Importance of Short-Term Neointimal Coverage of Drug-Eluting Stents in the Duration of Dual Antiplatelet Therapy
Abstract
:1. Introduction
2. Materials and Methods
3. Histological Studies of Healing Patterns in Different Stent Types
4. Neointimal Coverage Assessed by OCT
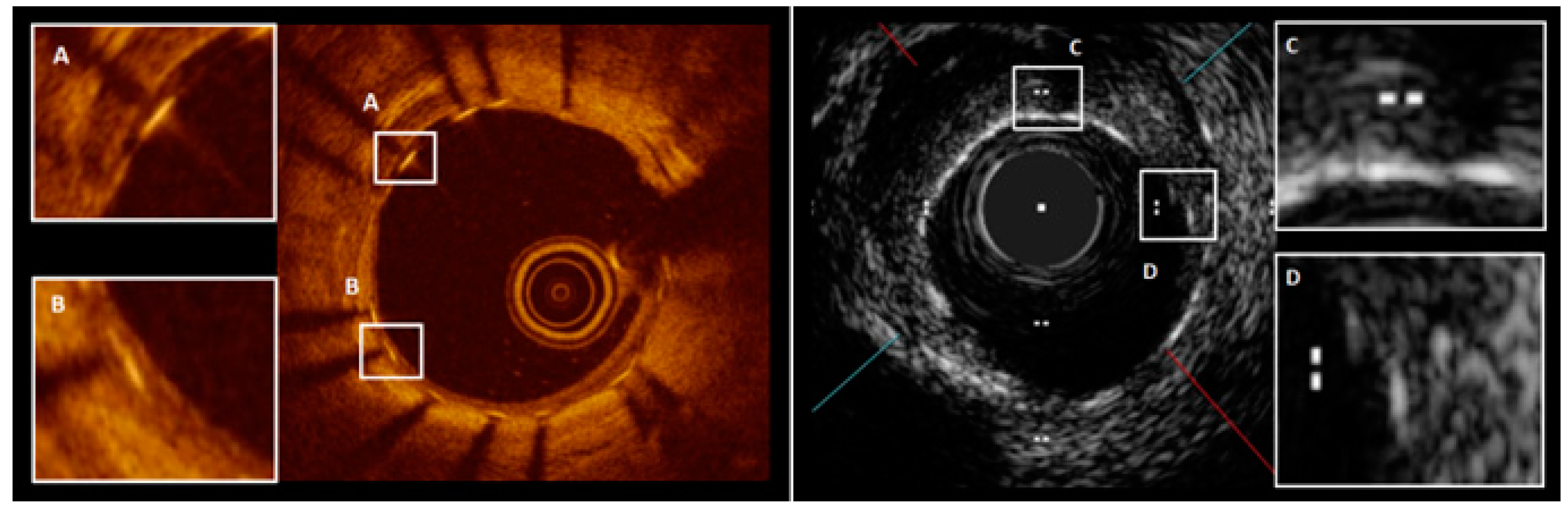
Optical Coherence Tomography (OCT) and High-Definition Intravascular Ultrasound (HD-IVUS)
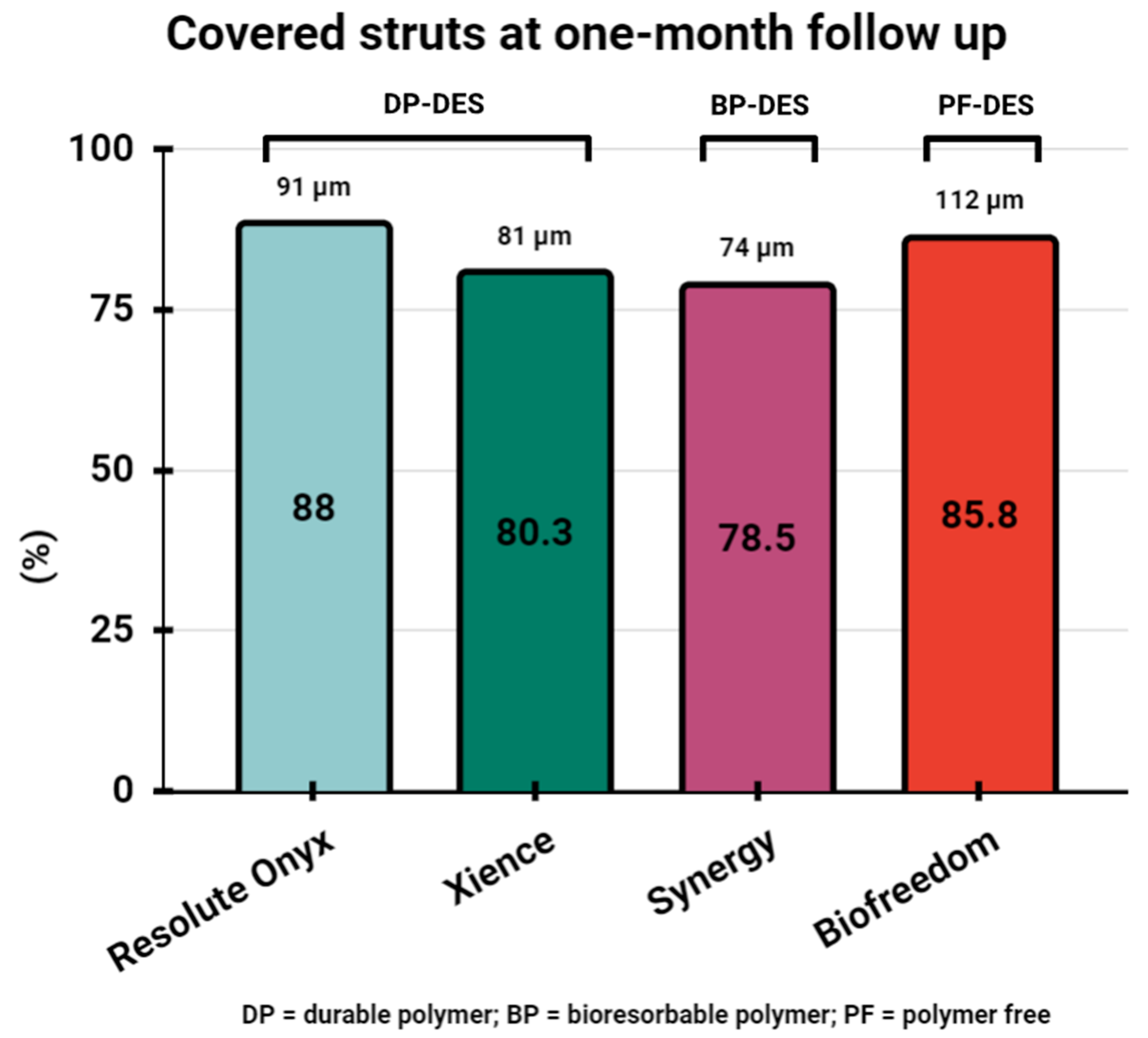
5. Assessment of Stent Healing by OCT One Month following PCI and Safety of One-Month DAPT
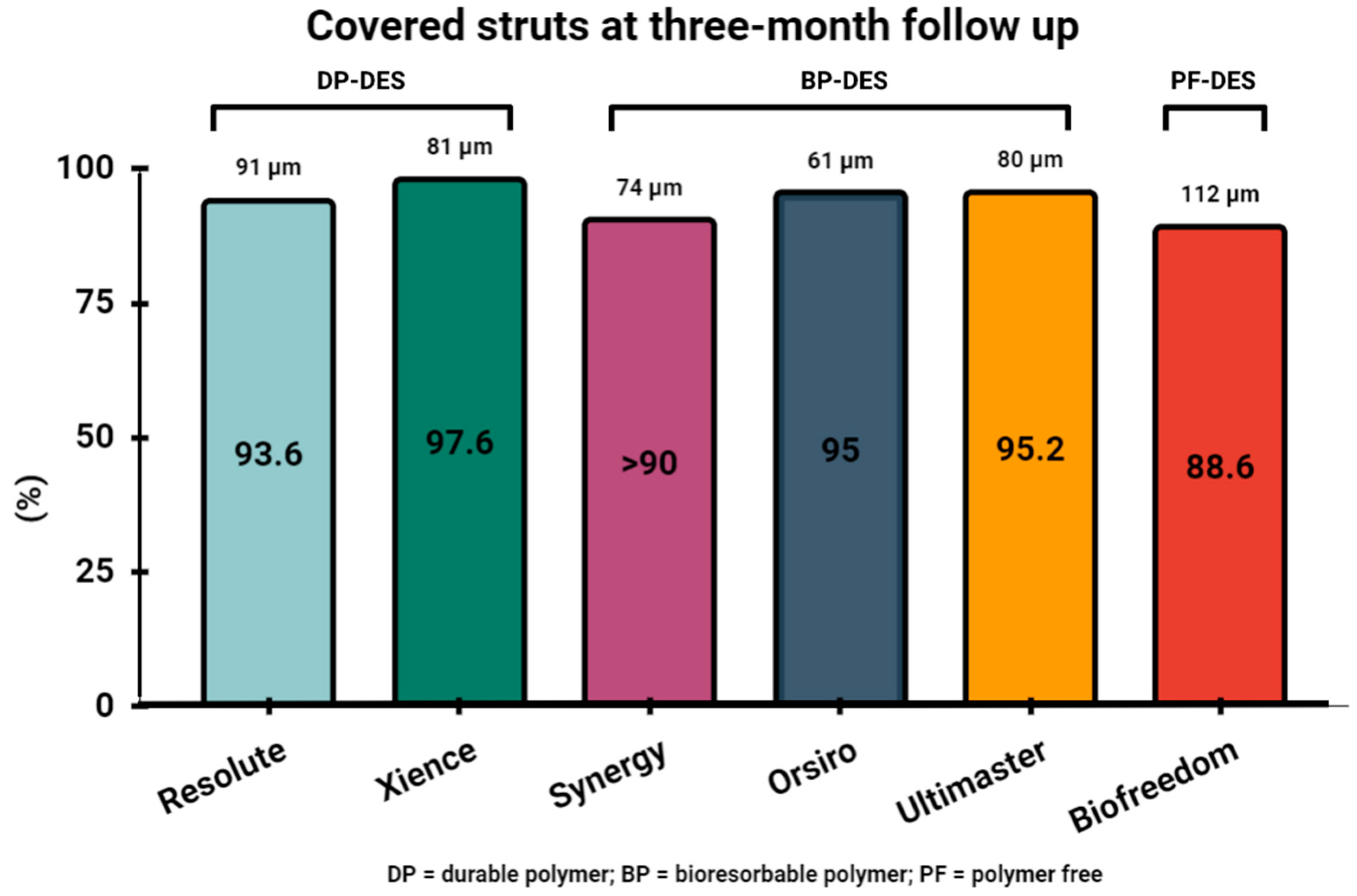
6. Assessment of Stent Healing by OCT Three Months following PCI and Safety of Three-Month DAPT
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navarese, E.P.; Andreotti, F.; Schulze, V.; Kołodziejczak, M.; Buffon, A.; Brouwer, M.; Costa, F.; Kowalewski, M.; Parati, G.; Lip, G.Y.H.; et al. Optimal Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention with Drug Eluting Stents: Meta-Analysis of Randomised Controlled Trials. BMJ 2015, 350, h1618. [Google Scholar] [CrossRef]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.-L.T.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; Hinterbuchner, L.; Jankowska, E.A.; Jüni, P.; Leosdottir, M.; Lorusso, R.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 55–161. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes The Task Force for the Diagnosis and Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Ueki, Y.; Bär, S.; Losdat, S.; Otsuka, T.; Zanchin, C.; Zanchin, T.; Gragnano, F.; Gargiulo, G.; Siontis, G.C.M.; Praz, F.; et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) Criteria in Patients Undergoing Percutaneous Coronary Intervention and Comparison with Contemporary Bleeding Risk Scores. EuroIntervention 2020, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Shiomi, H.; Yamaji, K.; Watanabe, H.; Shizuta, S.; Kato, T.; Ando, K.; Nakagawa, Y.; Furukawa, Y.; et al. Application of the Academic Research Consortium High Bleeding Risk Criteria in an All-Comers Registry of Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2019, 12, e008307. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention: A Consensus Document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 2019, 40, 2632–2653. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Van Klaveren, D.; Feres, F.; James, S.; Räber, L.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; Steg, P.G.; et al. Dual Antiplatelet Therapy Duration Based on Ischemic and Bleeding Risks After Coronary Stenting. J. Am. Coll. Cardiol. 2019, 73, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Costa, F.; Lokhnygina, Y.; Clare, R.M.; Wallentin, L.; Moliterno, D.J.; Armstrong, P.W.; White, H.D.; Held, C.; Aylward, P.E.; et al. Trade-off of Myocardial Infarction vs. Bleeding Types on Mortality after Acute Coronary Syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) Randomized Trial. Eur. Heart J. 2016, 38, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Giustino, G.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Rinaldi, M.J.; Neumann, F.-J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2015, 66, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, T.; Benedetto, U.; Bacchi-Reggiani, L.; Riva, D.D.; Biondi-Zoccai, G.; Feres, F.; Abizaid, A.; Hong, M.-K.; Kim, B.-K.; Jang, Y.; et al. Mortality in Patients Treated with Extended Duration Dual Antiplatelet Therapy after Drug-Eluting Stent Implantation: A Pairwise and Bayesian Network Meta-Analysis of Randomised Trials. Lancet 2015, 385, 2371–2382. [Google Scholar] [CrossRef]
- Kedhi, E.; Joesoef, K.S.; McFadden, E.; Wassing, J.; Van Mieghem, C.; Goedhart, D.; Smits, P.C. Second-Generation Everolimus-Eluting and Paclitaxel-Eluting Stents in Real-Life Practice (COMPARE): A Randomised Trial. Lancet 2010, 375, 201–209. [Google Scholar] [CrossRef]
- Räber, L.; Magro, M.; Stefanini, G.G.; Kalesan, B.; Van Domburg, R.T.; Onuma, Y.; Wenaweser, P.; Daemen, J.; Meier, B.; Jüni, P.; et al. Very Late Coronary Stent Thrombosis of a Newer-Generation Everolimus-Eluting Stent Compared with Early-Generation Drug-Eluting Stents: A Prospective Cohort Study. Circulation 2012, 125, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of Stent Thrombosis Among Bare-Metal Stents, First-Generation Drug-Eluting Stents, and Second-Generation Drug-Eluting Stents. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Lu, S.; Li, Y.; Deng, M.; Wang, Z.; Mao, X. A Comparison of the Main Outcomes from BP-BES and DP-DES at Five Years of Follow-up: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 14997. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of Drug-Eluting Stents in Humans. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef]
- Finn, A.V.; Joner, M.; Nakazawa, G.; Kolodgie, F.; Newell, J.; John, M.C.; Gold, H.K.; Virmani, R. Pathological Correlates of Late Drug-Eluting Stent Thrombosis: Strut Coverage as a Marker of Endothelialization. Circulation 2007, 115, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, G.; Finn, A.V.; Joner, M.; Ladich, E.; Kutys, R.; Mont, E.K.; Gold, H.K.; Burke, A.P.; Kolodgie, F.D.; Virmani, R. Delayed Arterial Healing and Increased Late Stent Thrombosis at Culprit Sites After Drug-Eluting Stent Placement for Acute Myocardial Infarction Patients: An Autopsy Study. Circulation 2008, 118, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Hao, H.; Imanaka, T.; Shibuya, M.; Ueda, Y.; Tsujimoto, M.; Ishibashi-Ueda, H.; Hirota, S. Initial Pathological Responses of Second-Generation Everolimus-Eluting Stents Implantation in Japanese Coronary Arteries: Comparison with First-Generation Sirolimus-Eluting Stents. J. Cardiol. 2018, 71, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Vorpahl, M.; Nakano, M.; Foerst, J.; Newell, J.B.; Sakakura, K.; Kutys, R.; Ladich, E.; Finn, A.V.; Kolodgie, F.D.; et al. Pathology of Second-Generation Everolimus-Eluting Stents Versus First-Generation Sirolimus- and Paclitaxel-Eluting Stents in Humans. Circulation 2014, 129, 211–223. [Google Scholar] [CrossRef]
- Kawagoe, Y.; Otsuka, F.; Onozuka, D.; Ishibashi-Ueda, H.; Ikeda, Y.; Ohta-Ogo, K.; Matsumoto, M.; Amemiya, K.; Asaumi, Y.; Kataoka, Y.; et al. Early Vascular Responses to Abluminal Biodegradable Polymer-Coated versus Circumferential Durable Polymer- Coated Newer-Generation Drug-Eluting Stents in Humans: A Pathological Study. EuroIntervention 2023, 18, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Prati, F.; Pawlowsky, T.; Cera, M.; La Manna, A.; Albertucci, M.; Tamburino, C. Comparison of Optical Coherence Tomography and Intravascular Ultrasound for the Assessment of In-Stent Tissue Coverage after Stent Implantation. EuroIntervention 2009, 5, 538–543. [Google Scholar] [CrossRef]
- Kochman, J.; Pietrasik, A.; Rdzanak, A.; Jąkała, J.; Zasada, W.; Ścibisz, A.; Kołtowski, Ł.; Proniewska, K.; Pociask, E.; Legutko, J. Comparison between Optical Coherence Tomography and Intravascular Ultrasound in Detecting Neointimal Healing Patterns after Stent Implantation. Kardiol. Pol. 2014, 72, 534–540. [Google Scholar] [CrossRef]
- Dobrolińska, M.; Gąsior, P.; Roleder, T.; Ochała, A.; Wojakowski, W. Short-Term Stent Strut Coverage: Optical Coherence Tomography vs High-Definition Intravascular Ultrasound. Kardiol. Pol. 2021, 79, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Wallace-Bradley, D.; Tellez, A.; Alviar, C.; Aboodi, M.; Sheehy, A.; Coleman, L.; Perkins, L.; Nakazawa, G.; Mintz, G.; et al. Accuracy of Optical Coherence Tomography in the Evaluation of Neointimal Coverage After Stent Implantation. JACC Cardiovasc. Imaging 2010, 3, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Zimarino, M.; Stabile, E.; Pizzicannella, G.; Fouad, T.; Rabozzi, R.; Filippini, A.; Pizzicannella, J.; Cera, M.; De Caterina, R. Does Optical Coherence Tomography Identify Arterial Healing after Stenting? An in Vivo Comparison with Histology, in a Rabbit Carotid Model. Heart 2008, 94, 217–221. [Google Scholar] [CrossRef]
- Fu, Q.; Hu, H.; Chen, W.; Tan, Z.; Li, L.; Wang, D.; Chen, B. Histological Validation of Frequency Domain Optical Coherence Tomography for the Evaluation of Neointimal Formation after a Novel Polymer-Free Sirolimus-Eluting Stent Implantation. J. Clin. Exp. Pathol. 2015, 8, 11068. [Google Scholar]
- Jinnouchi, H.; Otsuka, F.; Sato, Y.; Bhoite, R.R.; Sakamoto, A.; Torii, S.; Yahagi, K.; Cornelissen, A.; Mori, M.; Kawakami, R.; et al. Healthy Strut Coverage After Coronary Stent Implantation: An Ex Vivo Human Autopsy Study. Circ. Cardiovasc. Interv. 2020, 13, e008869. [Google Scholar] [CrossRef]
- Gonzalo, N.; Garcia-Garcia, H.; Serruys, P.; Commissaris, K.; Bezerra, H.; Gobbens, P.; Costa, M.; Regar, E. Reproducibility of Quantitative Optical Coherence Tomography for Stent Analysis. EuroIntervention 2009, 5, 224–232. [Google Scholar] [CrossRef]
- Antonsen, L.; Thayssen, P.; Junker, A.; Veien, K.T.; Hansen, H.S.; Hansen, K.N.; Hougaard, M.; Jensen, L.O. Intra- and Interobserver Reliability and Intra-Catheter Reproducibility Using Frequency Domain Optical Coherence Tomography for the Evaluation of Morphometric Stent Parameters and Qualitative Assessment of Stent Strut Coverage. Cardiovasc. Revasc. Med. 2015, 16, 469–477. [Google Scholar] [CrossRef]
- Guagliumi, G.; Sirbu, V.; Musumeci, G.; Gerber, R.; Biondi-Zoccai, G.; Ikejima, H.; Ladich, E.; Lortkipanidze, N.; Matiashvili, A.; Valsecchi, O.; et al. Examination of the In Vivo Mechanisms of Late Drug-Eluting Stent Thrombosis. JACC Cardiovasc. Interv. 2012, 5, 12–20. [Google Scholar] [CrossRef]
- Won, H.; Shin, D.-H.; Kim, B.-K.; Mintz, G.S.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; Jang, Y.; Hong, M.-K. Optical Coherence Tomography Derived Cut-off Value of Uncovered Stent Struts to Predict Adverse Clinical Outcomes after Drug-Eluting Stent Implantation. Int. J. Cardiovasc. Imaging 2013, 29, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Abizaid, A.; Mehran, R.; Schofer, J.; Schuler, G.C.; Hauptmann, K.E.; Magalhães, M.A.; Parise, H.; Grube, E. Polymer-Free Biolimus A9-Coated Stents in the Treatment of De Novo Coronary Lesions. JACC Cardiovasc. Interv. 2016, 9, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Tam, F.C.C.; Chan, K.K.W.; Lam, S.C.C.; Kong, S.-L.; Shea, C.P.; Wong, M.K.L.; Wong, A.Y.T.; Yung, A.S.Y.; Zhang, L.-W.; et al. Establishment of Healing Profile and Neointimal Transformation in the New Polymer-Free Biolimus A9-Coated Coronary Stent by Longitudinal Sequential Optical Coherence Tomography Assessments: The EGO-BIOFREEDOM Study. EuroIntervention 2018, 14, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.N.; Antonsen, L.; Maehara, A.; Mæng, M.; Ellert, J.; Ahlehoff, O.; Veien, K.T.; Hansen, K.N.; Noori, M.; Fallesen, C.O.; et al. Influence of Plaque Characteristics on Early Vascular Healing in Patients with ST-Elevation Myocardial Infarction. Cardiovasc. Revasc. Med. 2021, 30, 50–58. [Google Scholar] [CrossRef]
- Urban, P.; Abizaid, A.; Chevalier, B.; Greene, S.; Meredith, I.; Morice, M.-C.; Pocock, S. Rationale and Design of the LEADERS FREE Trial: A Randomized Double-Blind Comparison of the BioFreedom Drug-Coated Stent vs the Gazelle Bare Metal Stent in Patients at High Bleeding Risk Using a Short (1 Month) Course of Dual Antiplatelet Therapy. Am. Heart J. 2013, 165, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-Free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Garot, P.; Morice, M.-C.; Tresukosol, D.; Pocock, S.J.; Meredith, I.T.; Abizaid, A.; Carrié, D.; Naber, C.; Iñiguez, A.; Talwar, S.; et al. 2-Year Outcomes of High Bleeding Risk Patients After Polymer-Free Drug-Coated Stents. J. Am. Coll. Cardiol. 2017, 69, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Krucoff, M.W.; Urban, P.; Tanguay, J.-F.; McAndrew, T.; Zhang, Y.; Rao, S.V.; Morice, M.-C.; Price, M.J.; Cohen, D.J.; Abdel-Wahab, M.; et al. Global Approach to High Bleeding Risk Patients with Polymer-Free Drug-Coated Coronary Stents: The LF II Study. Circ. Cardiovasc. Interv. 2020, 13, e008603. [Google Scholar] [CrossRef]
- Eberli, F.R.; Stoll, H.; Urban, P.; Morice, M.; Brunel, P.; Maillard, L.; Lipiecki, J.; Cook, S.; Berland, J.; Hovasse, T.; et al. Polymer -free Biolimus-A9 Coated Thin Strut Stents for Patients at High Bleeding Risk 1-year Results from the Leaders Free III Study. Catheter. Cardiovasc. Interv. 2022, 99, 593–600. [Google Scholar] [CrossRef]
- Price, M.J.; Saito, S.; Shlofmitz, R.A.; Spriggs, D.J.; Attubato, M.; McLaurin, B.; Popma Almonacid, A.; Brar, S.; Liu, M.; Moe, E.; et al. First Report of the Resolute Onyx 2.0-Mm Zotarolimus-Eluting Stent for the Treatment of Coronary Lesions with Very Small Reference Vessel Diameter. JACC Cardiovasc. Interv. 2017, 10, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, H.; Sato, Y.; Cheng, Q.; Janifer, C.; Kutyna, M.; Cornelissen, A.; Wijeratne, R.; Sakamoto, A.; Guo, L.; Kolodgie, F.D.; et al. Thromboresistance and Endothelial Healing in Polymer-Coated versus Polymer-Free Drug-Eluting Stents: Implications for Short-Term Dual Anti-Platelet Therapy. Int. J. Cardiol. 2021, 327, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Roleder, T.; Kedhi, E.; Berta, B.; Gasior, P.; Wanha, W.; Roleder, M.; Fluder, J.; Smolka, G.; Ochala, A.; Wojakowski, W. Short-Term Stent Coverage of Second-Generation Zotarolimus-Eluting Durable Polymer Stents: Onyx One-Month Optical Coherence Tomography Study. Adv. Interv. Cardiol. 2019, 15, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.C.; Chan, K.; Lam, S.; Yung, A.; Lam, Y.M.; Chan, C.; Siu, D.; Tse, H.F. One-Year Clinical Outcomes of Patients Implanted with a Resolute OnyxTM Zotarolimus-Eluting Stent. J. Int. Med. Res. 2018, 46, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kedhi, E.; Latib, A.; Abizaid, A.; Kandzari, D.; Kirtane, A.J.; Mehran, R.; Price, M.J.; Simon, D.; Worthley, S.; Zaman, A.; et al. Rationale and Design of the Onyx ONE Global Randomized Trial: A Randomized Controlled Trial of High-Bleeding Risk Patients after Stent Placement with 1 Month of Dual Antiplatelet Therapy. Am. Heart J. 2019, 214, 134–141. [Google Scholar] [CrossRef]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-Based or Polymer-Free Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2020, 382, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-Based Versus Polymer-Free Stents in High Bleeding Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 1153–1163. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Kirtane, A.J.; Windecker, S.; Latib, A.; Kedhi, E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. For the Onyx ONE US/Japan Investigators. One-Month Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention with Zotarolimus-Eluting Stents in High-Bleeding-Risk Patients. Circ. Cardiovasc. Interv. 2020, 13, e009565. [Google Scholar] [CrossRef]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. For the STOPDAPT-2 Investigators. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414. [Google Scholar] [CrossRef]
- Watanabe, H.; Morimoto, T.; Natsuaki, M.; Yamamoto, K.; Obayashi, Y.; Ogita, M.; Suwa, S.; Isawa, T.; Domei, T.; Yamaji, K.; et al. Comparison of Clopidogrel Monotherapy After 1 to 2 Months of Dual Antiplatelet Therapy with 12 Months of Dual Antiplatelet Therapy in Patients with Acute Coronary Syndrome: The STOPDAPT-2 ACS Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 407. [Google Scholar] [CrossRef]
- Mehran, R.; Cao, D.; Angiolillo, D.J.; Bangalore, S.; Bhatt, D.L.; Ge, J.; Hermiller, J.; Makkar, R.R.; Neumann, F.-J.; Saito, S.; et al. 3- or 1-Month DAPT in Patients at High Bleeding Risk Undergoing Everolimus-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 1870–1883. [Google Scholar] [CrossRef]
- Ishihara, T.; Mizote, I.; Nakamura, D.; Okamoto, N.; Shiraki, T.; Itaya, N.; Tsujimura, T.; Takahara, M.; Nakayoshi, T.; Iida, O.; et al. The COLLABORATION Investigators. Comparison of 1-Month and 12-Month Vessel Responses Between the Polymer-Free Biolimus A9-Coated Stent and the Durable Polymer Everolimus-Eluting Stent. Circ. J. 2022, 86, 1397–1408. [Google Scholar] [CrossRef]
- Oka, N.; Fujii, K.; Kadohira, T.; Kitahara, H.; Fujimoto, Y.; Takahara, M.; Himi, T.; Ishikawa, K.; Sano, K.; Kobayashi, Y. Assessment of Strut Coverage of Everolimus-Eluting Platinum Chromium Stent 2 Weeks after Implantation by Optical Coherence Tomography. Heart Vessel. 2019, 34, 1258–1265. [Google Scholar] [CrossRef]
- Shimoda, M.; Ando, H.; Naito, K.; Suzuki, A.; Sakurai, S.; Nakano, Y.; Kurita, A.; Waseda, K.; Takashima, H.; Murotani, K.; et al. Early-Phase Vascular Healing of Bioabsorbable vs. Durable Polymer-Coated Everolimus-Eluting Stents in Patients with ST-Elevation Myocardial Infarction―2-Week and 4-Month Analyses with Optical Coherence Tomography. Circ. J. 2018, 82, 2594–2601. [Google Scholar] [CrossRef]
- Laine, M.; Dabry, T.; Combaret, N.; Motreff, P.; Puymirat, E.; Paganelli, F.; Thuny, F.; Cautela, J.; Peyrol, M.; Mancini, J.; et al. OCT Analysis of Very Early Strut Coverage of the Synergy Stent in Non-ST Segment Elevation Acute Coronary Syndrome Patients. J. Invasive Cardiol. 2019, 31, 10–14. [Google Scholar]
- Pivato, C.A.; Reimers, B.; Testa, L.; Pacchioni, A.; Briguori, C.; Musto, C.; Esposito, G.; Piccolo, R.; Lucisano, L.; De Luca, L.; et al. One-Month Dual Antiplatelet Therapy After Bioresorbable Polymer Everolimus-Eluting Stents in High Bleeding Risk Patients. J. Am. Heart Assoc. 2022, 11, e023454. [Google Scholar] [CrossRef]
- Varenne, O.; Cook, S.; Sideris, G.; Kedev, S.; Cuisset, T.; Carrié, D.; Hovasse, T.; Garot, P.; El Mahmoud, R.; Spaulding, C.; et al. Drug-Eluting Stents in Elderly Patients with Coronary Artery Disease (SENIOR): A Randomised Single-Blind Trial. Lancet 2018, 391, 41–50. [Google Scholar] [CrossRef]
- Koiwaya, H.; Nishihira, K.; Kadooka, K.; Kuriyama, N.; Shibata, Y. Vascular Healing in High-Bleeding-Risk Patients at 3-Month after Everolimus-Eluting Stent versus Biolimus A9-Coated Stent Implantation: Insights from Analysis of Optical Coherence Tomography and Coronary Angioscopy. Cardiovasc. Interv. Ther. 2023, 38, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Hashikata, T.; Tojo, T.; Namba, S.; Kitasato, L.; Hashimoto, T.; Kameda, R.; Shimohama, T.; Yamaoka-Tojo, M.; Ako, J. Neointimal Coverage of Zotarolimus-Eluting Stent at 1, 2, and 3 Months’ Follow-up: An Optical Coherence Tomography Study. Heart Vessel. 2016, 31, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, P.P.; Varho, V.; Nammas, W.; Mikkelsson, J.; Pietilä, M.; Ylitalo, A.; Airaksinen, J.K.E.; Sia, J.; Nyman, K.; Biancari, F.; et al. Early Neointimal Coverage and Vasodilator Response Following Biodegradable Polymer Sirolimus-Eluting vs. Durable Polymer Zotarolimus-Eluting Stents in Patients with Acute Coronary Syndrome: HATTRICK-OCT Trial. Circ. J. 2015, 79, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Secco, G.G.; Mattesini, A.; Fattori, R.; Parisi, R.; Castriota, F.; Vercellino, M.; Dall’Ara, G.; Uguccioni, L.; Marinucci, L.; De Luca, G.; et al. Time-Related Changes in Neointimal Tissue Coverage of a Novel Sirolimus Eluting Stent. Cardiovasc. Revasc. Med. 2016, 17, 38–43. [Google Scholar] [CrossRef]
- Kretov, E.; Naryshkin, I.; Baystrukov, V.; Grazhdankin, I.; Prokhorikhin, A.; Zubarev, D.; Biryukov, A.; Verin, V.; Boykov, A.; Malaev, D.; et al. Three-months Optical Coherence Tomography Analysis of a Biodegradable Polymer, Sirolimus-eluting Stent. J. Intervent. Cardiol. 2018, 31, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, L.N.; Balleby, I.R.; Barkholt, T.Ø.; Hebsgaard, L.; Terkelsen, C.J.; Holck, E.N.; Jensen, L.O.; Maeng, M.; Dijkstra, J.; Antonsen, L.; et al. Early Healing after Treatment of Coronary Lesions by Thin Strut Everolimus, or Thicker Strut Biolimus Eluting Bioabsorbable Polymer Stents: The SORT-OUT VIII OCT Study. Catheter. Cardiovasc. Interv. 2023, 101, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Lhermusier, T.; Ohayon, P.; Boudou, N.; Bouisset, F.; Campelo-Parada, F.; Roncalli, J.; Elbaz, M.; Carrié, D. Re-Endothelialisation after Synergy Stent and Absorb Bioresorbable Vascular Scaffold Implantation in Acute Myocardial Infarction: COVER-AMI Study. Trials 2019, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- De La Torre Hernández, J.M.; Tejedor, P.; Camarero, T.G.; Duran, J.M.; Lee, D.; Monedero, J.; Laso, F.S.; Calderón, M.A.; Veiga, G.; Zueco, J. Early Healing Assessment with Optical Coherence Tomography of Everolimus-eluting Stents with Bioabsorbable Polymer (SynergyTM) at 3 and 6 Months after Implantation. Catheter. Cardiovasc. Interv. 2016, 88, E67–E73. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, B.; Smits, P.C.; Carrié, D.; Mehilli, J.; Van Boven, A.J.; Regar, E.; Sawaya, F.J.; Chamié, D.; Kraaijeveld, A.O.; Hovasse, T.; et al. Serial Assessment of Strut Coverage of Biodegradable Polymer Drug-Eluting Stent at 1, 2, and 3 Months After Stent Implantation by Optical Frequency Domain Imaging: The DISCOVERY 1TO3 Study (Evaluation with OFDI of Strut Coverage of Terumo New Drug Eluting Stent with Biodegradable Polymer at 1, 2, and 3 Months). Circ. Cardiovasc. Interv. 2017, 10, e004801. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Sotomi, Y.; Suzuki, S.; Hamanaka, Y.; Nakatani, S.; Dijkstra, J.; Onuma, Y.; Serruys, P.W.; Sakata, Y.; Hirayama, A.; et al. Neointimal Characteristics Comparison between Biodegradable-Polymer and Durable-Polymer Drug-Eluting Stents: 3-Month Follow-up Optical Coherence Tomography Light Property Analysis from the RESTORE Registry. Int. J. Cardiovasc. Imaging 2020, 36, 205–215. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Sotomi, Y.; Kobayashi, T.; Omatsu, T.; Dijkstra, J.; Sakata, Y.; Hirayama, A.; Hirata, A.; Higuchi, Y. Comparable Neointimal Healing in Patients with Stable Coronary Lesions and Acute Coronary Syndrome: 3-Month Optical Coherence Tomography Analysis. Int. J. Cardiovasc. Imaging 2021, 37, 2095–2105. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, J.-S.; Yoon, H.-J.; Hur, S.-H.; Lee, S.-G.; Kim, J.W.; Hong, Y.J.; Kim, K.-S.; Choi, S.-Y.; Shin, D.-H.; et al. Early Strut Coverage in Patients Receiving Drug-Eluting Stents and Its Implications for Dual Antiplatelet Therapy. JACC Cardiovasc. Imaging 2018, 11, 1810–1819. [Google Scholar] [CrossRef]
- Kim, B.-K.; Hong, M.-K.; Shin, D.-H.; Nam, C.-M.; Kim, J.-S.; Ko, Y.-G.; Choi, D.; Kang, T.-S.; Park, B.-E.; Kang, W.-C.; et al. A New Strategy for Discontinuation of Dual Antiplatelet Therapy. J. Am. Coll. Cardiol. 2012, 60, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Yamamoto, E.; Shiomi, H.; Furukawa, Y.; Abe, M.; Nakao, K.; Ishikawa, T.; Kawai, K.; Yunoki, K.; et al. One-Year Outcome of a Prospective Trial Stopping Dual Antiplatelet Therapy at 3 Months after Everolimus-Eluting Cobalt-Chromium Stent Implantation: ShortT and OPtimal Duration of Dual AntiPlatelet Therapy after Everolimus-Eluting Cobalt-Chromium Stent (STOPDAPT) Trial. Cardiovasc. Interv. Ther. 2016, 31, 196–209. [Google Scholar] [CrossRef]
- Hahn, J.-Y.; Song, Y.B.; Oh, J.-H.; Chun, W.J.; Park, Y.H.; Jang, W.J.; Im, E.-S.; Jeong, J.-O.; Cho, B.R.; Oh, S.K.; et al. For the SMART-CHOICE Investigators. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 2019, 321, 2428. [Google Scholar] [CrossRef]
- Kirtane, A.J.; Stoler, R.; Feldman, R.; Neumann, F.-J.; Boutis, L.; Tahirkheli, N.; Toelg, R.; Othman, I.; Stein, B.; Choi, J.W.; et al. Primary Results of the EVOLVE Short DAPT Study: Evaluation of 3-Month Dual Antiplatelet Therapy in High Bleeding Risk Patients Treated with a Bioabsorbable Polymer-Coated Everolimus-Eluting Stent. Circ. Cardiovasc. Interv. 2021, 14, e010144. [Google Scholar] [CrossRef]
- Kim, B.-K.; Hong, S.-J.; Cho, Y.-H.; Yun, K.H.; Kim, Y.H.; Suh, Y.; Cho, J.Y.; Her, A.-Y.; Cho, S.; Jeon, D.W.; et al. For the TICO Investigators. Effect of Ticagrelor Monotherapy vs Ticagrelor with Aspirin on Major Bleeding and Cardiovascular Events in Patients with Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 2020, 323, 2407. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Ughi, G.J.; Adriaenssens, T.; Onsea, K.; Kayaert, P.; Dubois, C.; Sinnaeve, P.; Coosemans, M.; Desmet, W.; D’hooge, J. Automatic Segmentation of In-Vivo Intra-Coronary Optical Coherence Tomography Images to Assess Stent Strut Apposition and Coverage. Int. J. Cardiovasc. Imaging 2012, 28, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Kim, C.; Lee, J.J.; Song, J.W.; Kim, J.W.; Yoo, H. Automated Detection of Vessel Lumen and Stent Struts in Intravascular Optical Coherence Tomography to Evaluate Stent Apposition and Neointimal Coverage. Med. Phys. 2016, 43, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lee, J.; Jakl, M.; Wang, Z.; Cervinka, P.; Bezerra, H.G.; Wilson, D.L. Application and Evaluation of Highly Automated Software for Comprehensive Stent Analysis in Intravascular Optical Coherence Tomography. Sci. Rep. 2020, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Mehanna, E.; Li, C.; Zhu, H.; He, C.; Lu, F.; Zhao, K.; Gong, Y.; Wang, Z. Stent Detection with Very Thick Tissue Coverage in Intravascular OCT. Biomed. Opt. Express 2021, 12, 7500. [Google Scholar] [CrossRef]
| Trial | Design | Stent Type | P2Y12 Inhibitors | Endpoints | Outcomes | Duration of the Study |
|---|---|---|---|---|---|---|
| LEADERS FREE I [36,37,38] | 1 M DAPT following DCS implantation vs. 1 M DAPT following BMS implantation, both followed by ASA | SS-DCS, BMS | Clopidogrel | Primary efficacy: clinically driven TLR at one-year follow-up Primary safety: composite of CD, MI, and ST at one-year follow-up Secondary: bleeding events | The use of DCS followed by 1 M DAPT in HBR patients is superior to BMS in regard to safety and efficacy endpoints. | 2012–2015 |
| LEADERS FREE II [39] | 1 M DAPT following DCS implantation vs. 1 M DAPT following BMS implantation (BMS group from LF I trial), both followed by aspirin | SS-DCS, BMS | Clopidogrel | Primary efficacy: clinically indicated TLR at one-year follow-up Primary safety: composite of MI and CD at one-year follow-up Secondary: composite of CD, MI, or ST | The use of DCS followed by 1 M DAPT in HBR patients is superior to BMS in regard to safety and efficacy endpoints. | 2017–2018 |
| LEADERS FREE III [40] | 1 M DAPT following CoCr-DCS implantation vs. 1 M DAPT following BMS implantation and 1 M DAPT following CoCr-DCS implantation vs. 1 M DAPT following DCS implantation (BMS and DCS group taken from LF I trial) followed by aspirin | CoCr-DCS, BMS, SS-DCS | Clopidogrel | Primary efficacy: clinically indicated TLR Primary safety: composite of CD, MI, and definite/probable ST | CoCr-DCS proved noninferior to the SS-DCS for safety and superior to the BMS for efficacy in HBR patients treated with 1 M of DAPT. | 2017–2019 |
| ONYX ONE Global [45,46,47] | 1 M DAPT following ZES implantation vs. 1 M DAPT following DCS implantation, both followed by long-term aspirin or clopidogrel alone | ZES, DCS | Clopidogrel, prasugrel, ticagrelor | Primary: composite of CD, MI, or ST at 1-year follow-up Secondary: target lesion failure at 1 year | ZES proved to be noninferior to DCS in HBR patients with regard to primary and secondary endpoints at one-year follow-up. At 2-year follow-up, ZES and DCS had similar outcomes for the primary and secondary endpoints. | 2017–2018 |
| ONYX ONE CLEAR [48] | Multicenter, nonrandomized study evaluating the safety and effectiveness of 1 M DAPT followed by SAPT in patients who were event-free before DAPT discontinuation | ZES | Clopidogrel, prasugrel, ticagrelor | Primary: composite of CD or MI between 1 M and 1 year Secondary: rates of all-cause death, CD, major adverse cardiac events, target vessel failure, target lesion failure, any coronary revascularization procedure, definite/probable ST, stroke, and bleeding | Favorable safety and effectiveness between 1 M and 1 year were demonstrated among HBR patients unable to undergo prolonged DAPT therapy who were treated with Onyx Resolute ZES. | 2018–2020 |
| STOPDAPT-2 [49] | 1 M DAPT followed by clopidogrel monotherapy vs. 12 M DAPT | CoCr-EES | Clopidogrel, prasugrel | Primary: composite of cardiovascular death, MI, definite ST, ischemic or hemorrhagic stroke, or major or minor bleeding at 12 M follow-up Secondary: a composite of cardiovascular death, MI, definite ST, ischemic or hemorrhagic stroke, and the bleeding endpoint of major or minor bleeding assessed at 12 M follow-up | 1 M of DAPT followed by clopidogrel monotherapy was noninferior and superior to 12 M of DAPT. | 2015–2018 |
| STOPDAPT 2 ACS [50] | 1–2 M DAPT followed by clopidogrel monotherapy vs. 12 M DAPT in ACS patients | CoCr-EES | Clopidogrel, prasugrel | Primary: composite of cardiovascular death, MI, definite ST, ischemic or hemorrhagic stroke, or major or minor bleeding at 12 M follow-up Secondary: a composite of cardiovascular death, MI, definite ST, ischemic or hemorrhagic stroke, and the bleeding endpoint of major or minor bleeding assessed at 12 M follow-up | In ACS patients, clopidogrel monotherapy after 1 to 2 M of DAPT failed to prove noninferiority to 12 M DAPT, with an increase in cardiovascular events (2.76% vs. 1.86%), despite a lower occurrence of major bleeding events (0.54% vs. 1.17%) at 12 M follow-up. | 2018–2021 |
| POEM [56] | 1 M DAPT followed by ASA or OAC monotherapy following Synergy PtCr-EES implementation vs. OPC | PtCr-EES | Clopidogrel, prasugrel, ticagrelor | Primary: composite of CD, MI, or definite or probable ST at 12 M Secondary: all-cause death, CD, MI, ST, target vessel revascularization, TLR, major bleeding according to BARC criteria, cerebrovascular events, and target lesion failure | 1 M DAPT followed by ASA or OAC monotherapy was noninferior to OPC based on the occurrence of the primary endpoint (4.82% vs. 9.4% with a noninferiority margin of 3.85%) at one year follow-up. | 2017–2020 |
| SENIOR [57] | CCS: EES implantation followed by 1 M DAPT vs. BMS implantation followed by 1 M DAPT ACS: EES implantation followed by 6 M DAPT vs. BMS implantation followed by 6 M DAPT | EES, BMS | Clopidogrel, prasugrel, ticagrelor | Primary: composite of all-cause mortality, MI, stroke, or IDTLR at 1-year follow-up Secondary: bleeding complications (BARC 2–5 and BARC 3–5); definite or probable ST, all revascularisations; all components of the primary endpoint; and cardiovascular death, at 30 days, 180 days, 365 days, and 2 years | EES implantation followed by short-duration DAPT resulted in a lower occurrence of the primary endpoint than BMS implantation followed by short-duration DAPT (12% vs. 16%) at 1-year follow-up, proving to be better for people 75 years or older. | 2014–2017 |
| DETECT-OCT [69] | OCT-guided PCI vs. angiographically guided PCI, according to the percentage of uncovered struts: 3 M DAPT followed by ASA (<6% uncovered struts at 3 M follow-up) vs. 12 M DAPT (>6% uncovered struts at 3 M follow-up) | EES, BES | Clopidogrel | Primary: differences in 3 M coverage between EES vs. BES and OCT-guided and angiographic PCI Secondary: a composite of CD, MI, definite or probable ST, and major bleeding during 12 M follow-up |
| 2013–2017 |
| RESET [70] | 3 M DAPT followed by ASA monotherapy following E-ZES implantation vs. 12 M DAPT standard therapy | ZES, EES, SES | Clopidogrel | Composite of death from cardiovascular causes, MI, ST, IDTR, or bleeding at 1-year follow-up | 3 M DAPT following E-ZES implantation was noninferior to standard therapy. | 2009–2012 |
| STOPDAPT [71] | 3 M DAPT followed by ASA vs. 12 M DAPT in historical group (the CoCr-EES group in the RESET trial) | CO-Cr EES | Clopidogrel | Primary: Composite of cardiovascular death, MI, stroke, definite ST, and major/minor bleeding at 1 year Secondary: death, MI, stroke, possible/probable/definite ST, bleeding, TLR, TVR, coronary artery bypass grafting, and any coronary revascularization | 3 M DAPT was at least as safe as 12 M DAPT. | 2012–2014 |
| SMART-CHOICE [72] | 3 M DAPT followed by P2Y12 inhibitor vs. 12 M DAPT | EES, ZES, SES | Clopidogrel, prasugrel, ticagrelor | Primary: composite of all-cause death, MI, or stroke at 12 M following PCI Secondary: the components of the primary endpoint; CD; TLR; TVR; any revascularization; ST; BARC bleeding type of at least 2 or 3; a composite of death, MI, cerebrovascular events, or any revascularization at 12 M follow-up’ and each component of primary and secondary endpoints at 2 and 3 years. | 3 M DAPT followed by P2Y12 inhibitor monotherapy was noninferior to 12 M DAPT in regard to the occurrence of major adverse cardiac and cerebrovascular events. | 2014–2018 |
| EVOLVE Short DAPT [73] | 3 M DAPT followed by ASA (event-free patients at 3 M follow-up) vs. 12 M DAPT | EES | Clopidogrel, prasugrel or ticagrelor | Primary: all-cause death/MI and study stent-related definite/probable ST Secondary: the rate of bleeding (types 2, 3, and 5 according to BARC) in patients not receiving chronic anticoagulation | 3 M DAPT was associated with favorable rates of ischemic events in HBR patients. | 2016–2019 |
| XIENCE Short DAPT [51] | XIENCE 28: 1 M DAPT followed by ASA vs. 6 M DAPT XIENCE 90: 3 M DAPT followed by ASA vs. 12 M DAPT | EES | Clopidogrel, prasugrel or ticagrelor | Primary: the composite of all-cause death or MI Secondary: Bleeding types 2–5 according to BARC and definite or probable stent thrombosis | 1–3 M DAPT in HBR patients was noninferior in ischemic outcomes, and the incidence of ST was low, which may be associated with a lower incidence of major bleeding. | 2017–2020 |
| TICO [74] | 3 M DAPT followed by ticagrelor vs. 12 M DAPT in ACS patients | BP-SES | Ticagrelor | Primary: a composite of major bleeding and adverse cardiac and cerebrovascular events within 1 year Secondary: major or minor bleeding, death, MI, ST, stroke, and TVR | 3 M DAPT was associated with a statistically significant reduction in the composite outcome of major bleeding and cardiovascular events at 1 year. | 2015–2023 |
| TWILIGHT [75] | 3 M DAPT followed by ticagrelor vs. 12 M DAPT | DES | Ticagrelor | Primary: bleeding types 2, 3, and 5 according to BARC Secondary: death from any cause, nonfatal MI, or nonfatal stroke | 3 M DAPT followed by ticagrelor resulted in reduction in rates of bleeding, without an increase in ischemic events. | 2015–2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fluder-Wlodarczyk, J.; Pawłowski, S.; Chuchra, P.J.; Pawłowski, T.; Wojakowski, W.; Gasior, P. Importance of Short-Term Neointimal Coverage of Drug-Eluting Stents in the Duration of Dual Antiplatelet Therapy. J. Clin. Med. 2024, 13, 1730. https://doi.org/10.3390/jcm13061730
Fluder-Wlodarczyk J, Pawłowski S, Chuchra PJ, Pawłowski T, Wojakowski W, Gasior P. Importance of Short-Term Neointimal Coverage of Drug-Eluting Stents in the Duration of Dual Antiplatelet Therapy. Journal of Clinical Medicine. 2024; 13(6):1730. https://doi.org/10.3390/jcm13061730
Chicago/Turabian StyleFluder-Wlodarczyk, Joanna, Sławomir Pawłowski, Piotr J. Chuchra, Tomasz Pawłowski, Wojciech Wojakowski, and Pawel Gasior. 2024. "Importance of Short-Term Neointimal Coverage of Drug-Eluting Stents in the Duration of Dual Antiplatelet Therapy" Journal of Clinical Medicine 13, no. 6: 1730. https://doi.org/10.3390/jcm13061730
APA StyleFluder-Wlodarczyk, J., Pawłowski, S., Chuchra, P. J., Pawłowski, T., Wojakowski, W., & Gasior, P. (2024). Importance of Short-Term Neointimal Coverage of Drug-Eluting Stents in the Duration of Dual Antiplatelet Therapy. Journal of Clinical Medicine, 13(6), 1730. https://doi.org/10.3390/jcm13061730






