Comparison between a Novel Radiofrequency-Balloon and a Standard Cryo-Balloon in Pulmonary Vein Isolation: A Propensity-Score-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Matching
2.3. Ablation Procedure
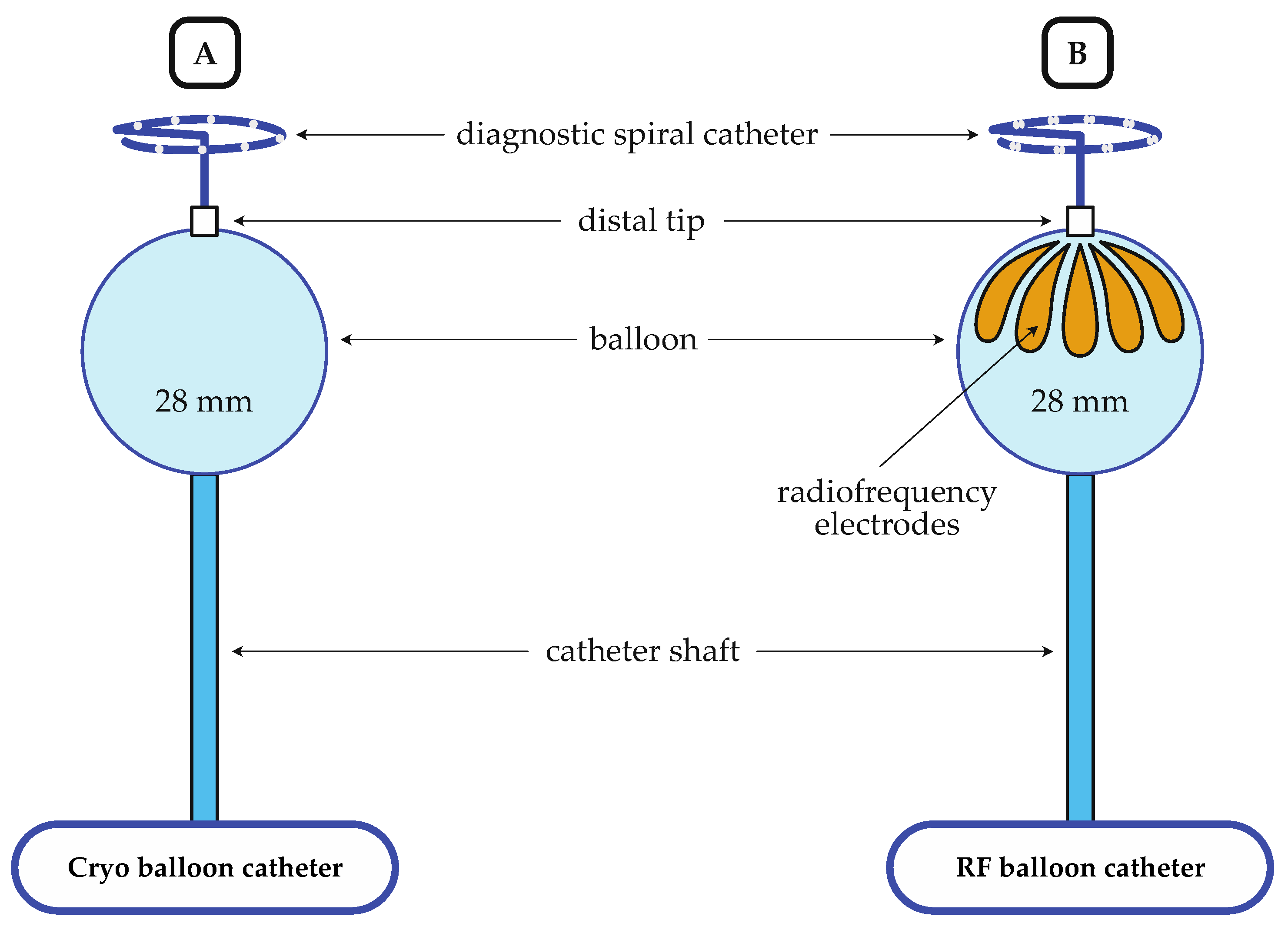
2.3.1. Cryo-Balloon Ablation
2.3.2. Radiofrequency-Balloon Ablation
2.4. Phrenic Nerve Monitoring
2.5. Periprocedural Management and Follow-Up
2.6. Study Endpoints
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural Characteristics and Ablation Data
3.3. Safety
3.4. Outcome Data
4. Discussion
4.1. Efficiency
4.2. Safety
4.3. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Niebauer, M.; Makati, K.; Halperin, B.; Gauri, A.; et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Albenque, J.-P.; Chun, K.R.J.; Fürnkranz, A.; Busch, M.; Elvan, A.; Schlüter, M.; Braegelmann, K.M.; Kueffer, F.J.; Hemingway, L.; et al. Repeat Ablation for Atrial Fibrillation Recurrence Post Cryoballoon or Radiofrequency Ablation in the FIRE AND ICE Trial. Circ. Arrhythm. Electrophysiol. 2019, 12, e007247. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Straube, F.; Wegscheider, K.; Kuniss, M.; Andresen, D.; Wu, L.-Q.; Tebbenjohanns, J.; Noelker, G.; Tilz, R.R.; Chun, J.K.R.; et al. Outcomes of Cryoballoon or Radiofrequency Ablation in Symptomatic Paroxysmal or Persistent Atrial Fibrillation. EP Eur. 2019, 21, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Poudyal, A.; Pulipati, P.; Larsen, T.; Krishnan, K.; Trohman, R.G.; Sharma, P.S.; Huang, H.D. A Systematic Review and Meta-analysis Comparing Second-generation Cryoballoon and Contact Force Radiofrequency Ablation for Initial Ablation of Paroxysmal and Persistent Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Schilling, R.; Dhillon, G.S.; Tondo, C.; Riva, S.; Grimaldi, M.; Quadrini, F.; Neuzil, P.; Chierchia, G.-B.; de Asmundis, C.; Abdelaal, A.; et al. Safety, Effectiveness, and Quality of Life Following Pulmonary Vein Isolation with a Multi-Electrode Radiofrequency Balloon Catheter in Paroxysmal Atrial Fibrillation: 1-Year Outcomes from SHINE. EP Eur. 2021, 23, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Honarbakhsh, S.; Di Monaco, A.; Coling, A.E.; Lenka, K.; Pizzamiglio, F.; Hunter, R.J.; Horton, R.; Mansour, M.; Natale, A.; et al. Use of a Multi-electrode Radiofrequency Balloon Catheter to Achieve Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation: 12-Month Outcomes of the RADIANCE Study. J. Cardiovasc. Electrophysiol. 2020, 31, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, A.; Almorad, A.; Pannone, L.; Della Rocca, D.G.; Bisignani, A.; Monaco, C.; Mouram, S.; Ramak, R.; Gauthey, A.; Overeinder, I.; et al. Pulmonary Vein Isolation with the Radiofrequency Balloon Catheter: A Single Centre Prospective Study. EP Eur. 2023, 25, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Teumer, Y.; Miesbichler, C.; Hauke, A.; Katov, L.; Bothner, C.; Pott, A.; Müller, M.; Walter, B.; Rottbauer, W.; Dahme, T.; et al. Atrial Fibrillation Ablation with a Novel Fully 3D-Mapping-Integrated Multi-Electrode Radiofrequency Balloon Catheter. J. Clin. Med. 2023, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Tadler, S.; Rothenbacher, D.; Seeger, J.; Wöhrle, J. A Hierarchical Algorithm for Multicentric Matched Cohort Study Designs. Curr. Med. Res. Opin. 2020, 36, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Kuss, O.; Blettner, M.; Börgermann, J. Propensity Score: An Alternative Method of Analyzing Treatment Effects: Part 23 of a series on evaluation of scientific Publications. Dtsch. Ärztebl. Int. 2016, 113, 597. [Google Scholar] [PubMed]
- Pott, A.; Kraft, C.; Stephan, T.; Petscher, K.; Rottbauer, W.; Dahme, T. Time-to-Isolation Guided Titration of Freeze Duration in 3rd Generation Short-Tip Cryoballoon Pulmonary Vein Isolation—Comparable Clinical Outcome and Shorter Procedure Duration. Int. J. Cardiol. 2018, 255, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Almorad, A.; Del Monte, A.; Della Rocca, D.G.; Pannone, L.; Ramak, R.; Overeinder, I.; Bala, G.; Ströker, E.; Sieira, J.; Dubois, A.; et al. Outcomes of Pulmonary Vein Isolation with Radiofrequency Balloon vs. Cryoballoon Ablation: A Multi-Centric Study. Europace 2023, 25, euad252. [Google Scholar] [CrossRef] [PubMed]
- My, I.; Bordignon, S.; Butt, M.; Rottner, L.; Marc, L.; Moser, F.; Wenzel, J.-P.; Obergassel, J.; Schleberger, R.; Moser, J.; et al. Novel Radiofrequency Balloon Catheter—Impact of Ablation Parameters on Single-Shot Isolation. Circ. J. 2023, 87, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, S.; My, I.; Tohoku, S.; Rillig, A.; Schaack, D.; Chen, S.; Reißmann, B.; Urbanek, L.; Hirokami, J.; Efe, T.; et al. Efficacy and Safety in Patients Treated with a Novel Radiofrequency Balloon: A Two Centres Experience from the AURORA Collaboration. Europace 2023, 25, euad106. [Google Scholar] [CrossRef] [PubMed]
- Heeger, C.-H.; Sohns, C.; Pott, A.; Metzner, A.; Inaba, O.; Straube, F.; Kuniss, M.; Aryana, A.; Miyazaki, S.; Cay, S.; et al. Phrenic Nerve Injury During Cryoballoon-Based Pulmonary Vein Isolation: Results of the Worldwide YETI Registry. Circ. Arrhythm. Electrophysiol. 2022, 15, e010516. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, V.; Breitenstein, A.; Schilling, R.; Hofer, D.; Tiongco, B.; Ang, R.; Hunter, R.; Earley, M.; Ahsan, S.; Mangiafico, V.; et al. Catheter Ablation of Atrial Fibrillation with a Multi-electrode Radiofrequency Balloon; First and Early Two Centre Experience in Europe. J. Cardiovasc. Electrophysiol. 2023, 34, 1350–1359. [Google Scholar] [CrossRef] [PubMed]


| RF Balloon | Cryo Balloon | |
|---|---|---|
| Ablation energy | RF | Cryo |
| Ablation time [s] | 60 | 180 1 |
| 3D mapping integration | + | - |
| Compliant balloon | + | - |
| Segmental ablation possible | + | - |
| PN pacing from the balloon possible | + | - |
| Baseline Characteristics | RFB Group n = 171 | CB Group n = 342 | p Value |
|---|---|---|---|
| Age [years] (mean ± SD) | 68.5 ± 10.2 | 67.9 ± 11.8 | 0.938 |
| Female, n (%) | 63 (36.8) | 126 (36.8) | 1.000 |
| BMI [kg/m2] (mean ± SD) | 28.5 ± 5.5 | 28.4 ± 5.2 | 0.847 |
| Paroxysmal AF, n (%) | 108 (63.2) | 216 (63.2) | 1.000 |
| CHA2DS2-VASc score (mean ± SD) | 3.8 ± 2.0 | 3.6 ± 1.9 | 0.155 |
| Reduced ejection fraction 1, n (%) | 69 (40.4) | 112 (32.7) | 0.096 |
| Arterial hypertension, n (%) | 129 (75.4) | 272 (79.5) | 0.308 |
| Diabetes mellitus, n (%) | 35 (20.5) | 67 (19.6) | 0.907 |
| Prior stroke or TIA, n (%) | 23 (13.5) | 39 (11.4) | 0.566 |
| CAD, n (%) | 91 (53.2) | 173 (50.6) | 0.649 |
| LAD [mm] (mean ± SD) | 45 ± 6 | 45 ± 6 | 0.609 |
| Procedural Characteristics | RFB Group n = 171 | CB Group n = 342 | p Value |
|---|---|---|---|
| Procedure duration (skin to skin) [min], median (IQR) | 88 (70–115) | 73 (54–97) | <0.001 |
| Dwell time [min], median (IQR) | 23 (15–36) | 28 (20–41) | 0.006 |
| Fluoroscopy time [min], median (IQR) | 18.9 (13.9–29.6) | 14.5 (9.8–21.4) | <0.001 |
| Ablation Data | RFB Group n = 171 | CB Group n = 342 | p Value |
|---|---|---|---|
| Treated PVs (overall), n | 669 | 1355 | 0.891 |
| LSPV, n | 161 | 324 | |
| Single shot isolation, n (%) | 128 (79.5) | 259 (80.2) | 0.968 |
| TTI [s] (mean ± SD) | 15.6 ± 8.6 | 47.9 ± 25.0 | <0.001 |
| TTI observational rate, n (%) | 126 (78.3) | 268 (82.7) | 0.352 |
| energy applications (mean ± SD) | 1.7 ± 1.3 | 1.6 ± 1.1 | 0.807 |
| LIPV, n | 161 | 324 | |
| Single shot isolation, n (%) | 125 (77.6) | 299 (92.6) | <0.001 |
| TTI [s] (mean ± SD) | 12.5 ± 6.2 | 39.4 ± 21.3 | <0.001 |
| TTI observational rate, n (%) | 128 (79.5) | 245 (75.6) | 0.255 |
| energy applications (mean ± SD) | 1.7 ± 1.0 | 1.4 ± 0.7 | 0.002 |
| LPV, n | 8 | 18 | |
| Single shot isolation, n (%) | 5 (62.5) | 6 (33.3) | - 2 |
| TTI [s] (mean ± SD) | 11.7 ± 7.2 | 61.9 ± 37.3 | - 2 |
| TTI observational rate, n (%) | 3 (37.5) | 12 (66.7) | - 2 |
| energy applications (mean ± SD) | 3.0 ± 2.1 | 3.6 ± 2.2 | 0.629 |
| RSPV, n | 169 | 339 | |
| Single shot isolation, n (%) | 114 (67.5) | 216 (63.9) | 0.437 |
| TTI [s] (mean ± SD) | 10.8 ± 5.4 | 43.4 ± 24.5 | <0.001 |
| TTI observational rate, n (%) | 133 (78.7) | 279 (82.3) | 0.355 |
| energy applications (mean ± SD) | 1.4 ± 1.1 | 1.5 ± 0.9 | 0.306 |
| RIPV, n | 169 | 339 | |
| Single shot isolation, n (%) | 99 (58.6) | 203 (60.1) | 0.753 |
| TTI [s] (mean ± SD) | 11.0 ± 5.0 | 46.5 ± 25.2 | <0.001 |
| TTI observational rate, n (%) | 130 (76.9) | 282 (83.2) | 0.109 |
| energy applications (mean ± SD) | 1.3 ± 0.6 | 1.6 ± 1.1 | 0.005 |
| RMPV, n | 1 | 8 | |
| Single shot isolation, n (%) | 1 (100) | 6 (75.0) | - 2 |
| TTI [s] (mean ± SD) | 35 1 | 35.5 ± 26.2 | - 2 |
| TTI observational rate, n (%) | 1 (100) | 2 (25.0) | - 2 |
| energy applications (mean ± SD) | 1 | 1.9 ± 0.6 | - 2 |
| RPV, n | 0 | 3 | |
| Single shot isolation, n (%) | - | 3 (100) | - 2 |
| TTI [s] | - | 49 1 | - 2 |
| TTI observational rate, n (%) | - | 1 (33.3%) | - 2 |
| energy applications (mean ± SD) | - | 1.7 ± 0.6 | - 2 |
| Complication Data | RFB Group n = 171 | CB Group n = 342 | p Value |
|---|---|---|---|
| Major complication, n (%) | 0 (0) | 4 (1.2) | 0.307 |
| Fatality | 0 (0) | 0 (0) | |
| Pericardial tamponade 1, n (%) | 0 (0) | 1 (0.3) | |
| Stroke or TIA, n (%) | 0 (0) | 1 (0.3) | |
| Persistent PNP, n (%) | 0 (0) | 2 (0.6) | |
| Atrio-esophageal fistula, n (%) | 0 (0) | 0 (0) | |
| Vascular access complication 1, n (%) | 0 (0) | 0 (0) | |
| Minor complication, n (%) | 6 (3.5) | 7 (2.0) | 0.374 |
| Pericardial effusion 2, n (%) | 0 (0) | 0 (0) | |
| Transient PNP, n (%) | 1 (0.6) | 4 (1.2) | |
| Vascular access complication 2, n (%) | 0 (0) | 3 (0.9) | |
| EDEL, n (%) | 5 (2.9) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teumer, Y.; Miesbichler, C.; Katov, L.; Mayer, B.; Rottbauer, W.; Bothner, C.; Weinmann, K. Comparison between a Novel Radiofrequency-Balloon and a Standard Cryo-Balloon in Pulmonary Vein Isolation: A Propensity-Score-Matched Analysis. J. Clin. Med. 2024, 13, 963. https://doi.org/10.3390/jcm13040963
Teumer Y, Miesbichler C, Katov L, Mayer B, Rottbauer W, Bothner C, Weinmann K. Comparison between a Novel Radiofrequency-Balloon and a Standard Cryo-Balloon in Pulmonary Vein Isolation: A Propensity-Score-Matched Analysis. Journal of Clinical Medicine. 2024; 13(4):963. https://doi.org/10.3390/jcm13040963
Chicago/Turabian StyleTeumer, Yannick, Clemens Miesbichler, Lyuboslav Katov, Benjamin Mayer, Wolfgang Rottbauer, Carlo Bothner, and Karolina Weinmann. 2024. "Comparison between a Novel Radiofrequency-Balloon and a Standard Cryo-Balloon in Pulmonary Vein Isolation: A Propensity-Score-Matched Analysis" Journal of Clinical Medicine 13, no. 4: 963. https://doi.org/10.3390/jcm13040963
APA StyleTeumer, Y., Miesbichler, C., Katov, L., Mayer, B., Rottbauer, W., Bothner, C., & Weinmann, K. (2024). Comparison between a Novel Radiofrequency-Balloon and a Standard Cryo-Balloon in Pulmonary Vein Isolation: A Propensity-Score-Matched Analysis. Journal of Clinical Medicine, 13(4), 963. https://doi.org/10.3390/jcm13040963






