Fatigue Item Response among Hemoglobin-Normalized Patients with Paroxysmal Nocturnal Hemoglobinuria: PEGASUS Trial Results at 16 and 48 Weeks
Abstract
Highlights
- Patients who switched from Ecu to Peg, reported improvements in fatigue, with greater improvements for those whose hemoglobin normalized
- We demonstrate treatment-related reductions in fatigue by highlighting the items most responsive to clinically important changes in a patient-reported outcome measure
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Sample
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Sample
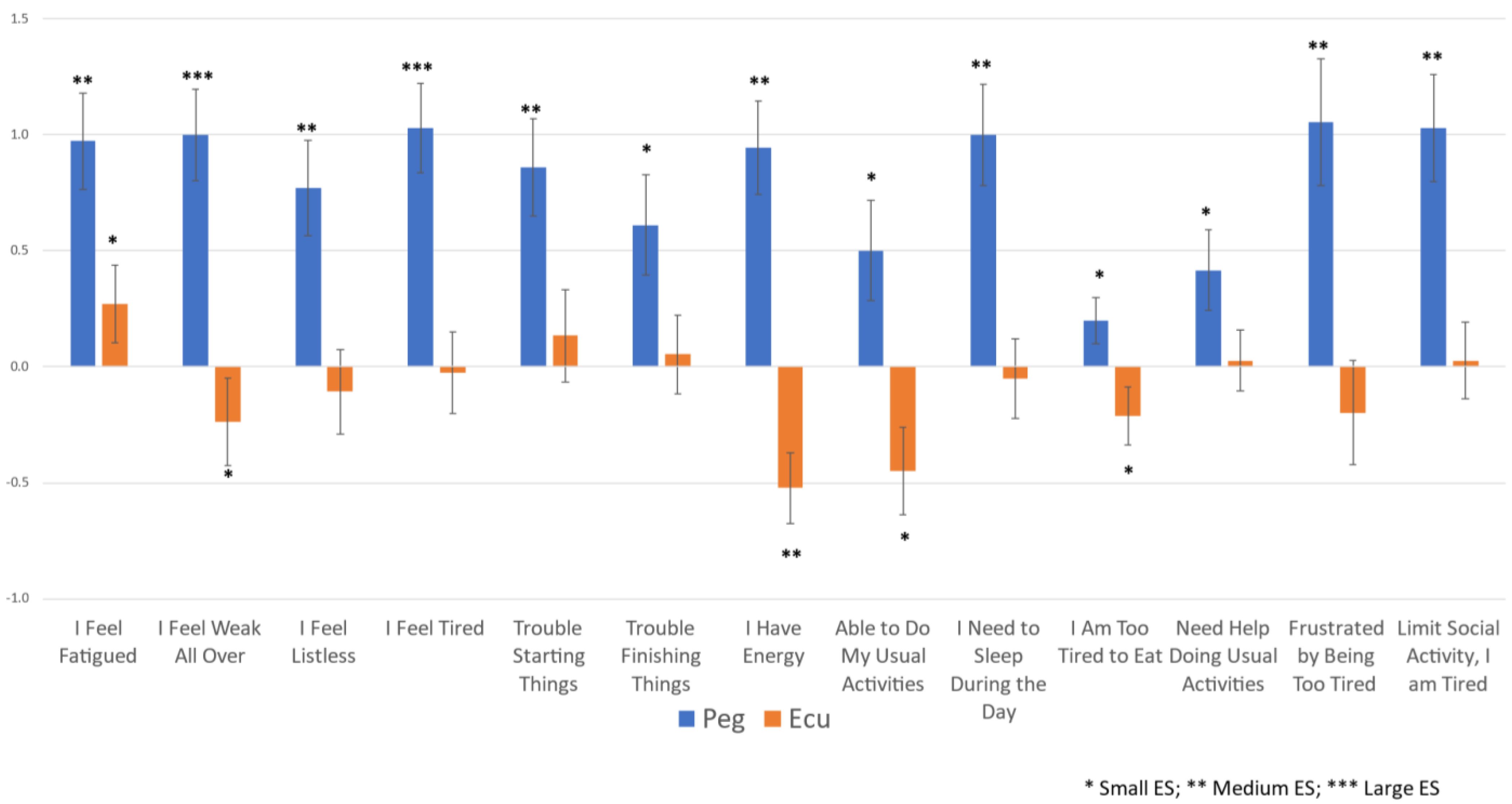
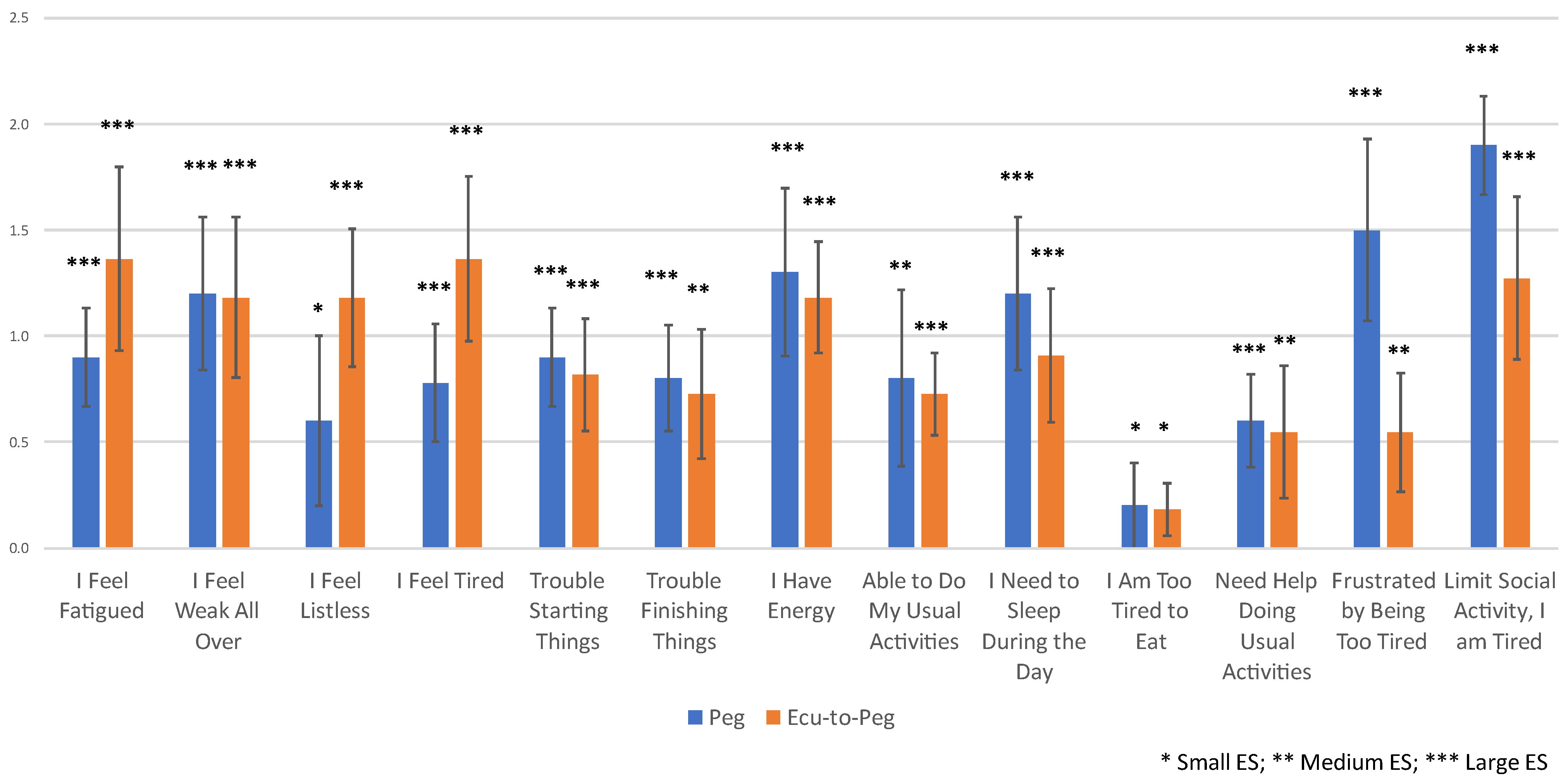
3.2. Fatigue Changes over Time
3.3. Relationship of Hb Normalization to Fatigue Changes over Time
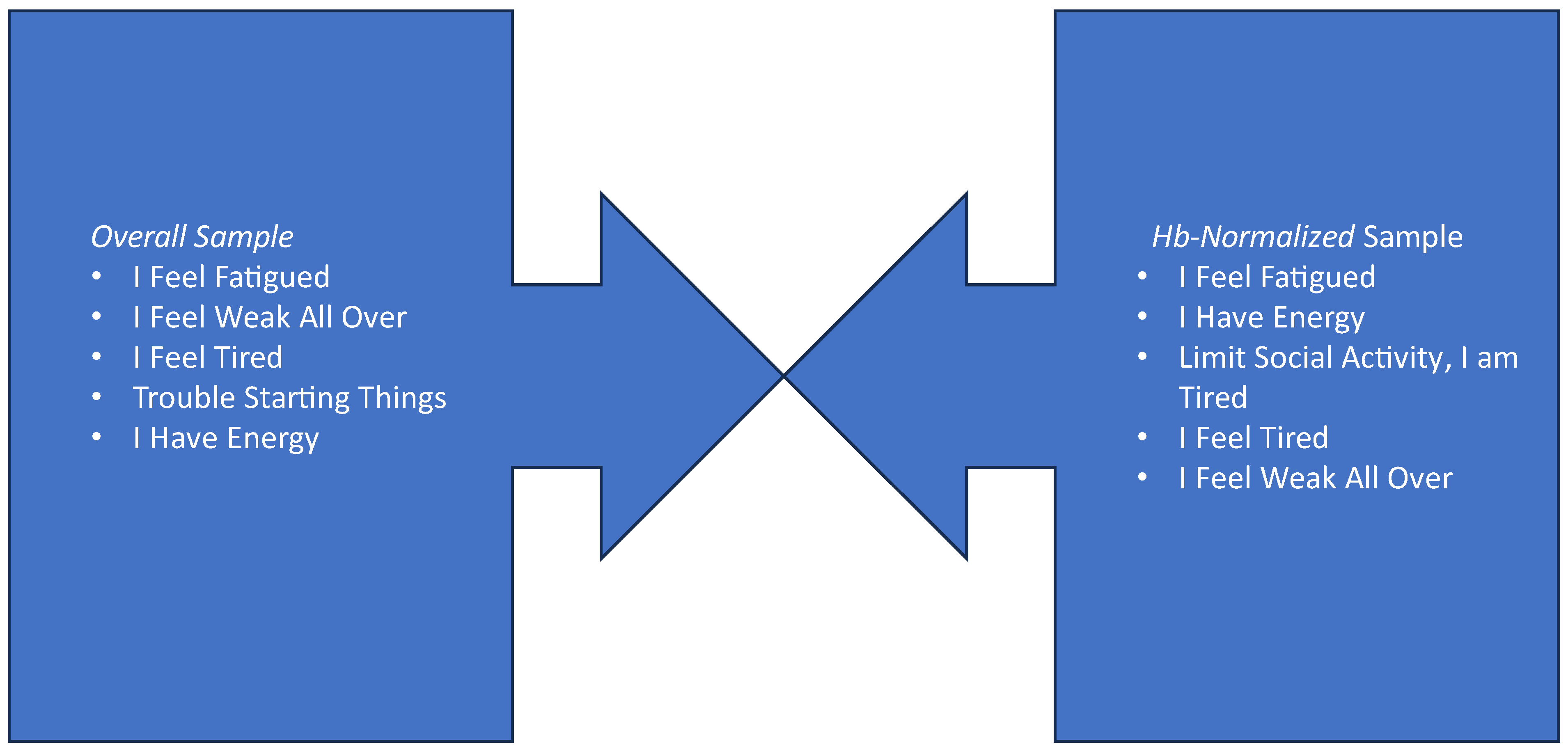
3.4. Item Content Most Reflective of Changes in Fatigue among Hb-Normalized Patients
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luzzatto, L. Recent advances in the pathogenesis and treatment of paroxysmal nocturnal hemoglobinuria. F1000Research 2016, 5, 209. [Google Scholar] [CrossRef]
- Schrezenmeier, H.; Muus, P.; Socié, G.; Szer, J.; Urbano-Ispizua, A.; Maciejewski, J.P.; Brodsky, R.A.; Bessler, M.; Kanakura, Y.; Rosse, W.; et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica 2014, 99, 922. [Google Scholar] [CrossRef]
- Hillmen, P.; Lewis, S.; Bessler, M.; Luzzatto, L.; Dacie, J.V. Natural history of paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 1995, 333, 1253–1258. [Google Scholar] [CrossRef]
- Nishimura, J.-I.; Kanakura, Y.; Ware, R.E.; Shichishima, T.; Nakakuma, H.; Ninomiya, H.; Decastro, C.M.; Hall, S.; Kanamaru, A.; Sullivan, K.M.; et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine 2004, 83, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Rother, R.P.; Wang, X.; Morris, J.S.M.; Quinn-Senger, K.; Kelly, R.; Richards, S.J.; Bessler, M.; Bell, L.; Hillmen, P.; et al. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2010, 149, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Elebute, M.; Kelly, R.; Urbano-Ispizua, A.; Hill, A.; Rother, R.P.; Khursigara, G.; Fu, C.; Omine, M.; Browne, P.; et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am. J. Hematol. 2010, 85, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Omine, M.; Richards, S.; Nishimura, J.-I.; Bessler, M.; Ware, R.; Hillmen, P.; Luzzatto, L.; Young, N.; Kinoshita, T.; et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005, 106, 3699–3709. [Google Scholar] [CrossRef] [PubMed]
- Weitz, I.; Meyers, G.; Lamy, T.; Cahn, J.; Uranga, M.T.; Vela, J.A.G.; Sanz, M.A.; Severino, B.; Kelly, R.J.; Hillmen, P.; et al. Cross-sectional validation study of patient-reported outcomes in patients with paroxysmal nocturnal haemoglobinuria. Intern. Med. J. 2013, 43, 298–307. [Google Scholar] [CrossRef]
- de Latour, R.P.; Mary, J.Y.; Salanoubat, C.; Terriou, L.; Etienne, G.; Mohty, M.; Roth, S.; de Guilbert, S.; Maury, S.; Cahn, J.Y. Paroxysmal nocturnal hemoglobinuria: Natural history of disease subcategories. Blood J. Am. Soc. Hematol. 2008, 112, 3099–3106. [Google Scholar] [CrossRef]
- Hillmen, P.; Muus, P.; Röth, A.; Elebute, M.O.; Risitano, A.M.; Schrezenmeier, H.; Szer, J.; Browne, P.; Maciejewski, J.P.; Schubert, J.; et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013, 162, 62–73. [Google Scholar] [CrossRef]
- Loschi, M.; Porcher, R.; Barraco, F.; Terriou, L.; Mohty, M.; de Guibert, S.; Mahe, B.; Lemal, R.; Dumas, P.; Etienne, G.; et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: A treatment versus no-treatment study. Am. J. Hematol. 2016, 91, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Mary, J.-Y.; de Gramont, A.; Rio, B.; Leporrier, M.; Rose, C.; Heudier, P.; Rochant, H.; Cahn, J.-Y.; Gluckman, E. Paroxysmal nocturnal haemoglobinuria: Long-term follow-up and prognostic factors. Lancet 1996, 348, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Hill, A.; Arnold, L.M.; Brooksbank, G.L.; Richards, S.J.; Cullen, M.; Mitchell, L.D.; Cohen, D.R.; Gregory, W.M.; Hillman, P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: Sustained efficacy and improved survival. Blood 2011, 117, 6786–6792. [Google Scholar] [CrossRef] [PubMed]
- Yenerel, M.N.; Muus, P.; Wilson, A.; Szer, J. Clinical course and disease burden in patients with paroxysmal nocturnal hemoglobinuria by hemolytic status. Blood Cells Mol. Dis. 2017, 65, 29–34. [Google Scholar] [CrossRef]
- Lee, J.W.; Jang, J.H.; Kim, J.S.; Yoon, S.-S.; Lee, J.-H.; Kim, Y.-K.; Jo, D.-Y.; Chung, J.; Sohn, S.K. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int. J. Hematol. 2013, 97, 749–757. [Google Scholar] [CrossRef]
- Yu, F.; Du, Y.; Han, B. A comparative analysis of clinical characteristics of patients with paroxysmal nocturnal hemoglobinuria between Asia and Europe/America. Int. J. Hematol. 2016, 103, 649–654. [Google Scholar] [CrossRef]
- Schrezenmeier, H.; Röth, A.; Araten, D.J.; Kanakura, Y.; Larratt, L.; Shammo, J.M.; Wilson, A.; Shayan, G.; Maciejewski, J.P. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): Updated analysis from the International PNH Registry. Ann. Hematol. 2020, 99, 1505–1514. [Google Scholar] [CrossRef]
- Jalbert, J.J.; Chaudhari, U.; Zhang, H.; Weyne, J.; Shammo, J.M. Epidemiology of PNH and real-world treatment patterns following an incident PNH diagnosis in the US. Blood 2019, 134, 3407. [Google Scholar] [CrossRef]
- Richards, S.J.; Painter, D.; Dickinson, A.J.; Griffin, M.; Munir, T.; Arnold, L.; Payne, D.; Pike, A.; Muus, P.; Hill, A.; et al. The incidence and prevalence of patients with paroxysmal nocturnal haemoglobinuria and aplastic anaemia PNH syndrome: A retrospective analysis of the UK’s population-based haematological malignancy research network 2004-2018. Eur. J. Haematol. 2021, 107, 211–218. [Google Scholar] [CrossRef]
- Glaus, A.; Müller, S. Hemoglobin and fatigue in cancer patients: Inseparable twins? Schweiz. Med. Wochenschr. 2000, 130, 471–477. [Google Scholar]
- Beutler, E.; Waalen, J. The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef]
- Holzner, B.; Kemmler, G.; Greil, R.; Kopp, M.; Zeimet, A.; Raderer, M.; Hejna, M.; Zöchbauer, S.; Krajnik, G.; Huber, H.; et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann. Oncol. 2002, 13, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Kallich, J.; McDermott, A.; Xu, X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: Results from five randomized clinical trials. Ann. Oncol. 2004, 15, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Holers, V.M.; Rotoli, B. TT30, a novel regulator of the Complement Alternative Pathway (CAP), inhibits hemolysis of Paroxysmal Nocturnal Hemoglobinuria (PNH) erythrocytes and prevents upstream C3 binding on their sSurface in an in vitro model. Blood 2009, 114, 158. [Google Scholar] [CrossRef]
- Stern, R.M.; Connell, N.T. Ravulizumab: A novel C5 inhibitor for the treatment of paroxysmal nocturnal hemoglobinuria. Ther. Adv. Hematol. 2019, 10, 2040620719874728. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, R.A.; De Latour, R.P.; Rottinghaus, S.T.; Röth, A.; Risitano, A.M.; Weitz, I.C.; Hillmen, P.; Maciejewski, J.P.; Szer, J.; Lee, J.W.; et al. Characterization of breakthrough hemolysis events observed in the phase III randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica 2021, 106, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Stark, R.B.; Borowiec, K.; Nolte, S.; Myren, K.-J. Norm-based comparison of the quality-of-life impact of ravulizumab and eculizumab in paroxysmal nocturnal hemoglobinuria. Orphanet J. Rare Dis. 2021, 16, 389. [Google Scholar] [CrossRef]
- Panse, J.; Wilson, K.; Fishman, J.; Wojciechowski, P.; Wdowiak, M.; Horneff, R.; Patriquin, C.J.; Oliver, M.; Hakimi, Z. Fatigue and health-related quality of life in paroxysmal nocturnal haemoglobinuria: A post hoc analysis of the pegcetacoplan PEGASUS trial data. Eur. J. Haematol. 2023, 111, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Szer, J.; Weitz, I.; Röth, A.; Höchsmann, B.; Panse, J.; Usuki, K.; Griffin, M.; Kiladjian, J.-J.; de Castro, C.; et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2021, 384, 1028–1037. [Google Scholar] [CrossRef]
- de Latour, R.P.; Szer, J.; Weitz, I.C.; Röth, A.; Höchsmann, B.; Panse, J.; Usuki, K.; Griffin, M.; Kiladijan, J.-J.; de Castro, C.M.; et al. Pegcetacoplan versus eculizumab in patients with paroxysmal nocturnal haemoglobinuria (PEGASUS): 48-week follow-up of a randomised, open-label, phase 3, active-comparator, controlled trial. Lancet Haematol. 2022, 9, e648–e659. [Google Scholar] [CrossRef]
- Cella, D.; Sarda, S.P.; Hsieh, R.; Fishman, J.; Hakimi, Z.; Hoffman, K.; Al-Adhami, M.; Nazir, J.; Cutts, K.; Lenderking, W.R. Changes in hemoglobin and clinical outcomes drive improvements in fatigue, quality of life, and physical function in patients with paroxysmal nocturnal hemoglobinuria: Post hoc analyses from the phase III PEGASUS study. Ann. Hematol. 2022, 101, 1905–1914. [Google Scholar] [CrossRef]
- Wong, R.S. Safety and efficacy of pegcetacoplan in paroxysmal nocturnal hemoglobinuria. Ther. Adv. Hematol. 2022, 13, 20406207221114673. [Google Scholar] [CrossRef]
- Cella, D.; Webster, K.; Beaumont, J. The FACIT-Fatigue Scale: Description, Reliability and Validity; Center on Outcomes Research and Education: Chicago, IL, USA, 2003; p. 1001. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Heymans, M.W.; Twisk, J.W. Handling missing data in clinical research. J. Clin. Epidemiol. 2022, 151, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Heron, J.; Sterne, J.A.; Tilling, K. Accounting for missing data in statistical analyses: Multiple imputation is not always the answer. Int. J. Epidemiol. 2019, 48, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- IBM. IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2021. [Google Scholar]

| Characteristic | Pegcetacoplan (n = 38) | Eculizumab (n = 38) |
|---|---|---|
| Age in years: mean (SD) | 49.9 (19–81) | 47.7 (23–78) |
| Female sex: no. (%) | 25 (66) | 22 (58) |
| Race: no (%) | ||
| Asian | 5 (13) | 7 (18) |
| Black | 2 (5) | 0 |
| White | 21 (55) | 25 (66) |
| Other | 0 | 1 (3) |
| Not reported | 10 (26) | 5 (13) |
| Body mass index: mean ± SD | 26.7 ± 3.9 | 25.9 ± 4.3 |
| Zero transfusions within previous 12 mo.: no. (%) | 9 (24) | 10 (26) |
| Median time since PNH diagnosis: no. years (range) | 6.0 (1–31) | 8.9 (1–38) |
| Hemoglobin: mean g/dL ± SD | 8.80 ± 1.00 | 8.67 ± 0.89 |
| FACIT-F score: mean ± SD | 31.1 ± 11.1 | 31.6 ± 12.5 |
| All Patients | Hb-Normalized Patients | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to Week 16 | Baseline to Week 48 | Baseline to Week 16 | Baseline to Week 48 | ||||||||||||||||
| FACIT-F Item No. and Content | Peg | Ecu * | Peg | Ecu-to-Peg | Peg | Ecu | Peg | Ecu-to-Peg | |||||||||||
| (n = 36) | (n = 37) | (n = 30) | (n = 29) | (n = 14) | (n = 10) | (n = 12) | |||||||||||||
| d | Rank | d | Rank | d | Rank | d | Rank | d | Rank | d | d | Rank | d | Rank | |||||
| 1 | I Feel Fatigued | 0.793 | 4 | 0.266 | 3 | 0.880 | 5 | 0.683 | 6 | 1.558 | 2 | NA | 1.220 | 2 | 0.951 | 6 | |||
| 2 | I Feel Weak All Over | 0.860 | 2 | −0.236 | 4 | 0.984 | 2 | 0.550 | 11 | 1.203 | 5 | NA | 1.057 | 5 | 0.945 | 7 | |||
| 3 | I Feel Listless | 0.635 | 9 | −0.098 | 8 | 0.492 | 10 | 0.953 | 1 | 0.888 | 9 | NA | 0.474 | 12 | 1.096 | 3 | |||
| 4 | I Feel Tired | 0.894 | 1 | −0.025 | 13 | 0.949 | 3 | 0.893 | 3 | 1.558 | 1 | NA | 0.933 | 9 | 1.060 | 4 | |||
| 5 | Trouble Starting Things | 0.691 | 7 | 0.134 | 7 | 0.933 | 4 | 0.933 | 2 | 0.964 | 8 | NA | 1.220 | 3 | 0.936 | 8 | |||
| 6 | Trouble Finishing Things | 0.472 | 10 | 0.053 | 9 | 0.537 | 9 | 0.643 | 7 | 0.559 | 11 | NA | 1.014 | 8 | 0.721 | 10 | |||
| 7 | I Have Energy | 0.794 | 3 | −0.522 | 1 | 0.816 | 7 | 0.573 | 9 | 1.406 | 3 | NA | 1.039 | 7 | 1.352 | 1 | |||
| 8 | Able to Do My Usual Activities | 0.385 | 12 | −0.448 | 2 | 0.446 | 12 | 0.381 | 13 | 0.334 | 13 | NA | 0.608 | 11 | 1.125 | 2 | |||
| 9 | I Need to Sleep During the Day | 0.764 | 5 | −0.051 | 10 | 0.787 | 8 | 0.700 | 5 | 1.062 | 7 | NA | 1.057 | 6 | 0.870 | 9 | |||
| 10 | I Am Too Tired to Eat | 0.342 | 13 | −0.212 | 5 | 0.385 | 13 | 0.573 | 10 | 0.393 | 12 | NA | 0.316 | 13 | 0.449 | 13 | |||
| 11 | Need Help Doing Usual Activities | 0.396 | 11 | 0.034 | 11 | 0.479 | 11 | 0.402 | 12 | 0.884 | 10 | NA | 0.858 | 10 | 0.527 | 12 | |||
| 12 | Frustrated by Being Too Tired | 0.644 | 8 | −0.198 | 6 | 0.834 | 6 | 0.596 | 8 | 1.362 | 4 | NA | 1.108 | 4 | 0.584 | 11 | |||
| 13 | Limit Social Activity, I am Tired | 0.743 | 6 | 0.027 | 12 | 1.075 | 1 | 0.762 | 4 | 1.068 | 6 | NA | 2.575 | 1 | 1.001 | 5 | |||
| Conditional formatting reflects magnitude and direction of Cohen’s d effect size (see Legend). For the sake of interpretation, Cohen’s d = 0.2–0.49 is a small effect, 0.5–0.79 is a medium effect, and >0.8 is a large effect. * Ranking based on absolute value of effect size. FACIT items are copyrighted by David Cella, PhD, and reprinted with permission. |  | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, C.E.; Borowiec, K.; Min, J.; Fishman, J. Fatigue Item Response among Hemoglobin-Normalized Patients with Paroxysmal Nocturnal Hemoglobinuria: PEGASUS Trial Results at 16 and 48 Weeks. J. Clin. Med. 2024, 13, 1703. https://doi.org/10.3390/jcm13061703
Schwartz CE, Borowiec K, Min J, Fishman J. Fatigue Item Response among Hemoglobin-Normalized Patients with Paroxysmal Nocturnal Hemoglobinuria: PEGASUS Trial Results at 16 and 48 Weeks. Journal of Clinical Medicine. 2024; 13(6):1703. https://doi.org/10.3390/jcm13061703
Chicago/Turabian StyleSchwartz, Carolyn E., Katrina Borowiec, Jinny Min, and Jesse Fishman. 2024. "Fatigue Item Response among Hemoglobin-Normalized Patients with Paroxysmal Nocturnal Hemoglobinuria: PEGASUS Trial Results at 16 and 48 Weeks" Journal of Clinical Medicine 13, no. 6: 1703. https://doi.org/10.3390/jcm13061703
APA StyleSchwartz, C. E., Borowiec, K., Min, J., & Fishman, J. (2024). Fatigue Item Response among Hemoglobin-Normalized Patients with Paroxysmal Nocturnal Hemoglobinuria: PEGASUS Trial Results at 16 and 48 Weeks. Journal of Clinical Medicine, 13(6), 1703. https://doi.org/10.3390/jcm13061703





