Bisphosphonate-Related Atypical Femoral Fractures in Patients with Autoimmune Disease Treated with Glucocorticoids: Surgical Results for 20 Limbs
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Patient Demographics
2.3. Treatment Strategies
2.4. Assessment Methods
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ensrud, K.E.; Thompson, D.E.; Cauley, J.A.; Nevitt, M.C.; Kado, D.M.; Hochberg, M.C.; Santora, A.C., 2nd; Black, D.M. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J. Am. Geriatr. Soc. 2000, 48, 241–249. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos. Int. 2010, 21, 71–79. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nawata, H.; Soen, S.; Fujiwara, S.; Nakayama, H.; Tanaka, I.; Ozono, K.; Sagawa, A.; Takayanagi, R.; Tanaka, H.; et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J. Bone Miner. Metab. 2014, 32, 337–350. [Google Scholar] [CrossRef]
- Odvina, C.V.; Zerwekh, J.E.; Rao, D.S.; Maalouf, N.; Gottschalk, F.A.; Pak, C.Y. Severely suppressed bone turnover: A potential complication of alendronate therapy. J. Clin. Endocrinol. Metab. 2005, 90, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S. Glucocorticoid-induced osteoporosis. Rev. Endocr. Metab. Disord. 2001, 2, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Nishino, T.; Kamada, H.; Nozawa, D.; Mishima, H.; Yamazaki, M. Location of fractures and the characteristics of patients with atypical femoral fractures: Analyses of 38 Japanese cases. J. Bone Miner. Metab. 2017, 35, 209–214. [Google Scholar] [CrossRef]
- Egol, K.A.; Park, J.H.; Rosenberg, Z.S.; Peck, V.; Tejwani, N.C. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin. Orthop. Relat. Res. 2014, 472, 2728–2734. [Google Scholar] [CrossRef]
- Nishino, T.; Hyodo, K.; Matsumoto, Y.; Yanagisawa, Y.; Yoshizawa, T.; Yamazaki, M. Surgical results of atypical femoral fractures in long-term bisphosphonate and glucocorticoid users–Relationship between fracture reduction and bone union. J. Orthop. 2020, 19, 143–149. [Google Scholar] [CrossRef]
- Oh, Y.; Wakabayashi, Y.; Kurosa, Y.; Fujita, K.; Okawa, A. Potential pathogenic mechanism for stress fractures of the bowed femoral shaft in the elderly: Mechanical analysis by the CT-based finite element method. Injury 2014, 45, 1764–1771. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Eda, Y.; Yamazaki, M. Technical note on the removal of a “cold-welded” lag screw from a Trigen Meta-Tan nail. Trauma Case Rep. 2021, 35, 100526. [Google Scholar] [CrossRef]
- Papakostidis, C.; Psyllakis, I.; Vardakas, D.; Grestas, A.; Giannoudis, P.V. Femoral-shaft fractures and nonunions treated with intramedullary nails: The role of dynamisation. Injury 2011, 42, 1353–1361. [Google Scholar] [CrossRef]
- Goh, S.K.; Yang, K.Y.; Koh, J.S.; Wong, M.K.; Chua, S.Y.; Chua, D.T.; Howe, T.S. Subtrochanteric insufficiency fractures in patients on alendronate therapy: A caution. J. Bone Jt. Surg. Br. Vol. 2007, 89, 349–353. [Google Scholar] [CrossRef]
- Gedmintas, L.; Solomon, D.H.; Kim, S.C. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: A systematic review and meta-analysis. J. Bone Miner. Res. 2013, 28, 1729–1737. [Google Scholar] [CrossRef]
- Girgis, C.M.; Sher, D.; Seibel, M.J. Atypical femoral fractures and bisphosphonate use. N. Engl. J. Med. 2010, 362, 1848–1849. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Huang, S.Y.; Lee, G.A.; Khandelwal, S.; Provus, J.; Ettinger, B.; Gonzalez, J.R.; Hui, R.L.; Grimsrud, C.D. Clinical correlates of atypical femoral fracture. Bone 2012, 51, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kondo, N.; Takai, C.; Kurosawa, Y.; Hasegawa, E.; Wakamatsu, A.; Kobayashi, D.; Nakatsue, T.; Abe, A.; Kazama, J.J.; et al. Risks of femoral localized periosteal thickening in patients with autoimmune inflammatory rheumatic diseases. Mod. Rheumatol. 2023, 33, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Management of glucocorticoid-induced osteoporosis. Nat. Rev. Rheumatol. 2010, 6, 82–88. [Google Scholar] [CrossRef]
- Koh, A.; Guerado, E.; Giannoudis, P.V. Atypical femoral fractures related to bisphosphonate treatment: Issues and controversies related to their surgical management. Bone Jt. J. 2017, 99, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Garrison, I.; Domingue, G.; Honeycutt, M.W. Subtrochanteric femur fractures: Current review of management. EFORT Open Rev. 2021, 6, 145–151. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ha, Y.C.; Kang, B.J.; Chang, J.S.; Koo, K.H. Predicting need for fixation of atypical femoral fracture. J. Clin. Endocrinol. Metab. 2013, 98, 2742–2745. [Google Scholar] [CrossRef] [PubMed]
- Cermak, K.; Shumelinsky, F.; Alexiou, J.; Gebhart, M.J. Case reports: Subtrochanteric femoral stress fractures after prolonged alendronate therapy. Clin. Orthop. Relat. Res. 2010, 468, 1991–1996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, H.S.; Kim, C.K.; Park, Y.S.; Moon, Y.W.; Lim, S.J.; Kim, S.M. Factors Associated with Increased Healing Time in Complete Femoral Fractures After Long-Term Bisphosphonate Therapy. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.E.; Lee, K.J.; Shin, W.C.; Moon, N.H.; Kim, C.H. The effect of teriparatide on fracture healing after atypical femoral fracture: A systematic review and meta-analysis. Osteoporos. Int. 2023, 34, 1323–1334. [Google Scholar] [CrossRef]
- Lee, S.Y.; Niikura, T.; Iwakura, T.; Fukui, T.; Kuroda, R. Clinical Experience With the Use of Low-Intensity Pulsed Ultrasound (LIPUS) in the Treatment of Atypical Femoral Fractures. J. Orthop. Trauma 2017, 31, S2. [Google Scholar] [CrossRef]
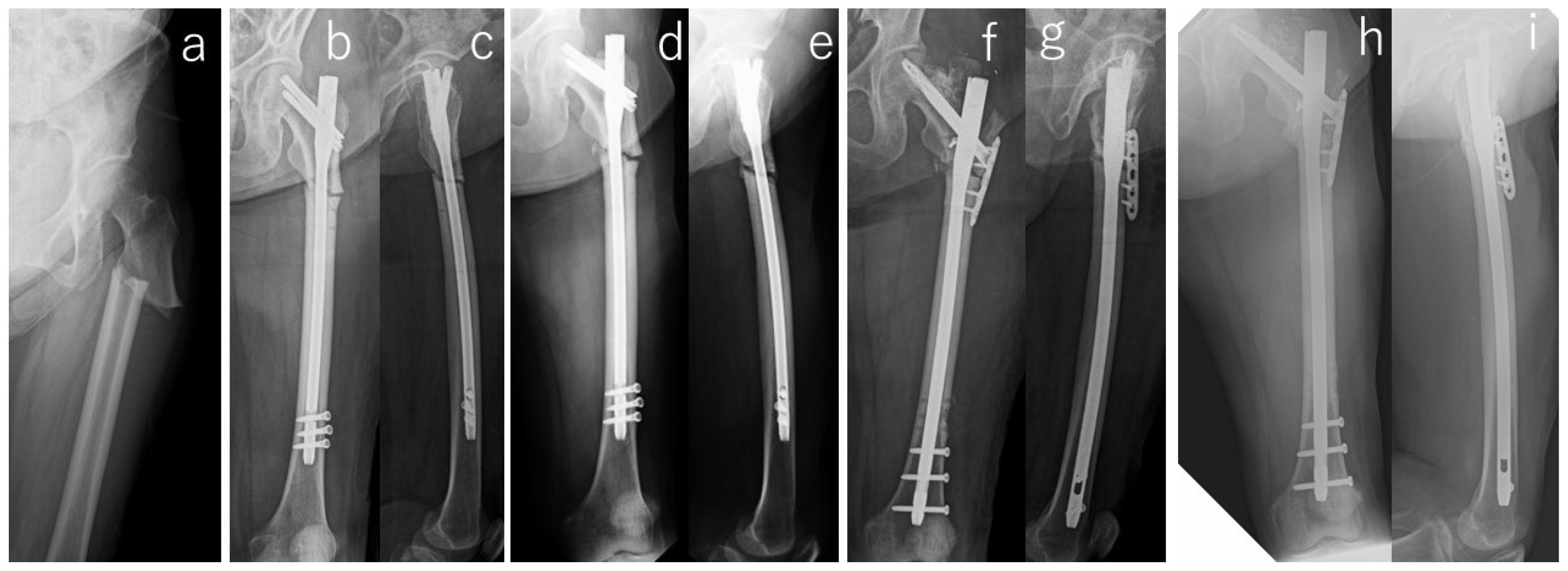
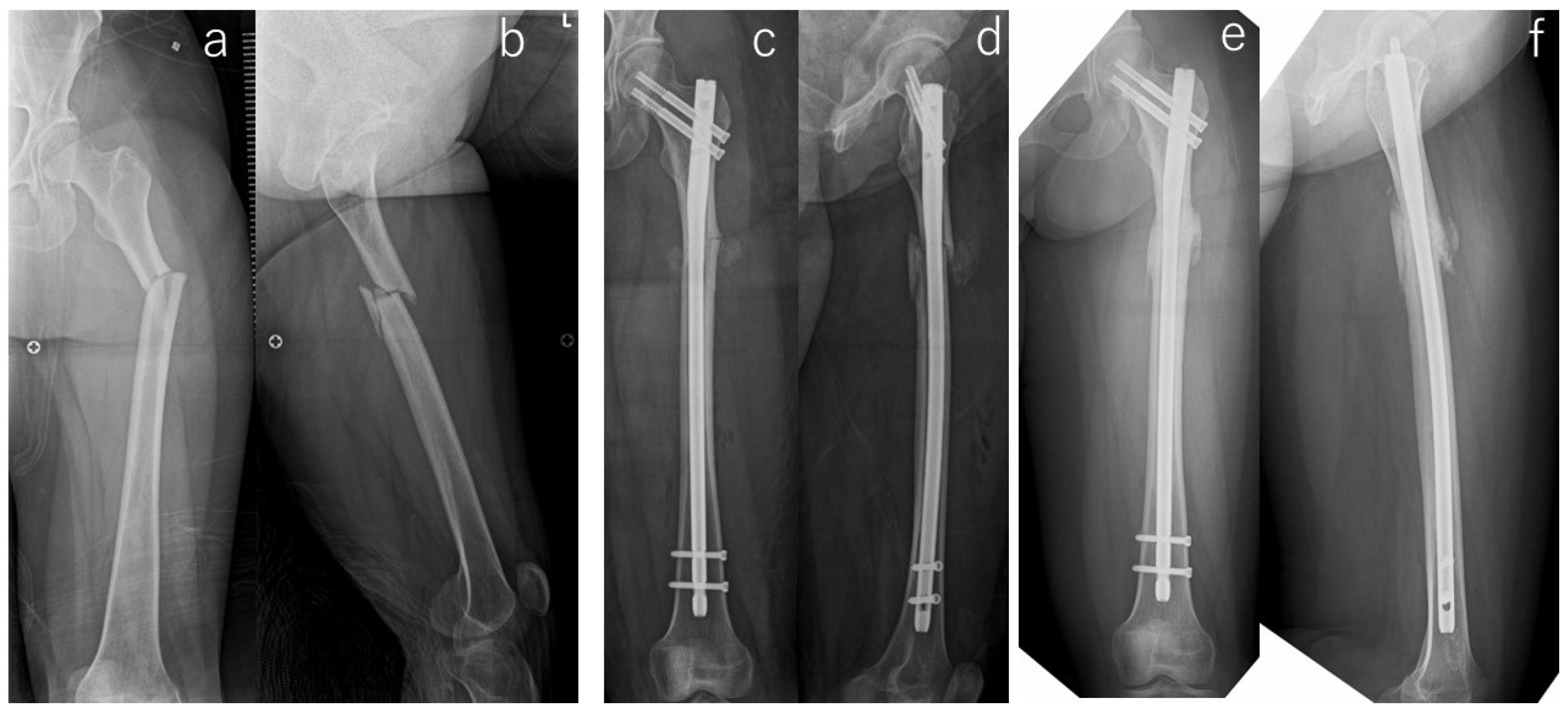
| # | Age (yr) | Gender | Body Mass Index (kg/m2) | Follow-Up Period (mo) | Affected Side | Location | Bilateral Lesion | Prodrome | Comorbities | Duration of GC Use (yrs) | Kind of BP | Duration of BP Use (yrs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | Female | 29.4 | 60 | Right | Subtrochanteric | + | + | Myasthenia gravis, Diabetes mellitus | 28 | Risedronate | 5 |
| 2 | 54 | Female | 24.5 | 18 | Right | Subtrochanteric | + | + | Dermatomyositis, Interstitial pneumonia | 18 | Alendronate | 4 |
| 3 | 58 | Female | 23.4 | 146 | Right | Subtrochanteric | + | - | Rheumatoid arthritis | 12 | Alendronate | 6.5 |
| 4 | 54 | Female | 25.0 | 112 | Left | Subtrochanteric | + | - | Rheumatoid arthritis | 9 | Alendronate | 5.5 |
| 5 | 48 | Female | 29.4 | 127 | Right | Femoral diaphysis | + | + | Systemic lupus erythematosus | 21 | Alendronate | 10 |
| 6 | 67 | Female | 27.7 | 128 | Left | Femoral diaphysis | - | - | Rheumatoid arthritis | 30 | Alendronate | 5 |
| 7 | 78 | Female | 28.0 | 61 | Right | Subtrochanteric | + | + | Adult Still’s disease | 11 | Alendronate | 7 |
| 8 | 78 | Female | 28.0 | 61 | Left | Subtrochanteric | + | + | Adult Still’s disease | 11 | Alendronate | 7 |
| 9 | 50 | Female | 20.4 | 90 | Left | Femoral diaphysis | + | + | Rheumatoid arthritis | 20 | Alendronate/Minodronate | 6.5 |
| 10 | 79 | Female | 23.5 | 75 | Left | Subtrochanteric | + | + | Adult Still’s disease | 11 | Etidronate/Alendronate | 7 |
| 11 | 76 | Male | 18.7 | 77 | Right | Subtrochanteric | - | + | Rheumatoid arthritis | 14 | Alendronate/Minodronate | 7 |
| 12 | 67 | Female | 27.1 | 74 | Left | Subtrochanteric | + | + | Interstitial pneumonia, Diabetes mellitus | 9 | Alendronate | 9 |
| 13 | 73 | Female | 24.4 | 46 | Left | Subtrochanteric | + | - | Systemic lupus erythematosus, Rheumatoid arthritis | 20 | Etidronate/Alendronate | 12 |
| 14 | 58 | Female | 24.7 | 44 | Left | Subtrochanteric | + | + | Systemic lupus erythematosus, Basedow’s disease | 34 | Alendronate | 20 |
| 15 | 58 | Female | 29.1 | 43 | Left | Subtrochanteric | + | + | Rheumatoid arthritis | 19 | Alendronate | 19.5 |
| 16 | 56 | Female | 24.4 | 41 | Right | Femoral diaphysis | + | - | IgG4-related disease | 10 | Alendronate | 10 |
| 17 | 85 | Female | 27.4 | 39 | Right | Femoral diaphysis | - | + | Myasthenia gravis | 7 | Alendronate | 7.5 |
| 18 | 49 | Female | 20.6 | 34 | Right | Subtrochanteric | + | + | Systemic lupus erythematosus, Rheumatoid arthritis | 11 | Minodronate | 5.6 |
| 19 | 41 | Female | 26.3 | 18 | Left | Subtrochanteric | + | + | Systemic lupus erythematosus, Glomerulonephritis | 18 | Alendronate/Minodronate | 13 |
| 20 | 76 | Female | 21.4 | 15 | Left | Subtrochanteric | - | + | Rheumatoid arthritis, Polymyositis | 26 | Alendronate/Minodronate | 14.5 |
| Bone-Related Markers: Unit (Normal Range) | Dual-Energy X-ray Absorptiometry | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | Ca * | IP | ALP | BAP | Intact-P1NP | Total-P1NP | Urinary NTX | TRACP-5b | Lumbar Spine | Contralateral Femoral Neck |
| mg/dL (8.5–10.5) | mg/dL (2.7–4.5) | U/I (104–338) | μg/L (3.8–22.6) | μg/L (27–109) | μg/L (18.1–74.1) | /mmol·Cre (14.3–89.0 ) | mU/dL (120–420) | BMD (g/cm2)/T-Score/YAM (%) | BMD (g/cm2)/T-Score/YAM (%) | |
| 1 | 9.7 | 2.5 | 219 | N.A. | N.A. | N.A. | 30.9 | N.A. | 1.068/2.2/106 | 0.867/2.4/100 |
| 2 | 9.6 | 2.5 | 118 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 3 | 9.1 | 3.4 | 218 | 10.9 | N.A. | N.A. | 45 | N.A. | 0.824/−1.7/81 | 0.718/−1.3/83 |
| 4 | 9.5 | 3.1 | 128 | 6.2 | N.A. | N.A. | 31.5 | 170 | 0.874/−1.2/86 | 0.700/−0.8/89 |
| 5 | 9.8 | 3.3 | 141 | N.A. | 35.3 | N.A. | N.A. | 162 | 0.987/−0.2/98 | 0.616/−1.6/78 |
| 6 | 9.3 | 4.3 | 335 | N.A. | 15.1 | N.A. | N.A. | 295 | 1.076/0.6/106 | 0.570/−2.0/72 |
| 7, 8 | 8.9 | 2.8 | 148 | N.A. | 12.6 | N.A. | N.A. | 147 | 1.209/1.8/120 | N.A. |
| 9 | 9.3 | N.A. | 248 | N.A. | N.A. | 11.8 | N.A. | 110 | 0.507/−4.6/49 | 0.476/−2.9/60 |
| 10 | 9.6 | 3.3 | 168 | N.A. | N.A. | 17.4 | N.A. | 321 | 0.705/−2.8/70 | 0.639/−1.4/81 |
| 11 | 9.1 | 3.2 | 122 | N.A. | N.A. | 11 | N.A. | N.A. | 0.841/−1.4/81 | 0.551/−2.5/64 |
| 12 | 9 | 3.6 | 174 | N.A. | N.A. | 13.7 | N.A. | 344 | 1.106/0.9/109 | 0.758/−0.3/96 |
| 13 | 9.3 | 3 | 207 | 8.9 | N.A. | 21.1 | 24.6 | 340 | N.A. | N.A. |
| 14 | 9.4 | 4 | 138 | N.A. | N.A. | 10.1 | N.A. | 231 | 1.067/0.5/106 | N.A. |
| 15 | 9.1 | 3.3 | 256 | N.A. | N.A. | 35.7 | N.A. | 665 | 1.156/1.3/114 | 0.885/0.9/112 |
| 16 | 8.9 | 3.2 | 184 | N.A. | N.A. | 10.7 | N.A. | 298 | 1.027/0.1/102 | 0.769/−0.2/98 |
| 17 | 9 | 4.1 | 286 | N.A. | N.A. | 23.8 | N.A. | 492 | 1.294/2.6/128 | 0.507/−2.6/64 |
| 18 | 8.9 | 4.7 | 27 * | N.A. | N.A. | 11.7 | N.A. | 63 | N.A. | N.A. |
| 19 | 8.9 | 2.3 | 66 * | N.A. | N.A. | 12.8 | N.A. | 170 | 0.841/−1.5/83 | 0.646/−1.3/82 |
| 20 | 9.2 | 3.6 | 69 * | N.A. | N.A. | 67 | N.A. | 300 | 0.712/−2.7/70 | 0.402/−3.5/51 |
| Implant | Operative Procedure | Adjuvant Therapy | Malalignment | Cortical Discontinuity (-/Direction) | Fracture Gap | Correction Loss | Duration of Bone Union/Nonunion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open Reduction | Bone Graft | Drilling | LIPUS # | Teriparatide | AP View | Lateral View | AP View | Lateral View | AP View | Lateral View | |||
| Antegrade intramedullary nail | - | - | - | - | - | - | - | - | Posterior | + | - | - | 6 months |
| Antegrade intramedullary nail | - | - | - | - | - | - | - | - | Posterior | + | - | - | 10 months |
| Cephalomedullary long nail | - | - | - | - | - | Varus | - | - | Posterior | - | - | - | Non-union (atrophic) * |
| Cephalomedullary long nail | - | - | - | - | - | - | - | - | Posterior | - | - | - | 16 months |
| Antegrade intramedullary nail | - | - | - | + | - | - | - | - | - | - | - | - | 18 months |
| Antegrade intramedullary nail | - | - | - | + | - | - | - | - | Posterior | - | + | + | Non-union (Hyper) * |
| Antegrade intramedullary nail | + | - | + | + | + | - | - | - | Posterior | + | - | - | Non-union (atrophic) |
| Cephalomedullary long nail | - | - | - | + | + | - | - | - | Posterior | + | - | - | 36 months |
| Antegrade intramedullary nail | - | - | - | + | + | - | - | - | Posterior | + | - | - | 10 months |
| Cephalomedullary long nail | + | + | + | + | - | - | - | - | - | + | - | - | Non-union (atrophic) * |
| Cephalomedullary long nail | - | - | - | + | + | - | Extension | - | Posterior | + | - | - | 21 months |
| Cephalomedullary long nail | - | + | + | + | + | Varus | - | - | - | - | - | - | 6 months |
| Cephalomedullary long nail | + | - | - | + | + | - | - | - | - | + | + | - | Non-union (atrophic) * |
| Antegrade intramedullary nail | + | - | - | + | + | - | - | - | - | - | - | - | 20 months |
| Cephalomedullary long nail | + | - | + | + | + | - | Extension | - | Posterior | + | - | - | Non-union (atrophic) * |
| Cephalomedullary long nail | - | - | - | + | + | - | - | - | - | - | - | - | 6 months |
| Antegrade intramedullary nail | - | - | - | + | + | - | - | - | - | + | - | - | 9 months |
| Antegrade intramedullary nail | + | - | + | + | + | - | - | - | - | - | - | - | Non-union (atrophic) |
| Antegrade intramedullary nail | - | - | - | + | - | Varus | - | - | Posterior | - | - | - | 5 months |
| Antegrade intramedullary nail | - | - | - | + | + | Varus | - | - | - | + | - | - | 8 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, T.; Hyodo, K.; Matsumoto, Y.; Yanagisawa, Y.; Yamazaki, M. Bisphosphonate-Related Atypical Femoral Fractures in Patients with Autoimmune Disease Treated with Glucocorticoids: Surgical Results for 20 Limbs. J. Clin. Med. 2024, 13, 1027. https://doi.org/10.3390/jcm13041027
Nishino T, Hyodo K, Matsumoto Y, Yanagisawa Y, Yamazaki M. Bisphosphonate-Related Atypical Femoral Fractures in Patients with Autoimmune Disease Treated with Glucocorticoids: Surgical Results for 20 Limbs. Journal of Clinical Medicine. 2024; 13(4):1027. https://doi.org/10.3390/jcm13041027
Chicago/Turabian StyleNishino, Tomofumi, Kojiro Hyodo, Yukei Matsumoto, Yohei Yanagisawa, and Masashi Yamazaki. 2024. "Bisphosphonate-Related Atypical Femoral Fractures in Patients with Autoimmune Disease Treated with Glucocorticoids: Surgical Results for 20 Limbs" Journal of Clinical Medicine 13, no. 4: 1027. https://doi.org/10.3390/jcm13041027
APA StyleNishino, T., Hyodo, K., Matsumoto, Y., Yanagisawa, Y., & Yamazaki, M. (2024). Bisphosphonate-Related Atypical Femoral Fractures in Patients with Autoimmune Disease Treated with Glucocorticoids: Surgical Results for 20 Limbs. Journal of Clinical Medicine, 13(4), 1027. https://doi.org/10.3390/jcm13041027







