Short- to Mid-Term Clinical and Radiological Results of Selective Laser Melting Highly Porous Titanium Cup in Primary Total Hip Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Inclusion, Demographics, and Perioperative Management
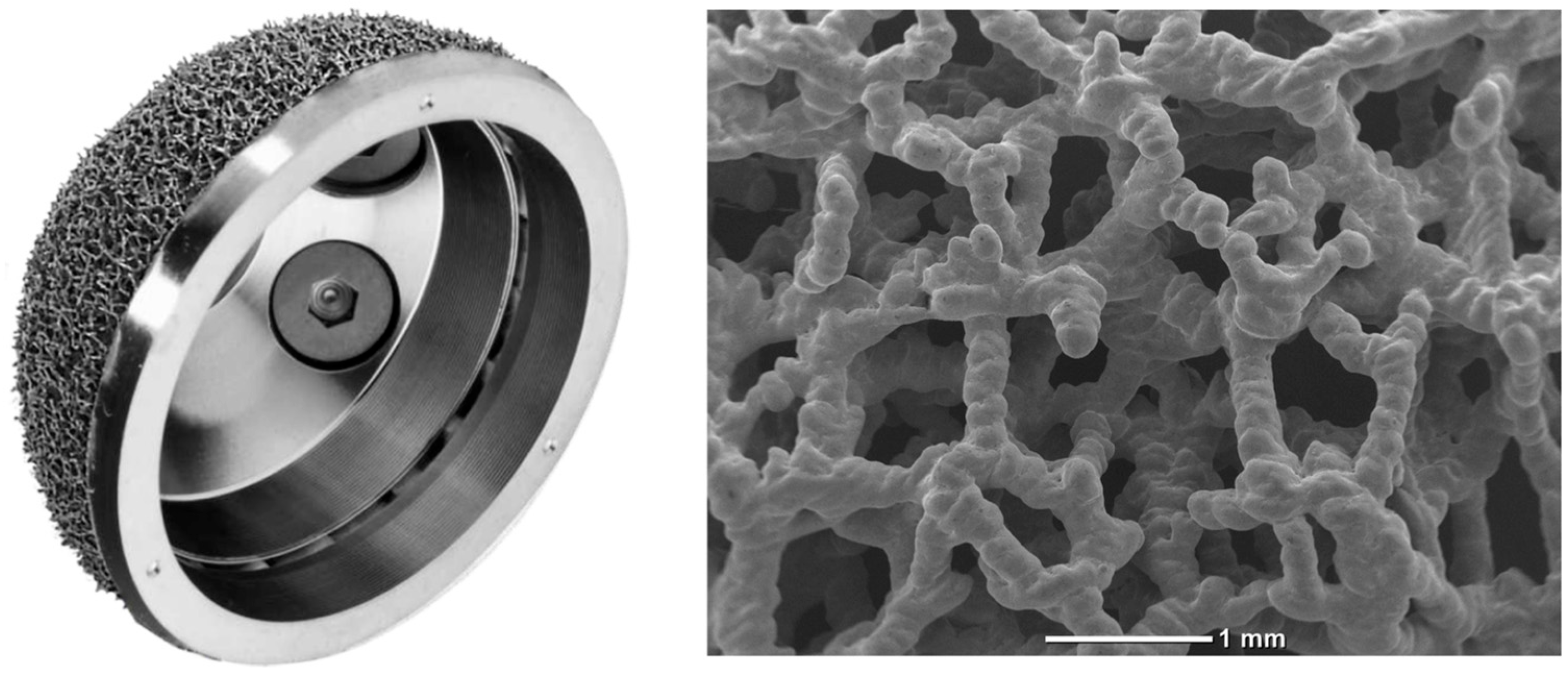
2.2. Implant Description
2.3. Functional Assessment
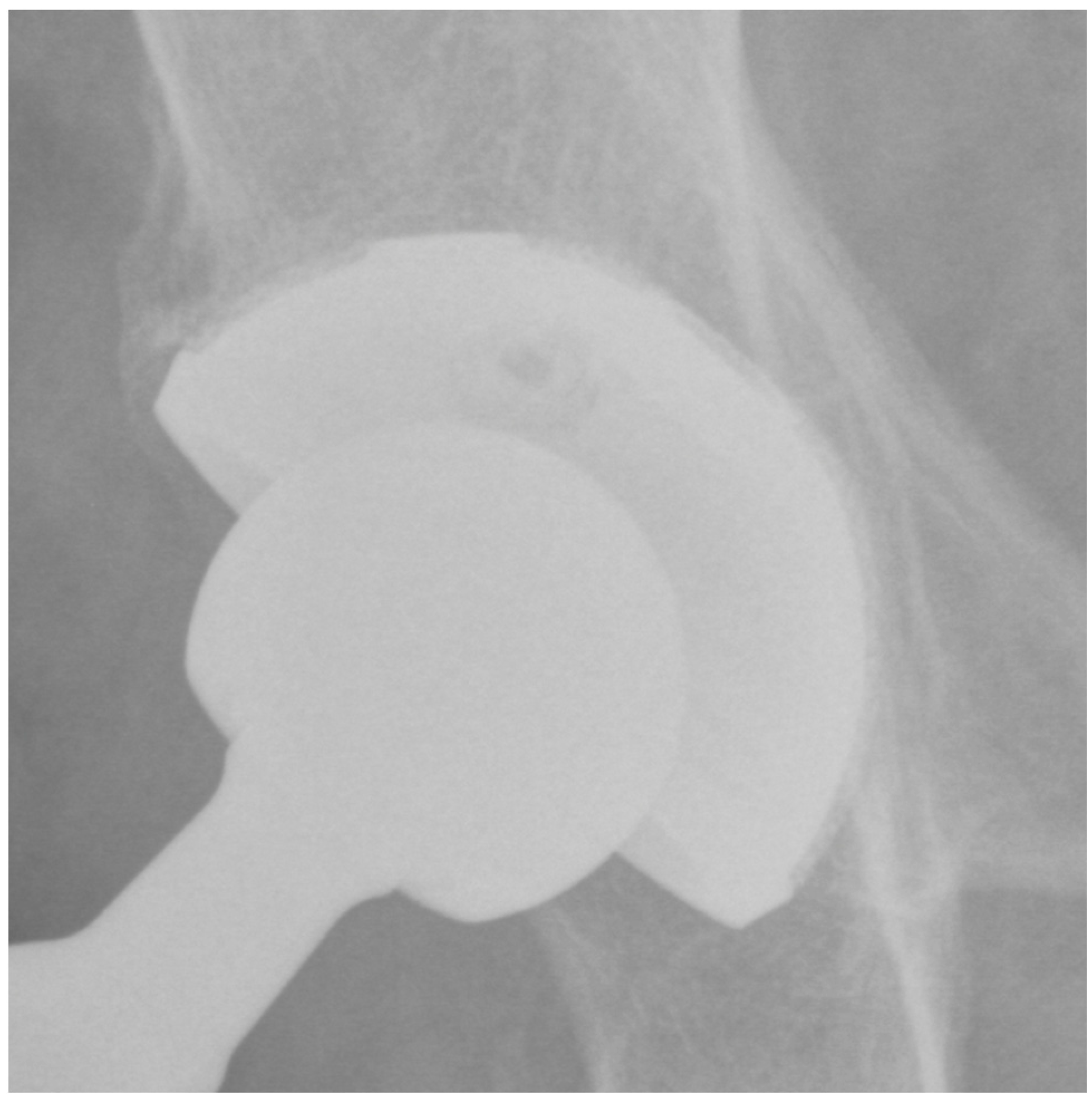
2.4. Imaging Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mercurio, M.; Gasparini, G.; Sanzo, V.; Familiari, F.; Castioni, D.; Galasso, O. Cemented Total Knee Arthroplasty Shows Less Blood Loss but a Higher Rate of Aseptic Loosening Compared with Cementless Fixation: An Updated Meta-Analysis of Comparative Studies. J. Arthroplast. 2022, 37, 1879–1887.e4. [Google Scholar] [CrossRef]
- De Martino, I.; Mancino, F.; Di Matteo, V.; Singlitico, A.; Maccauro, G.; Gasparini, G. Tantalum Cones for Severe Bone Defects in Revision Knee Arthroplasty: A Minimum 10-Year Follow-Up. J. Arthroplast. 2023, 38, 886–892. [Google Scholar] [CrossRef]
- Mancino, F.; Di Matteo, V.; Mocini, F.; Pietramala, S.; Singlitico, A.; De Fazio, A.; La Vergata, V.; Gasparini, G.; Maccauro, G.; De Martino, I. Short-Term Survivorship of 3D-Printed Titanium Metaphyseal Cones in Revision Total Knee Arthroplasty: A Systematic Review. Orthop. Rev. 2022, 14, 35891. [Google Scholar] [CrossRef] [PubMed]
- De Martino, I.; D’Apolito, R.; Sculco, P.K.; Poultsides, L.A.; Gasparini, G. Total Knee Arthroplasty Using Cementless Porous Tantalum Monoblock Tibial Component: A Minimum 10-Year Follow-Up. J. Arthroplast. 2016, 31, 2193–2198. [Google Scholar] [CrossRef]
- Macheras, G.A.; Lepetsos, P.; Leonidou, A.O.; Anastasopoulos, P.P.; Galanakos, S.P.; Poultsides, L.A. Survivorship of a Porous Tantalum Monoblock Acetabular Component in Primary Hip Arthroplasty with a Mean Follow-Up of 18 Years. J. Arthroplast. 2017, 32, 3680–3684. [Google Scholar] [CrossRef]
- De Martino, I.; De Santis, V.; Sculco, P.K.; D’Apolito, R.; Poultsides, L.A.; Gasparini, G. Long-Term Clinical and Radiographic Outcomes of Porous Tantalum Monoblock Acetabular Component in Primary Hip Arthroplasty: A Minimum of 15-Year Follow-Up. J. Arthroplast. 2016, 31, 110–114. [Google Scholar] [CrossRef]
- Galasso, O.; De Gori, M.; Cerbasi, S.; Familiari, F.; Recano, P.; Balato, G.; Gasparini, G.; Mariconda, M. Tantalum Monoblock Cups in Total Hip Arthroplasty: Clinical Results and Outcome Predictors. J. Biol. Regul. Homeost. Agents 2018, 32, 29–34. [Google Scholar] [PubMed]
- Hailer, N. 20 Years of Porous Tantalum in Primary and Revision Hip Arthroplasty-Time for a Critical Appraisal. Acta Orthop. 2018, 89, 254–255. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, H.; Peng, L.; Sun, Z. A Review of Research Progress in Selective Laser Melting (SLM). Micromachines 2022, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, Y.; Li, F.; Wang, X.; Zhang, K.; Liu, Z.; Tian, H. A New 3D Printing Porous Trabecular Titanium Metal Acetabular Cup for Primary Total Hip Arthroplasty: A Minimum 2-Year Follow-up of 92 Consecutive Patients. J. Orthop. Surg. 2020, 15, 383. [Google Scholar] [CrossRef]
- Naziri, Q.; Issa, K.; Pivec, R.; Harwin, S.F.; Delanois, R.E.; Mont, M.A. Excellent Results of Primary THA Using a Highly Porous Titanium Cup. Orthopedics 2013, 36, e390–e394. [Google Scholar] [CrossRef] [PubMed]
- Falck-Ytter, Y.; Francis, C.W.; Johanson, N.A.; Curley, C.; Dahl, O.E.; Schulman, S.; Ortel, T.L.; Pauker, S.G.; Colwell, C.W. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e278S–e325S. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Della Valle, C.J.; Suleiman, L.I.; Springer, B.D.; Gehrke, T.; Bini, S.A.; Segreti, J.; Chen, A.F.; Goswami, K.; Tan, T.L.; et al. Definition of Successful Infection Management and Guidelines for Reporting of Outcomes After Surgical Treatment of Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society (MSIS). J. Bone Jt. Surg. Am. 2019, 101, e69. [Google Scholar] [CrossRef] [PubMed]
- Grappiolo, G.; Bruno, C.F.; Loppini, M.; Mercurio, M.; Castioni, D.; Gasparini, G.; Galasso, O. Conversion of Fused Hip to Total Hip Arthroplasty: Long-Term Clinical and Radiological Outcomes. J. Arthroplast. 2021, 36, 1060–1066. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Neo, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Osteoinduction of Porous Bioactive Titanium Metal. Biomaterials 2004, 25, 443–450. [Google Scholar] [CrossRef]
- Kühne, J.H.; Bartl, R.; Frisch, B.; Hammer, C.; Jansson, V.; Zimmer, M. Bone Formation in Coralline Hydroxyapatite. Effects of Pore Size Studied in Rabbits. Acta Orthop. Scand. 1994, 65, 246–252. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Castioni, D.; Galasso, O.; Iannò, B.; Mercurio, M.; Gasparini, G. Posterior versus Lateral Surgical Approach: Functionality and Quality of Life after Total Hip Arthroplasty in a Matched Cohort Study. BMC Musculoskelet. Disord. 2021, 22, 932. [Google Scholar] [CrossRef]
- Ritter, M.A.; Fechtman, R.W.; Keating, E.M.; Faris, P.M. The Use of a Hip Score for Evaluation of the Results of Total Hip Arthroplasty. J. Arthroplast. 1990, 5, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Behrend, H.; Giesinger, K.; Giesinger, J.M.; Kuster, M.S. The “Forgotten Joint” as the Ultimate Goal in Joint Arthroplasty: Validation of a New Patient-Reported Outcome Measure. J. Arthroplast. 2012, 27, 430–436.e1. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.-H. A Simplified Method to Determine Acetabular Cup Anteversion from Plain Radiographs. J. Arthroplast. 2004, 19, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.S.; McAuley, J.P.; Young, A.M.; Engh, C.A. Radiographic Signs of Osseointegration in Porous-Coated Acetabular Components. Clin. Orthop. 2006, 444, 176–183. [Google Scholar] [CrossRef] [PubMed]
- DeLee, J.G.; Charnley, J. Radiological Demarcation of Cemented Sockets in Total Hip Replacement. Clin. Orthop. 1976, 121, 20–32. [Google Scholar] [CrossRef]
- Brooker, A.F.; Bowerman, J.W.; Robinson, R.A.; Riley, L.H. Ectopic Ossification following Total Hip Replacement. Incidence and a Method of Classification. J. Bone Jt. Surg. Am. 1973, 55, 1629–1632. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Lewinnek, G.E.; Lewis, J.L.; Tarr, R.; Compere, C.L.; Zimmerman, J.R. Dislocations after Total Hip-Replacement Arthroplasties. J. Bone Jt. Surg. Am. 1978, 60, 217–220. [Google Scholar] [CrossRef]
- de Filippis, R.; Mercurio, M.; Garcia, C.S.; De Fazio, P.; Gasparini, G.; Galasso, O. Defining the Minimum Clinically Important Difference (MCID) in the Hospital Anxiety and Depression Scale (HADS) in Patients Undergoing Total Hip and Knee Arthroplasty. Orthop. Traumatol. Surg. Res. OTSR 2023, 103689. [Google Scholar] [CrossRef]
- Tubach, F.; Wells, G.A.; Ravaud, P.; Dougados, M. Minimal Clinically Important Difference, Low Disease Activity State, and Patient Acceptable Symptom State: Methodological Issues. J. Rheumatol. 2005, 32, 2025–2029. [Google Scholar]
- Gasparini, G.; De Benedetto, M.; Cundari, A.; De Gori, M.; Orlando, N.; McFarland, E.G.; Galasso, O.; Castricini, R. Predictors of Functional Outcomes and Recurrent Shoulder Instability after Arthroscopic Anterior Stabilization. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 406–413. [Google Scholar] [CrossRef]
- Ciriello, V.; La China, R.; Chirillo, D.F.; Bianco, G.; Fusini, F.; Scarlato, U.; Albanese, C.; Bonzanini, G.; Banci, L.; Piovani, L. Is Modular Dual Mobility Superior to Standard Bearings for Reducing Dislocation Risk after Primary Total Hip Arthroplasty? A Retrospective Comparative Multicenter Study. J. Clin. Med. 2023, 12, 4200. [Google Scholar] [CrossRef]
- Dall’Ava, L.; Hothi, H.; Henckel, J.; Di Laura, A.; Tirabosco, R.; Eskelinen, A.; Skinner, J.; Hart, A. Osseointegration of Retrieved 3D-Printed, off-the-Shelf Acetabular Implants. Bone Jt. Res. 2021, 10, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, Y.-X.; Tian, H.; Wang, J.-W.; Liu, W.-G.; Li, H. Minimum 7-Year Follow-up of a Porous Coated Trabecular Titanium Cup Manufactured with Electron Beam Melting Technique in Primary Total Hip Arthroplasty. Orthop. Surg. 2021, 13, 817–824. [Google Scholar] [CrossRef]
- McLean, J.M.; Cappelletto, J.; Clarnette, J.; Hill, C.L.; Gill, T.; Mandziak, D.; Leith, J. Normal Population Reference Values for the Oxford and Harris Hip Scores-Electronic Data Collection and Its Implications for Clinical Practice. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2017, 27, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Yamamoto, Y.; Harada, Y.; Inoue, R.; Sasaki, E.; Ishibashi, Y. Radiographic Assessment of Radiolucent Lines around a Highly Porous Titanium Cup (Tritanium) Using Digital Tomosynthesis, after Total Hip Arthroplasty. J. Orthop. Surg. 2021, 16, 266. [Google Scholar] [CrossRef] [PubMed]
- Rambani, R.; Nayak, M.; Aziz, M.S.; Almeida, K. Tantalum versus Titanium Acetabular Cups in Primary Total Hip Arthroplasty: Current Concept and a Review of the Current Literature. Arch. Bone Jt. Surg. 2022, 10, 385–394. [Google Scholar] [CrossRef]
- Gkiatas, I.; Xiang, W.; Karasavvidis, T.; Windsor, E.N.; Malahias, M.-A.; Tarity, T.D.; Sculco, P.K. Relatively Low Rate of Heterotopic Ossification following Primary Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21.00096. [Google Scholar] [CrossRef]
- Kocic, M.; Lazovic, M.; Mitkovic, M.; Djokic, B. Clinical Significance of the Heterotopic Ossification after Total Hip Arthroplasty. Orthopedics 2010, 33, 16. [Google Scholar] [CrossRef]
- Mariconda, M.; Galasso, O.; Costa, G.G.; Recano, P.; Cerbasi, S. Quality of Life and Functionality after Total Hip Arthroplasty: A Long-Term Follow-up Study. BMC Musculoskelet. Disord. 2011, 12, 222. [Google Scholar] [CrossRef] [PubMed]
| Patients (n = 104) | Median (IQR) or n (%) |
|---|---|
| Sex | |
| Male | 41 (39.4%) |
| Female | 63 (60.6%) |
| Age at surgery (years) | 73 (65–79) |
| Side | |
| Right | 54 (51.9%) |
| Left | 50 (48.1%) |
| Follow-up (months) | 61 (52–69) |
| Moore Sign | N (%) |
|---|---|
| Absence of radiolucent lines | 104 (100%) |
| Presence of a superolateral buttress | 95 (91%) |
| Presence of medial stress-shielding | 85 (82%) |
| Presence of radial trabeculae | 70 (67%) |
| Presence of inferomedial buttress | 75 (72%) |
| Postop. FJS-12 | Postop. HHS | HHS var. | ||
|---|---|---|---|---|
| acetabular sclerosis | yes | 94 (50–94) | 85.8 (74.7–91.8) | 28.5 (25.1–30.6) |
| no | 98 (10–100) | 94.8 (38.2–95.8) | 26.6 (0–48.5) | |
| p = 0.145 | p = 0.096 | p = 0.938 | ||
| heterotopic ossifications | yes | 96 (90–100) | 95.8 (85.8–95.8) | 30.6 (23.6–47.1) |
| no | 96 (10–100) | 94.7 (38.2–95.8) | 26.6 (0–48.5) | |
| p = 0.383 | p = 0.452 | p = 0.46 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Familiari, F.; Barone, A.; De Gori, M.; Banci, L.; Palco, M.; Simonetta, R.; Gasparini, G.; Mercurio, M.; Calafiore, G. Short- to Mid-Term Clinical and Radiological Results of Selective Laser Melting Highly Porous Titanium Cup in Primary Total Hip Arthroplasty. J. Clin. Med. 2024, 13, 969. https://doi.org/10.3390/jcm13040969
Familiari F, Barone A, De Gori M, Banci L, Palco M, Simonetta R, Gasparini G, Mercurio M, Calafiore G. Short- to Mid-Term Clinical and Radiological Results of Selective Laser Melting Highly Porous Titanium Cup in Primary Total Hip Arthroplasty. Journal of Clinical Medicine. 2024; 13(4):969. https://doi.org/10.3390/jcm13040969
Chicago/Turabian StyleFamiliari, Filippo, Alessandro Barone, Marco De Gori, Lorenzo Banci, Michelangelo Palco, Roberto Simonetta, Giorgio Gasparini, Michele Mercurio, and Giuseppe Calafiore. 2024. "Short- to Mid-Term Clinical and Radiological Results of Selective Laser Melting Highly Porous Titanium Cup in Primary Total Hip Arthroplasty" Journal of Clinical Medicine 13, no. 4: 969. https://doi.org/10.3390/jcm13040969
APA StyleFamiliari, F., Barone, A., De Gori, M., Banci, L., Palco, M., Simonetta, R., Gasparini, G., Mercurio, M., & Calafiore, G. (2024). Short- to Mid-Term Clinical and Radiological Results of Selective Laser Melting Highly Porous Titanium Cup in Primary Total Hip Arthroplasty. Journal of Clinical Medicine, 13(4), 969. https://doi.org/10.3390/jcm13040969








