Changes in the Visual Field Test after Descemet Stripping Automated Endothelial Keratoplasty in Advanced Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Procedures and Postoperative Management
2.2. Intraoperative Complications
2.3. Data Collection and Analysis
2.4. Evaluation of the Visual Field
- (a)
- Humphery field analyzer (HFA)
- (b)
- Goldman perimetry (GP)
2.5. Definition of Advanced Glaucoma
3. Results
3.1. Patient Demographics
3.2. Visual Acuity
3.3. Postoperative IOP Control and Graft Outcomes
3.4. Visual Field Test
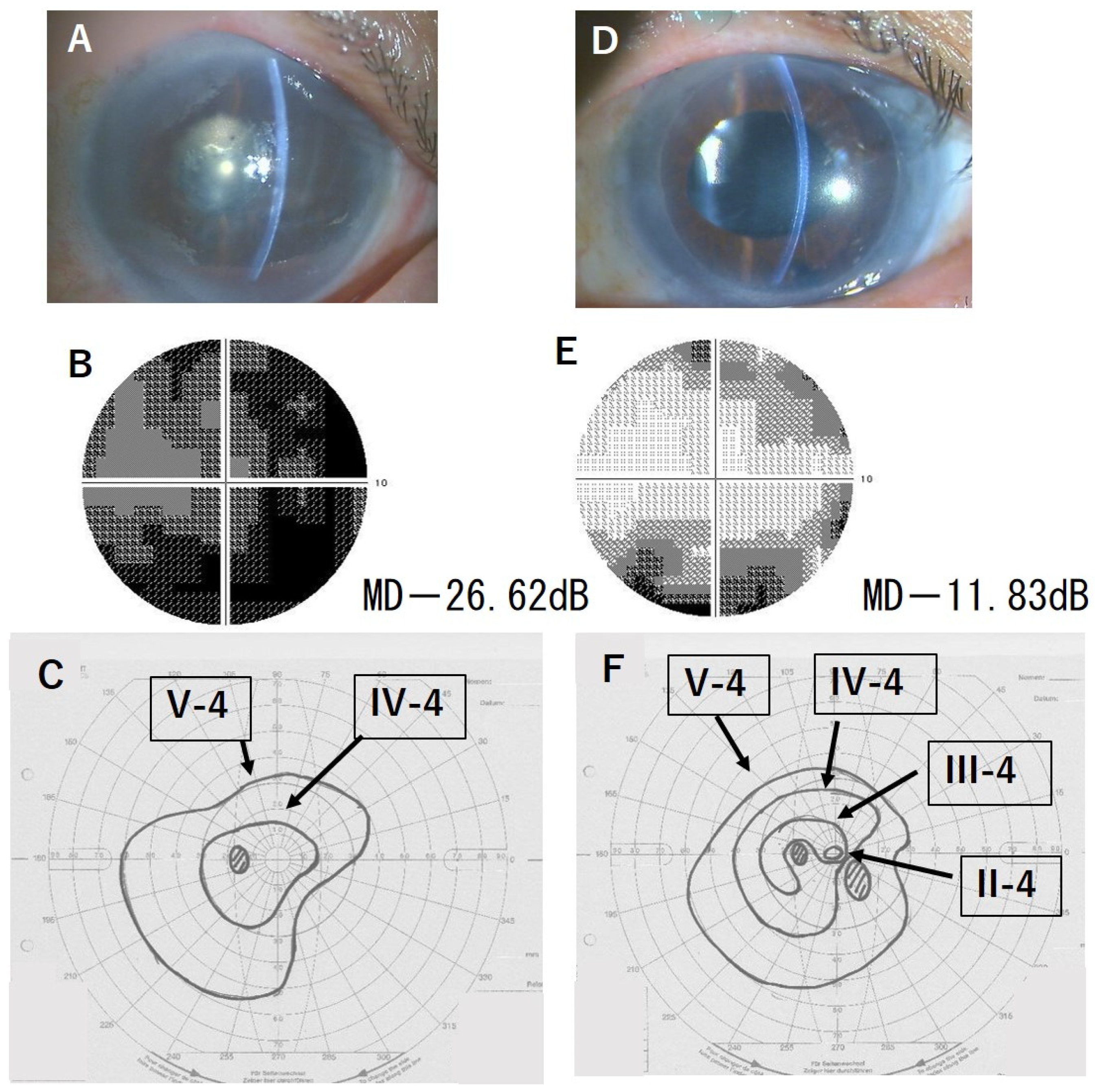
3.5. Representative Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vajaranant, T.S.; Price, M.O.; Price, F.W.; Gao, W.; Wilensky, J.T.; Edward, D.P. Visual acuity and intraocular pressure after Descemet’s stripping endothelial keratoplasty in eyes with and without preexisting glaucoma. Ophthalmology 2009, 116, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Wiaux, C.; Baghdasaryan, E.; Lee, O.L.; Bourges, J.-L.; Deng, S.X.; Yu, F.; Aldave, A.J. Outcomes after Descemet stripping endothelial keratoplasty in glaucoma patients with previous trabeculectomy and tube shunt implantation. Cornea 2011, 30, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Aldave, A.J.; Chen, J.L.; Zaman, A.S.; Deng, S.X.; Yu, F. Outcomes after DSEK in 101 eyes with previous trabeculectomy and tube shunt implantation. Cornea 2014, 33, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kurita, J.; Hayashi, T.; Shimizu, T.; Sunouchi, C.; Hara, Y.; Kobayashi, A.; Yamagami, S. Postoperative increase in intraocular pressure after penetrating keratoplasty and Descemet stripping automated endothelial keratoplasty in Asian patients. Eye Bank. Corneal Transplant. 2023, 2, e0009. [Google Scholar] [CrossRef]
- Anshu, A.; Price, M.O.; Price, F.W. Descemet’s stripping endothelial keratoplasty: Long-term graft survival and risk factors for failure in eyes with preexisting glaucoma. Ophthalmology 2012, 119, 1982–1987. [Google Scholar] [CrossRef]
- Kang, J.J.; Ritterband, D.C.; Atallah, R.T.; Liebmann, J.M.; Seedor, J.A. Clinical Outcomes of descemet stripping Endothelial Keratoplasty in Eyes with glaucoma drainage devices. J. Glaucoma 2019, 28, 601–605. [Google Scholar] [CrossRef]
- Ni, N.; Sperling, B.J.; Dai, Y.; Hannush, S.B. Outcomes after Descemet stripping endothelial keratoplasty in patients with glaucoma drainage devices. Cornea 2015, 34, 870–875. [Google Scholar] [CrossRef]
- Alshaker, S.; Mimouni, M.; Batawi, H.; Cohen, E.; Trinh, T.M.; Santaella, G.; Chan, C.C.M.; Slomovic, A.R.M.; Rootman, D.S.M.; Sorkin, N. Four-Year Survival Comparison of Endothelial Keratoplasty Techniques in Patients with Previous Glaucoma Surgery. Cornea 2021, 40, 1282–1289. [Google Scholar] [CrossRef]
- Takemori, H.; Higashide, T.; Kobayashi, A.; Yokogawa, H.; Sugiyama, K. Glaucoma-related risk factors for endothelial cell loss and graft failure after Descemet’s stripping automated endothelial keratoplasty. J. Glaucoma 2023, 32, e95–e102. [Google Scholar] [CrossRef]
- Kang, J.J.; Ritterband, D.C.; Lai, K.; Liebmann, J.M.; Seedor, J.A. Descemet stripping endothelial keratoplasty in eyes with previous glaucoma surgery. Cornea 2016, 35, 1520–1525. [Google Scholar] [CrossRef]
- Kim, P.; Amiran, M.D.; Lichtinger, A.; Yeung, S.N.; Slomovic, A.R.; Rootman, D.S. Outcomes of Descemet stripping automated endothelial keratoplasty in patients with glaucoma drainage devices. Cornea 2012, 31, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Feltgen, N.; Junker, B.; Schulze-Bonsel, K.; Bach, M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity test. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 247, 137–142. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Disease. Tenth Revision. Clinical Modification (ICD-10-CM); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Murata, H.; Hirasawa, H.; Aoyama, Y.; Sugisaki, K.; Araie, M.; Mayama, C.; Aihara, M.; Asaoka, R. Identifying areas of the visual field important for quality of life in patients with glaucoma. PLoS ONE 2013, 8, e58695. [Google Scholar] [CrossRef] [PubMed]
- McKean-Cowdin, R.; Wang, Y.; Wu, J.; Azen, S.P.; Varma, R.; Los Angeles Latino Eye Study Group. Impact of visual field loss on health-related quality of life in glaucoma: The Los Angeles Latino Eye Study. Ophthalmology 2008, 115, 941–948.e1. [Google Scholar] [CrossRef]
- Abe, R.Y.; Diniz-Filho, A.; Costa, V.P.; Gracitelli, C.P.; Baig, S.; Medeiros, F.A. The impact of location of progressive visual field loss on longitudinal changes in quality of life of patients with glaucoma. Ophthalmology 2016, 123, 552–557. [Google Scholar] [CrossRef]
- Sotimehin, A.E.; Ramulu, P.Y. Measuring disability in glaucoma. J. Glaucoma 2018, 27, 939–949. [Google Scholar] [CrossRef]
- Seol, B.R.; Jeoung, J.W.; Park, K.H. Changes of visual-field global indices after cataract surgery in primary open-angle glaucoma patients. Jpn. J. Ophthalmol. 2016, 60, 439–455. [Google Scholar] [CrossRef]
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar] [CrossRef]
- Yohannan, J.; Boland, M.V.; Ramulu, P. The association between intraocular pressure and visual field worsening in treated glaucoma patients. J. Glaucoma 2021, 30, 759–768. [Google Scholar] [CrossRef]
- Chang, D.T.; Pantcheva, M.B.; Noecker, R.J. Corneal thickness and intraocular pressure in edematous corneas before and after Descemet stripping with automated endothelial keratoplasty. Cornea 2010, 29, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, Y.; Kobayashi, A.; Yokogawa, H.; Sugiyama, K. Intraocular pressure after Descemet’s stripping and non-Descemet’s stripping automated endothelial keratoplasty. Jpn. J. Ophthalmol. 2011, 55, 98–102. [Google Scholar] [CrossRef]
- Elalfy, M.; Maqsoos, S.; Soliman, S.; Heazy, S.M.; Hannon, A.A.; Gatziufas, Z.; Lake, D.; Hamada, S. Incidence and risk factors of ocular hypertension/glaucoma after Descemet stripping endothelial keratoplasty. Clin. Ophthalmol. 2021, 15, 2179–2188. [Google Scholar] [CrossRef]
- Lehman, R.E.; Copeland, L.A.; Stock, E.M.; Fulcher, S.F. Graft deratchment rate in DSEK/DSAEK after same-day complete air removal. Cornea 2015, 34, 1358–1361. [Google Scholar] [CrossRef]
- Soleimani, M.; Alizadeh, A.; Irannejad, M.; Shahriari, M.; Cheraqpour, K. Management of lenticular detachment and epithelial downgrowth after Descemet stripping endothelial keratoplasty: A novel technique and brief literature review. BMC Ophthalmol. 2022, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Itty, S.; Proia, A.D.; DelMonte, D.W.; Santaella, R.M.; Carlson, A.; Allingham, R.R. Clinical course and origin of epithelium in cases of epithelial downgrowth after Descemet stripping endothelial keratoplasty. Cornea 2014, 33, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Rodler, K.D.; Pregun, T.; Andras, B.; Pluzsik, M.T.; Hargitai, J.; Kerenyi, A. Posterior chamber intraocular lens dislocation into the vitreous in association with Descemet stripping endothelial keratoplasty. Cornea 2022, 41, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Wakimasu, K.; Kitazawa, K.; Kayukawa, K.; Yokota, I.; Inatomi, T.; Hieda, O.; Sotozono, C.; Kinoshita, S. Five-year follow-up outcomes after Descemet’s stripping automated endothelial keratoplasty: A retrospective study. BMJ Open Ophthalmol. 2020, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Kuchtey, J.; Rezaei, K.A.; Jaru-Ampornpan, P.; Sternberg, P., Jr.; Kuchtey, R.W. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6441–6447. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Yamaguchi, T.; Nishisako, S.; Matsumura, T.; Fukui, M.; Higa, K.; Inoue, T.; Shimazaki, J. Aqueous Cytokine Levels Are Associated with Progression of Peripheral Anterior Synechiae after Descemet Stripping Automated Endothelial Keratoplasty. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Ratio/Percentage/Stages |
|---|---|
| Sex (male–female) | 14:5 |
| Age at the time of DSAEK (years) | 73 ± 11 (mean ± SD) |
Type of glaucoma
| 7 (36.8%) eyes Normal tension glaucoma in 1 eye 6 (31.6%) eyes 6 (31.6%) eyes |
| Lens status | Pseudophakic in all eyes |
| Glaucoma severity | Advanced in all eyes |
| Case | BCVA (logMAR) | VF MD (dB) | VF Improvement | ||
|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | (Difference in Value) | |
| 1 | 1.2 | 0.4 | −32.11 | −27.36 | I (+4.75) |
| 2 | 1.7 | 0.5 | −26.62 | −11.83 | I (+14.75) |
| 3 | 2.0 | 0.15 | −28.03 | −17.25 | I (+10.78) |
| 4 | 1.4 | 0.7 | −21.06 | −20.36 | N (+0.7) |
| 5 | 2.3 | 0.7 | −25.80 | −25.36 | N (+0.44) |
| 6 | 0.5 | 0.3 | −12.81 | −13.77 | N (−0.96) |
| 7 | 1.1 | 0.4 | −13.95 | −15.98 | W (−2.03) |
| 8 | 2.3 | 1.4 | −20.04 | −15.99 | I (+4.05) |
| 9 | 1.2 | 0.4 | −19.77 | −17.30 | I (+2.47) |
| Average 1.6 ± 0.6, 0.8 ± 0.5 | −22.24 ± 6.5, −18.36 ± 5.1 | ||||
| Case | BCVA (logMAR) | Total Degree of VF by the Target of I-4e | Detection of New Isopter * | VF Improvement | ||
|---|---|---|---|---|---|---|
| Baseline | After | Baseline | After | |||
| DSAEK | DSAEK | |||||
| 1 | 1.2 | 0.4 | 0 | 20 | + | I |
| 2 | 1.7 | 0.5 | 0 | 81 | + | I |
| 3 | 2 | 0.15 | 9 | 140 | + | I |
| 4 | 1.4 | 0.7 | 45 | 50 | − | N |
| 5 | 2.3 | 0.7 | 0 | 76 | + | I |
| 10 | 1.3 | 0.8 | 0 | 87 | + | I |
| 11 | 2.3 | 1.3 | 0 | 0 | + | I |
| 12 | 2.3 | 1.7 | 0 | 0 | − | N |
| 13 | 2.3 | 2.3 | 0 | 0 | − | N |
| 14 | 1.1 | 0.7 | 0 | 85 | + | I |
| 15 | 2.3 | 1.0 | 0 | 57 | + | I |
| 16 | 1.2 | 0.4 | 0 | 141 | + | I |
| 17 | 1.7 | 0.7 | 0 | 21 | + | I |
| 18 | 2.3 | 0.8 | 35 | 10 | − | W |
| 19 | 0.7 | 0.4 | 78 | 84 | − | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyokawa, N.; Araki-Sasaki, K.; Kimura, H.; Kuroda, S. Changes in the Visual Field Test after Descemet Stripping Automated Endothelial Keratoplasty in Advanced Glaucoma. J. Clin. Med. 2024, 13, 1431. https://doi.org/10.3390/jcm13051431
Toyokawa N, Araki-Sasaki K, Kimura H, Kuroda S. Changes in the Visual Field Test after Descemet Stripping Automated Endothelial Keratoplasty in Advanced Glaucoma. Journal of Clinical Medicine. 2024; 13(5):1431. https://doi.org/10.3390/jcm13051431
Chicago/Turabian StyleToyokawa, Noriko, Kaoru Araki-Sasaki, Hideya Kimura, and Shinichiro Kuroda. 2024. "Changes in the Visual Field Test after Descemet Stripping Automated Endothelial Keratoplasty in Advanced Glaucoma" Journal of Clinical Medicine 13, no. 5: 1431. https://doi.org/10.3390/jcm13051431
APA StyleToyokawa, N., Araki-Sasaki, K., Kimura, H., & Kuroda, S. (2024). Changes in the Visual Field Test after Descemet Stripping Automated Endothelial Keratoplasty in Advanced Glaucoma. Journal of Clinical Medicine, 13(5), 1431. https://doi.org/10.3390/jcm13051431






