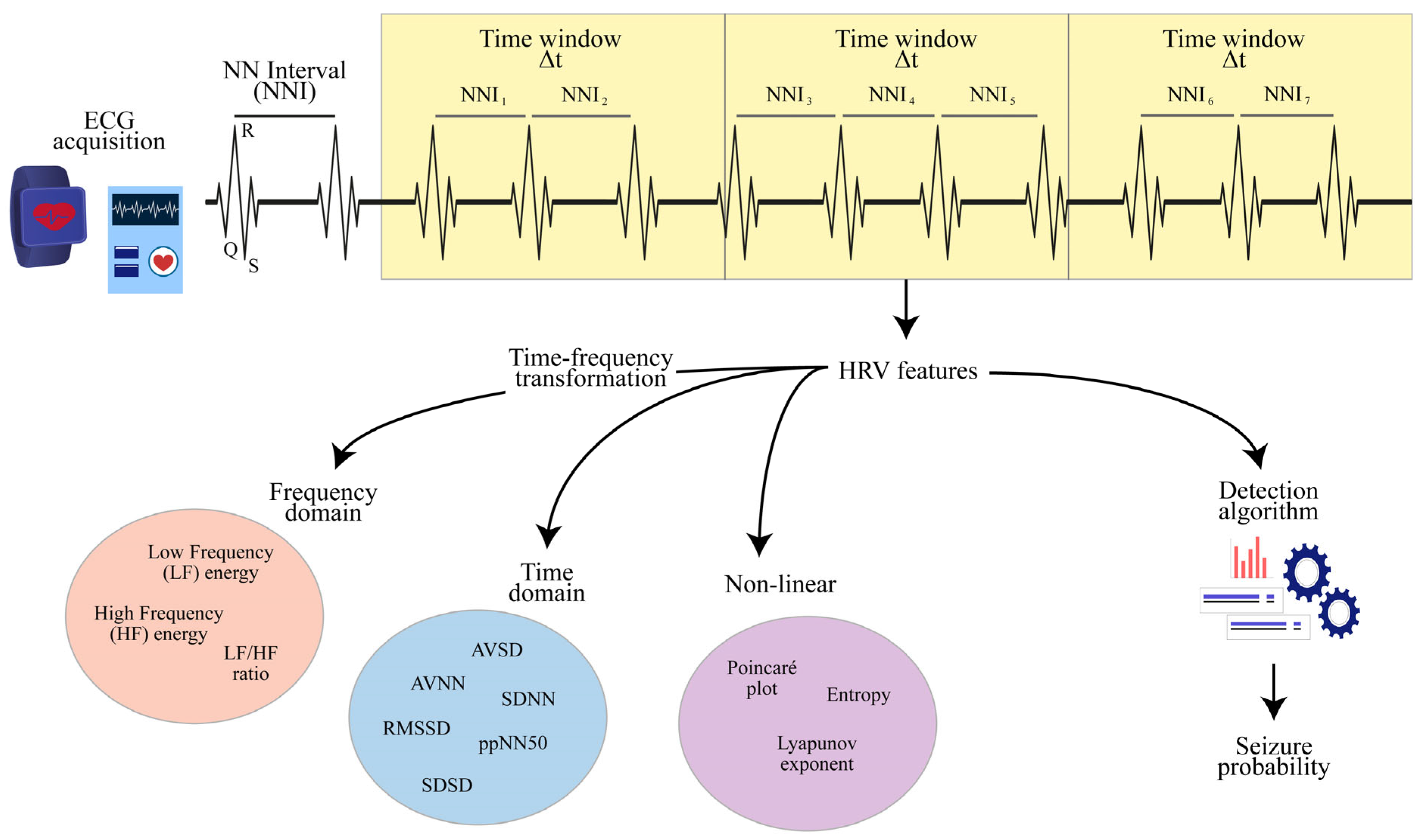
2.2. HRV Features
2.3. Detection Algorithms
3. Results
3.1. HRV Features
3.2. Detection Algorithms
3.3. Neonatal Population
3.4. Pediatric Population
3.5. Adult Population
3.6. Wearable Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- So, E.L. What is known about the mechanisms underlying SUDEP? Epilepsia 2008, 49, 93–98. [Google Scholar] [CrossRef]
- Guekht, A.B.; Mitrokhina, T.V.; Lebedeva, A.V.; Dzugaeva, F.K.; Milchakova, L.E.; Lokshina, O.B.; Feygina, A.A.; Gusev, E.I. Factors influencing on quality of life in people with epilepsy. Seizure 2007, 16, 128–133. [Google Scholar] [CrossRef]
- Stirling, R.E.; Cook, M.J.; Grayden, D.B.; Karoly, P.J. Seizure forecasting and cyclic control of seizures. Epilepsia 2021, 62, S2–S14. [Google Scholar] [CrossRef]
- Schernthaner, C.; Lindinger, G.; Pötzelberger, K.; Zeiler, K.; Baumgartner, C. Autonomic epilepsy—The influence of epileptic discharges on heart rate and rhythm. Wien. Klin. Wochenschr. 1999, 111, 392–401. [Google Scholar]
- Sevcencu, C.; Struijk, J.J. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010, 51, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Tinuper, P.; Bisulli, F.; Cerullo, A.; Carcangiu, R.; Marini, C.; Pierangeli, G.; Cortelli, P. Ictal bradycardia in partial epileptic seizures: Autonomic investigation in three cases and literature review. Brain 2001, 124, 2361–2371. [Google Scholar] [CrossRef]
- Stein, P.K.; Bosner, M.S.; Kleiger, R.E.; Conger, B.M. Heart rate variability: A measure of cardiac autonomic tone. Am. Heart J. 1994, 127, 1376–1381. [Google Scholar] [CrossRef]
- Cheshire, W.P.; Freeman, R.; Gibbons, C.H.; Cortelli, P.; Wenning, G.K.; Hilz, M.J.; Spies, J.M.; Lipp, A.; Sandroni, P.; Wada, N.; et al. Electrodiagnostic assessment of the autonomic nervous system: A consensus statement endorsed by the American Autonomic Society, American Academy of Neurology, and the International Federation of Clinical Neurophysiology. Clin. Neurophysiol. 2021, 132, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.H.; Martin, G.J.; Magid, N.; Weiss, J.S.; Schaad, J.W.; Kehoe, R.; Zheutlin, T.; Fintel, D.J.; Hsieh, A.M.; Lesch, M. Low heart rate variability and sudden cardiac death. J. Electrocardiol. 1988, 21, S46–S55. [Google Scholar] [CrossRef]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can wearable devices accurately measure heart rate variability? A systematic review. Folia Medica 2018, 60, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Cosoli, G.; Spinsante, S.; Scalise, L. Wrist-worn and chest-strap wearable devices: Systematic review on accuracy and metrological characteristics. Measurement 2020, 159, 107789. [Google Scholar] [CrossRef]
- Massetani, R.; Strata, G.; Galli, R.; Gori, S.; Gneri, C.; Limbruno, U.; Di Santo, D.; Mariani, M.; Murri, L. Alteration of cardiac function in patients with temporal lobe epilepsy: Different roles of EEG-ECG monitoring and spectral analysis of RR variability. Epilepsia 1997, 38, 363–369. [Google Scholar] [CrossRef]
- Tomson, T.; Ericson, M.; Ihrman, C.; Lindblad, L.E. Heart rate variability in patients with epilepsy. Epilepsy Res. 1998, 30, 77–83. [Google Scholar] [CrossRef]
- Zijlmans, M.; Flanagan, D.; Gotman, J. Heart rate changes and ECG abnormalities during epileptic seizures: Prevalence and definition of an objective clinical sign. Epilepsia 2002, 43, 847–854. [Google Scholar] [CrossRef]
- Opherk, C.; Coromilas, J.; Hirsch, L.J. Heart rate and EKG changes in 102 seizures: Analysis of influencing factors. Epilepsy Res. 2002, 52, 117–127. [Google Scholar] [CrossRef]
- Devinsky, O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr. 2004, 4, 43–46. [Google Scholar] [CrossRef]
- Leijten, F.S.; Consortium, D.T.; van Andel, J.; Ungureanu, C.; Arends, J.; Tan, F.; van Dijk, J.; Petkov, G.; Kalitzin, S.; Gutter, T. Multimodal seizure detection: A review. Epilepsia 2018, 59, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, M.; Gourvennec, B. Heart rate sensors acceptability: Data reliability vs. ease of use. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016; pp. 94–98. [Google Scholar]
- Statello, R.; Carnevali, L.; Sgoifo, A.; Miragoli, M.; Pisani, F. Heart rate variability in neonatal seizures: Investigation and implications for management. Neurophysiol. Clin. 2021, 51, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.A.; Sivathamboo, S.; Perucca, P. Heart rate variability measurement in epilepsy: How can we move from research to clinical practice? Epilepsia 2018, 59, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Arbune, A.A.; Jeppesen, J.; Ryvlin, P. Biomarkers of seizure severity derived from wearable devices. Epilepsia 2020, 61, S61–S66. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Lagae, L. Cardiac changes in epilepsy. Seizure 2010, 19, 455–460. [Google Scholar] [CrossRef]
- Mazzola, L.; Rheims, S. Ictal and Interictal Cardiac Manifestations in Epilepsy. A Review of Their Relation with an Altered Central Control of Autonomic Functions and With the Risk of SUDEP. Front. Neurol. 2021, 12, 642645. [Google Scholar] [CrossRef]
- van Westrhenen, A.; De Cooman, T.; Lazeron, R.H.C.; Van Huffel, S.; Thijs, R.D. Ictal autonomic changes as a tool for seizure detection: A systematic review. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2019, 29, 161–181. [Google Scholar] [CrossRef]
- Behbahani, S. A review of significant research on epileptic seizure detection and prediction using heart rate variability. Arch. Turk. Soc. Cardiol. 2018, 46, 414–421. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J. Heart rate variability. Clin. Cardiol. 1990, 13, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Brigham, E.O. The Fast Fourier Transform and Its Applications; Prentice-Hall, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Bentley, P.M.; McDonnell, J. Wavelet transforms: An introduction. Electron. Commun. Eng. J. 1994, 6, 175–186. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.; Van Roon, A.; Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2001, 48, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Bravi, A.; Longtin, A.; Seely, A.J. Review and classification of variability analysis techniques with clinical applications. Biomed. Eng. Online 2011, 10, 90. [Google Scholar] [CrossRef]
- Inouye, T.; Shinosaki, K.; Sakamoto, H.; Toi, S.; Ukai, S.; Iyama, A.; Katsuda, Y.; Hirano, M. Quantification of EEG irregularity by use of the entropy of the power spectrum. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 204–210. [Google Scholar] [CrossRef]
- Cencini, M.; Vulpiani, A. Finite size Lyapunov exponent: Review on applications. J. Phys. A Math. Theor. 2013, 46, 254019. [Google Scholar] [CrossRef]
- Henriques, T.; Ribeiro, M.; Teixeira, A.; Castro, L.; Antunes, L.; Costa-Santos, C. Nonlinear methods most applied to heart-rate time series: A review. Entropy 2020, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C. A tutorial on generalized linear models. J. Qual. Technol. 1997, 29, 274–291. [Google Scholar] [CrossRef]
- Kotsiantis, S.B.; Zaharakis, I.; Pintelas, P. Supervised machine learning: A review of classification techniques. Emerg. Artif. Intell. Appl. Comput. Eng. 2007, 160, 3–24. [Google Scholar]
- Grira, N.; Crucianu, M.; Boujemaa, N. Unsupervised and semi-supervised clustering: A brief survey. A Rev. Mach. Learn. Tech. Process. Multimed. Content 2004, 1, 9–16. [Google Scholar]
- Byvatov, E.; Schneider, G. Support vector machine applications in bioinformatics. Appl. Bioinform. 2003, 2, 67–77. [Google Scholar]
- Frassineti, L.; Lanata, A.; Manfredi, C. HRV analysis: A non-invasive approach to discriminate between newborns with and without seizures. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Conference, 1–5 November 2021; Volume 2021, pp. 52–55. [Google Scholar]
- Olmi, B.; Manfredi, C.; Frassineti, L.; Dani, C.; Lori, S.; Bertini, G.; Cossu, C.; Bastianelli, M.; Gabbanini, S.; Lanatà, A. Heart Rate Variability Analysis for Seizure Detection in Neonatal Intensive Care Units. Bioengineering 2022, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Frassineti, L.; Lanata, A.; Olmi, B.; Manfredi, C. Multiscale Entropy Analysis of Heart Rate Variability in Neonatal Patients with and without Seizures. Bioengineering 2021, 8, 122. [Google Scholar] [CrossRef]
- Malarvili, M.B.; Mesbah, M.; Boashash, B. Time-frequency analysis of heart rate variability for neonatal seizure detection. Australas. Phys. Eng. Sci. Med. 2006, 29, 67–72. [Google Scholar] [CrossRef]
- Statello, R.; Carnevali, L.; Alinovi, D.; Pisani, F.; Sgoifo, A. Heart rate variability in neonatal patients with seizures. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2018, 129, 2534–2540. [Google Scholar] [CrossRef]
- Malarvili, M.B.; Mesbah, M. Newborn seizure detection based on heart rate variability. IEEE Trans. Biomed. Eng. 2009, 56, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Frassineti, L.; Manfredi, C.; Olmi, B.; Lanata, A. A Generalized Linear Model for an ECG-based Neonatal Seizure Detector. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Conference, 1–5 November 2021; Volume 2021, pp. 471–474. [Google Scholar]
- Malarvili, M.B.; Mesbah, M. Combining newborn EEG and HRV information for automatic seizure detection. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; Volume 2008, pp. 4756–4759. [Google Scholar]
- Maruyama, S.; Jain, P.; Parbhoo, K.; Go, C.; Shibata, T.; Otsubo, H. Prolonged Video-EEG and Heart Rate Variability can Elucidate Autonomic Dysregulation in Infantile Apneic Seizures. Pediatr. Neurol. 2022, 127, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef] [PubMed]
- Pernice, R.; Faes, L.; Kotiuchyi, I.; Stivala, S.; Busacca, A.; Popov, A.; Kharytonov, V. Time, frequency and information domain analysis of short-term heart rate variability before and after focal and generalized seizures in epileptic children. Physiol. Meas. 2019, 40, 074003. [Google Scholar] [CrossRef] [PubMed]
- Giannakakis, G.; Tsiknakis, M.; Vorgia, P. Focal epileptic seizures anticipation based on patterns of heart rate variability parameters. Comput. Methods Programs Biomed. 2019, 178, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Schiecke, K.; Wacker, M.; Piper, D.; Benninger, F.; Feucht, M.; Witte, H. Time-variant, frequency-selective, linear and nonlinear analysis of heart rate variability in children with temporal lobe epilepsy. IEEE Trans. Biomed. Eng. 2014, 61, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Piper, D.; Schiecke, K.; Leistritz, L.; Pester, B.; Benninger, F.; Feucht, M.; Ungureanu, M.; Strungaru, R.; Witte, H. Synchronization analysis between heart rate variability and EEG activity before, during, and after epileptic seizure. Biomed. Technik. Biomed. Eng. 2014, 59, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Schiecke, K.; Pester, B.; Piper, D.; Benninger, F.; Feucht, M.; Leistritz, L.; Witte, H. Nonlinear Directed Interactions Between HRV and EEG Activity in Children with TLE. IEEE Trans. Biomed. Eng. 2016, 63, 2497–2504. [Google Scholar] [CrossRef]
- De Cooman, T.; Varon, C.; Van de Vel, A.; Jansen, K.; Ceulemans, B.; Lagae, L.; Van Huffel, S. Adaptive nocturnal seizure detection using heart rate and low-complexity novelty detection. Seizure 2018, 59, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Benninger, F.; Urak, L.; Plattner, B.; Geldner, J.; Feucht, M. EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology 2004, 63, 324–328. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, M.E.; Brown, J.K. Abnormalities in cardiac and respiratory function observed during seizures in childhood. Dev. Med. Child Neurol. 2005, 47, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, C.; Sinha, S.; Thennarasu, K.; Jagadisha, T. Quantitative analysis of heart rate variability in patients with absence epilepsy. Neurol. India 2011, 59, 25–29. [Google Scholar] [PubMed]
- Kolsal, E.; Serdaroğlu, A.; Cilsal, E.; Kula, S.; Soysal, A.Ş.; Kurt, A.N.; Arhan, E. Can heart rate variability in children with epilepsy be used to predict seizures? Seizure 2014, 23, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Mao, X.; Chen, Y.; Huang, L.; Liu, W.; Huang, X.; Tan, Z.; Wang, X.; Wu, W.; Chen, Q.; et al. The changes of HRV in refractory epilepsy: The potential index to predict the onset of epilepsy in children. J. X-ray Sci. Technol. 2016, 24, 309–317. [Google Scholar] [CrossRef]
- Assaf, N.; Weller, B.; Deutsh-Castel, T.; Cohen, A.; Tirosh, E. The relationship between heart rate variability and epileptiform activity among children–a controlled study. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2008, 25, 317–320. [Google Scholar] [CrossRef]
- Okanari, K.; Maruyama, S.; Suzuki, H.; Shibata, T.; Pulcine, E.; Donner, E.J.; Otsubo, H. Autonomic dysregulation in children with epilepsy with postictal generalized EEG suppression following generalized convulsive seizures. Epilepsy Behav. 2020, 102, 106688. [Google Scholar] [CrossRef]
- Sarkis, R.A.; Thome-Souza, S.; Poh, M.Z.; Llewellyn, N.; Klehm, J.; Madsen, J.R.; Picard, R.; Pennell, P.B.; Dworetzky, B.A.; Loddenkemper, T.; et al. Autonomic changes following generalized tonic clonic seizures: An analysis of adult and pediatric patients with epilepsy. Epilepsy Res. 2015, 115, 113–118. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.T.; Guo, C.L.; Zhang, S.J.; Zeng, X.W.; Meng, F.G. Comparison of heart rate changes with ictal tachycardia seizures in adults and children. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2016, 32, 689–695. [Google Scholar] [CrossRef]
- Brotherstone, R.; McLellan, A. Parasympathetic alteration during sub-clinical seizures. Seizure 2012, 21, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Qaraqe, M.; Ismail, M.; Serpedin, E.; Zulfi, H. Epileptic seizure onset detection based on EEG and ECG data fusion. Epilepsy Behav. 2016, 58, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Romigi, A.; Citi, L.; Placidi, F.; Izzi, F.; Albanese, M.; Scilingo, E.P.; Marciani, M.G.; Duggento, A.; Guerrisi, M.; et al. Predicting seizures in untreated temporal lobe epilepsy using point-process nonlinear models of heartbeat dynamics. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 985–988. [Google Scholar]
- Pavei, J.; Heinzen, R.G.; Novakova, B.; Walz, R.; Serra, A.J.; Reuber, M.; Ponnusamy, A.; Marques, J.L.B. Early Seizure Detection Based on Cardiac Autonomic Regulation Dynamics. Front. Physiol. 2017, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Karasmanoglou, A.; Antonakakis, M.; Zervakis, M. ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures. Int. J. Environ. Res. Public Health 2023, 20, 5000. [Google Scholar] [CrossRef]
- Page, T.; Rugg-Gunn, F.J. Bitemporal seizure spread and its effect on autonomic dysfunction. Epilepsy Behav. 2018, 84, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.; Jafarnia Dabanloo, N.; Nasrabadi, A.M.; Dourado, A. Epileptic seizure prediction based on features extracted from lagged Poincaré plots. Int. J. Neurosci. 2022, 2022, 2106435. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, T.; Miyajima, M.; Fujiwara, K.; Kano, M.; Suzuki, Y.; Watanabe, Y.; Watanabe, S.; Hoshida, T.; Inaji, M.; Maehara, T. Wearable Epileptic Seizure Prediction System with Machine-Learning-Based Anomaly Detection of Heart Rate Variability. Sensor 2020, 20, 3987. [Google Scholar] [CrossRef]
- Fujiwara, K.; Miyajima, M.; Yamakawa, T.; Abe, E.; Suzuki, Y.; Sawada, Y.; Kano, M.; Maehara, T.; Ohta, K.; Sasai-Sakuma, T.; et al. Epileptic Seizure Prediction Based on Multivariate Statistical Process Control of Heart Rate Variability Features. IEEE Trans. Biomed. Eng. 2016, 63, 1321–1332. [Google Scholar]
- Surges, R.; Scott, C.A.; Walker, M.C. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology 2010, 74, 421–426. [Google Scholar] [CrossRef]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Brugada, R.; Pedersen, B.; Rubboli, G.; Johansen, P.; Beniczky, S. Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy. Epilepsia 2014, 55, e67–e71. [Google Scholar] [CrossRef]
- Gaspard, N. Heartbreakers-Cardiac Stress After Uncomplicated Generalized Convulsive Seizures. Epilepsy Curr. 2019, 19, 246–248. [Google Scholar] [CrossRef]
- Arbune, A.A.; Jeppesen, J.; Conradsen, I.; Ryvlin, P.; Beniczky, S. Peri-ictal heart rate variability parameters as surrogate markers of seizure severity. Epilepsia 2020, 61, S55–S60. [Google Scholar] [CrossRef]
- Toth, V.; Hejjel, L.; Fogarasi, A.; Gyimesi, C.; Orsi, G.; Szucs, A.; Kovacs, N.; Komoly, S.; Ebner, A.; Janszky, J. Periictal heart rate variability analysis suggests long-term postictal autonomic disturbance in epilepsy. Eur. J. Neurol. 2010, 17, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Sivathamboo, S.; Constantino, T.N.; Chen, Z.; Sparks, P.B.; Goldin, J.; Velakoulis, D.; Jones, N.C.; Kwan, P.; Macefield, V.G.; O’Brien, T.J.; et al. Cardiorespiratory and autonomic function in epileptic seizures: A video-EEG monitoring study. Epilepsy Behav. 2020, 111, 107271. [Google Scholar] [CrossRef] [PubMed]
- Adjei, P.; Surges, R.; Scott, C.A.; Kallis, C.; Shorvon, S.; Walker, M.C. Do subclinical electrographic seizure patterns affect heart rate and its variability? Epilepsy Res. 2009, 87, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Forti, A.; Falla, M.; Scquizzato, T.; Strapazzon, G. A New Approach to Detect Nonconvulsive Seizures in Patients in a Cardiac Surgery Intensive Care Unit by Monitoring Heart Rate Variability. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2770–2774. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Beniczky, S.; Johansen, P.; Sidenius, P.; Fuglsang-Frederiksen, A. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure 2015, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jaychandran, R.; Chaitanya, G.; Satishchandra, P.; Bharath, R.D.; Thennarasu, K.; Sinha, S. Monitoring peri-ictal changes in heart rate variability, oxygen saturation and blood pressure in epilepsy monitoring unit. Epilepsy Res. 2016, 125, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.T.; Rodrigues, S.; Campelo, M.; Dias, D.; Rego, R.; Rocha, H.; Sá, F.; Tavares-Silva, M.; Pinto, R.; Pestana, G.; et al. Heart rate variability in patients with refractory epilepsy: The influence of generalized convulsive seizures. Epilepsy Res. 2021, 178, 106796. [Google Scholar] [CrossRef] [PubMed]
- Calandra-Buonaura, G.; Toschi, N.; Provini, F.; Corazza, I.; Bisulli, F.; Barletta, G.; Vandi, S.; Montagna, P.; Guerrisi, M.; Tinuper, P.; et al. Physiologic autonomic arousal heralds motor manifestations of seizures in nocturnal frontal lobe epilepsy: Implications for pathophysiology. Sleep Med. 2012, 13, 252–262. [Google Scholar] [CrossRef]
- You, S.M.; Jo, H.J.; Cho, B.H.; Song, J.Y.; Kim, D.Y.; Hwang, Y.H.; Shon, Y.M.; Seo, D.W.; Kim, I.Y. Comparing Ictal Cardiac Autonomic Changes in Patients with Frontal Lobe Epilepsy and Temporal Lobe Epilepsy by Ultra-Short-Term Heart Rate Variability Analysis. Medicina 2021, 57, 666. [Google Scholar] [CrossRef]
- Romigi, A.; Albanese, M.; Placidi, F.; Izzi, F.; Mercuri, N.B.; Marchi, A.; Liguori, C.; Campagna, N.; Duggento, A.; Canichella, A.; et al. Heart rate variability in untreated newly diagnosed temporal lobe epilepsy: Evidence for ictal sympathetic dysregulation. Epilepsia 2016, 57, 418–426. [Google Scholar] [CrossRef]
- Behbahani, S.; Dabanloo, N.J.; Nasrabadi, A.M.; Dourado, A. Classification of ictal and seizure-free HRV signals with focus on lateralization of epilepsy. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2016, 24, 43–56. [Google Scholar] [CrossRef]
- Shimmura, M.; Uehara, T.; Ogata, K.; Shigeto, H.; Maeda, T.; Sakata, A.; Yamasaki, R.; Kira, J.I. Higher postictal parasympathetic activity following greater ictal heart rate increase in right- than left-sided seizures. Epilepsy Behav. 2019, 97, 161–168. [Google Scholar] [CrossRef]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Johansen, P.; Christensen, J.; Wüstenhagen, S.; Tankisi, H.; Qerama, E.; Hess, A.; Beniczky, S. Seizure detection based on heart rate variability using a wearable electrocardiography device. Epilepsia 2019, 60, 2105–2113. [Google Scholar] [CrossRef]
- Ponnusamy, A.; Marques, J.L.; Reuber, M. Comparison of heart rate variability parameters during complex partial seizures and psychogenic nonepileptic seizures. Epilepsia 2012, 53, 1314–1321. [Google Scholar] [CrossRef]
- Hödl, S.; Olbert, E.; Mahringer, C.; Struhal, W.; Carrette, E.; Meurs, A.; Gadeyne, S.; Dauwe, I.; Goossens, L.; Raedt, R.; et al. Pre-ictal heart rate variability alterations in focal onset seizures and response to vagus nerve stimulation. Seizure 2021, 86, 175–180. [Google Scholar] [CrossRef]
- Hödl, S.; Olbert, E.; Mahringer, C.; Carrette, E.; Meurs, A.; Gadeyne, S.; Dauwe, I.; Goossens, L.; Raedt, R.; Boon, P.; et al. Severe autonomic nervous system imbalance in Lennox-Gastaut syndrome patients demonstrated by heart rate variability recordings. Epilepsy Res. 2021, 177, 106783. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Beniczky, S.; Fuglsang-Frederiksen, A.; Sidenius, P.; Jasemian, Y. Detection of epileptic-seizures by means of power spectrum analysis of heart rate variability: A pilot study. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2010, 18, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Beniczky, S.; Johansen, P.; Sidenius, P.; Fuglsang-Frederiksen, A. Using Lorenz plot and Cardiac Sympathetic Index of heart rate variability for detecting seizures for patients with epilepsy. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 4563–4566. [Google Scholar]
- Behbahani, S.; Dabanloo, N.J.; Nasrabadi, A.M.; Dourado, A. Prediction of epileptic seizures based on heart rate variability. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2016, 24, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Faber, R.; Stepan, H.; Baumert, M.; Voss, A.; Walther, T. Changes of blood pressure and heart rate variability precede a grand mal seizure in a pregnant woman. J. Perinat. Med. 2004, 32, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.; Dabanloo, N.J.; Nasrabadi, A.M.; Teixeira, C.A.; Dourado, A. Pre-ictal heart rate variability assessment of epileptic seizures by means of linear and non-linear analyses. Anadolu Kardiyol. Derg. 2013, 13, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.; Dabanloo, N.J.; Motie Nasrabadi, A.; Dourado, A. Gender-Related Differences in Heart Rate Variability of Epileptic Patients. Am. J. Men’s Health 2018, 12, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, L.; Assi, E.B.; Toffa, D.H.; Nguyen, D.K.; Sawan, M. Unsupervised Clustering of HRV Features Reveals Preictal Changes in Human Epilepsy. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 698–701. [Google Scholar]
- Leal, A.; Pinto, M.; Henriques, J.; Graca Ruano, M.D.; de Carvalho, P.; Teixeira, C. Preictal Time Assessment using Heart Rate Variability Features in Drug-resistant Epilepsy Patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 6776–6779. [Google Scholar]
- Leal, A.; Pinto, M.F.; Lopes, F.; Bianchi, A.M.; Henriques, J.; Ruano, M.G.; de Carvalho, P.; Dourado, A.; Teixeira, C.A. Heart rate variability analysis for the identification of the preictal interval in patients with drug-resistant epilepsy. Sci. Rep. 2021, 11, 5987. [Google Scholar] [CrossRef] [PubMed]
- Moridani, M.K.; Farhadi, H. Heart rate variability as a biomarker for epilepsy seizure prediction. Bratisl. Lek. Listy 2017, 118, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Christensen, J.; Johansen, P.; Beniczky, S. Personalized seizure detection using logistic regression machine learning based on wearable ECG-monitoring device. Seizure 2023, 107, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Beniczky, S.; Fuglsang Frederiksen, A.; Sidenius, P.; Johansen, P. Modified automatic R-peak detection algorithm for patients with epilepsy using a portable electrocardiogram recorder. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; Volume 2017, pp. 4082–4085. [Google Scholar]
- Kołodziej, M.; Majkowski, A.; Rak, R.J.; Świderski, B.; Rysz, A. System for automatic heart rate calculation in epileptic seizures. Australas. Phys. Eng. Sci. Med. 2017, 40, 555–564. [Google Scholar] [CrossRef]
- Zambrana-Vinaroz, D.; Vicente-Samper, J.M.; Sabater-Navarro, J.M. Validation of Continuous Monitoring System for Epileptic Users in Outpatient Settings. Sensors 2022, 22, 2900. [Google Scholar] [CrossRef]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Johansen, P.; Christensen, J.; Wüstenhagen, S.; Tankisi, H.; Qerama, E.; Beniczky, S. Seizure detection using heart rate variability: A prospective validation study. Epilepsia 2020, 61, S41–S46. [Google Scholar] [CrossRef]
- Jeppesen, J.; Christensen, J.; Mølgaard, H.; Beniczky, S. Automated detection of focal seizures using subcutaneously implanted electrocardiographic device: A proof-of-concept study. Epilepsia 2023, 64, S59–S64. [Google Scholar] [CrossRef]
- Zambrana-Vinaroz, D.; Vicente-Samper, J.M.; Manrique-Cordoba, J.; Sabater-Navarro, J.M. Wearable Epileptic Seizure Prediction System Based on Machine Learning Techniques Using ECG, PPG and EEG Signals. Sensors 2022, 22, 9372. [Google Scholar] [CrossRef] [PubMed]
- Bersimis, S.; Psarakis, S.; Panaretos, J. Multivariate statistical process control charts: An overview. Qual. Reliab. Eng. Int. 2007, 23, 517–543. [Google Scholar] [CrossRef]
- Greene, B.R.; de Chazal, P.; Boylan, G.; Reilly, R.B.; O’Brien, C.; Connolly, S. Heart and respiration rate changes in the neonate during electroencephalographic seizure. Med. Biol. Eng. Comput. 2006, 44, 27–34. [Google Scholar] [CrossRef]
- Faria, M.T.; Rodrigues, S.; Campelo, M.; Dias, D.; Rego, R.; Rocha, H.; Sá, F.; Tavares-Silva, M.; Pinto, R.; Pestana, G.; et al. Does the type of seizure influence heart rate variability changes? Epilepsy Behav. 2022, 126, 108453. [Google Scholar] [CrossRef]
- Au Yong, H.M.; Minato, E.; Paul, E.; Seneviratne, U. Can seizure-related heart rate differentiate epileptic from psychogenic nonepileptic seizures? Epilepsy Behav. 2020, 112, 107353. [Google Scholar] [CrossRef] [PubMed]
- Gregg, N.M.; Pal Attia, T.; Nasseri, M.; Joseph, B.; Karoly, P.; Cui, J.; Stirling, R.E.; Viana, P.F.; Richner, T.J.; Nurse, E.S.; et al. Seizure occurrence is linked to multiday cycles in diverse physiological signals. Epilepsia 2023, 64, 1627–1639. [Google Scholar] [CrossRef]
- Elger, C.E.; Hoppe, C. Diagnostic challenges in epilepsy: Seizure under-reporting and seizure detection. Lancet Neurol. 2018, 17, 279–288. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Lehnertz, K.; Richardson, M.P.; Schelter, B.; Zaveri, H.P. Seizure prediction—Ready for a new era. Nat. Rev. Neurol. 2018, 14, 618–630. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]

| Index | Search | Results |
|---|---|---|
| 1 | “Epilepsy” [Mesh] OR “Seizures” [Mesh] OR “Epilep*” [title/abstract] OR “Seizure*” [title/abstract] OR “Ictal” [title/abstract] OR “Pre-ictal” [title/abstract] OR “Post-ictal” [title/abstract] OR “Peri-ictal” [title/abstract] OR “Inter-ictal” [title/abstract] | 265,315 |
| 2 | (“Time analysis” [title/abstract] OR “Power analysis” [title/abstract] OR “Nonlinear” [title/abstract] OR “Non linear” [title/abstract] OR “Non-linear” [title/abstract]) AND (“Electrocardiography” [Mesh] OR “Electrocardiography” [title/abstract] OR “Electrocardiogram” [title/abstract] OR “EKG” [title/abstract] OR “ECG” [title/abstract]) | 1847 |
| 3 | “Heart rate variability” [title/abstract] OR “HR variability” [title/abstract] | 23,489 |
| 4 | #2 or #3 | 24,610 |
| 5 | #1 and #4 | 402 |
| Population | Study Number | Population Size | Clinical Setting | Seizure Detection Performance | Seizure Prediction Time | HRV Changes | Seizure Type | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Ictal | Ictal | Post-Ictal | ||||||||
| Neonates (0–1 month) | 8 | Total = 256 Min = 5 Max = 52 | NICU | ↑ AUC = 87% [41] ↓ AUC = 62% [42] | NA | + | +++ | + | Not specified | [41,42,43,44,45,46,47,48] |
| Infants (2–12 months) | 1 | 7 | EMU | NA | NA | +++ | NA | +++ | IAS | [49] |
| Children (1–18 years) | 18 | Total = 397 Min = 9 Max = 72 | EMU | ↑ Sens = 89.06% and FAR = 0.41/hour [50] ↓ Sens = 60.9% and Spec = 82.6% [51] | Min = 21.8 s [52] Max = 25 min [50] | +++ | +++ | +++ | CS > NCS > ES | [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] |
| Adults (>18 years) | 50 | Total = 941 Min = 1 Max = 70 | EMU | ↑ Sens = 100.0% and FAR = 0.90/hour [67] ↓ Sens = 60.0% and Spec = 84.62% [68] | Min = 5 min [69] Max = 30 min [70] | +++ | +++ | +++ (CS > NCS) | CS > F(A+) > F(A−) > ES | [50,64,65,66,67,68,69,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104] |
| Wearable | ↑ Sens = 93.10% and FAR = 0.04/hour [91] ↓ Sens = 78.20% and FAR = 0.03/hour [105] | NA | +++ | +++ | +++ | CS > F(A+) > F(A−) | [73,91,105,106,107,108,109,110,111] | |||
| ECG-Patch | Sens = 92.6% and FAR = 0.11/hour [110] | NA | +++ | +++ | +++ | F(A+) | [110] | |||
| Handy Tips | |
|---|---|
| Neonates (0–1 month) |
|
| Children (1–18 years) |
|
| Adults (>18 years) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, F.; Scarabello, A.; Taruffi, L.; Pasini, E.; Calandra-Buonaura, G.; Vignatelli, L.; Bisulli, F. Heart Rate Variability as a Tool for Seizure Prediction: A Scoping Review. J. Clin. Med. 2024, 13, 747. https://doi.org/10.3390/jcm13030747
Mason F, Scarabello A, Taruffi L, Pasini E, Calandra-Buonaura G, Vignatelli L, Bisulli F. Heart Rate Variability as a Tool for Seizure Prediction: A Scoping Review. Journal of Clinical Medicine. 2024; 13(3):747. https://doi.org/10.3390/jcm13030747
Chicago/Turabian StyleMason, Federico, Anna Scarabello, Lisa Taruffi, Elena Pasini, Giovanna Calandra-Buonaura, Luca Vignatelli, and Francesca Bisulli. 2024. "Heart Rate Variability as a Tool for Seizure Prediction: A Scoping Review" Journal of Clinical Medicine 13, no. 3: 747. https://doi.org/10.3390/jcm13030747





