The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome—Lessons Learned?
Abstract
1. Introduction
2. Defining ARDS
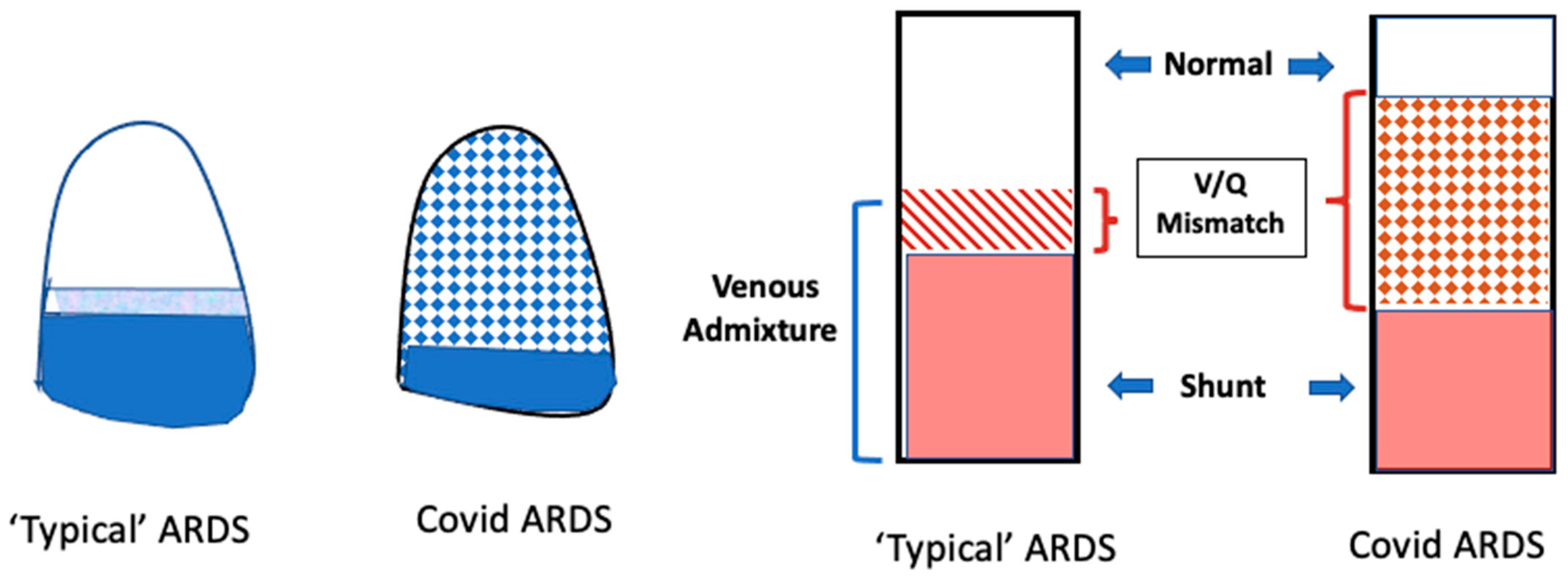
3. Understanding Hypoxemia in COVID-19 ARDS
4. Respiratory Mechanics and Lung Injury
5. Timing of Intubation in COVID-19 Pneumonia
6. Awake Prone Position
7. Ventilator Management in COVID-19 ARDS
7.1. Tidal Volume
7.2. Positive End Expiratory Pressure
7.3. Prone Position
7.4. Detecting End-Tidal Hyperinflation
8. Extracorporeal Membrane Oxygenation for COVID-19 ARDS
9. Conclusions
9.1. Oxygenation-Based Criteria for ARDS May Misdirect Ventilation Strategy
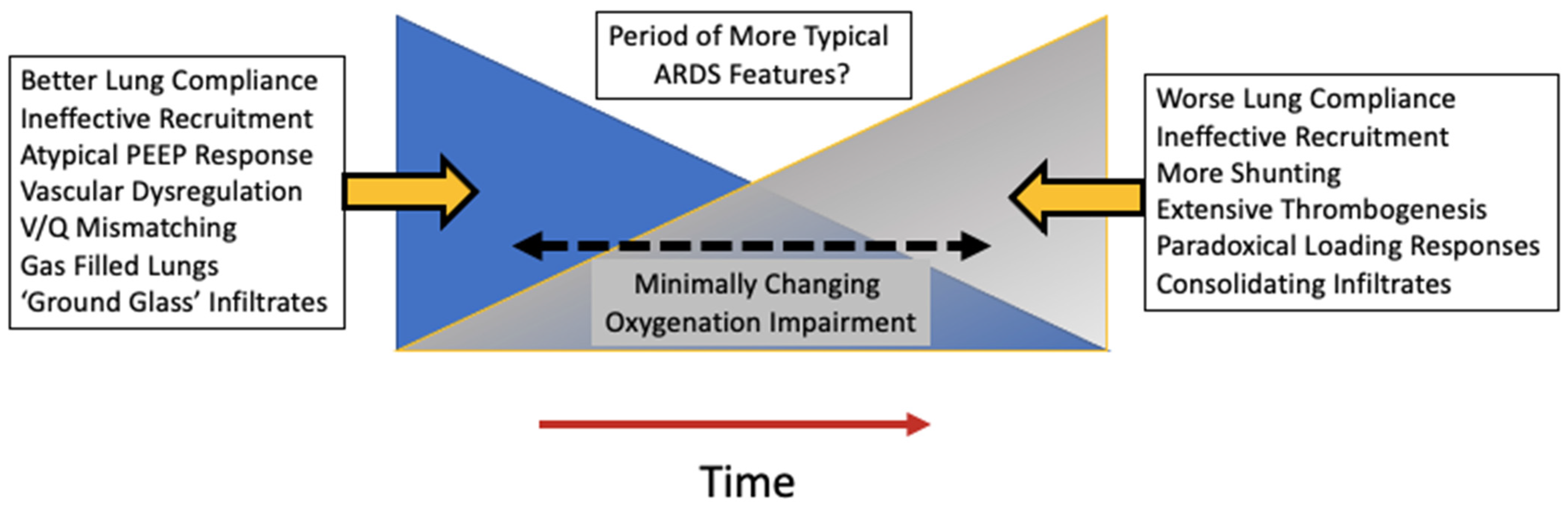
9.2. Mechanical Properties May Change Dramatically over Time
9.3. Personalize Ventilator Settings
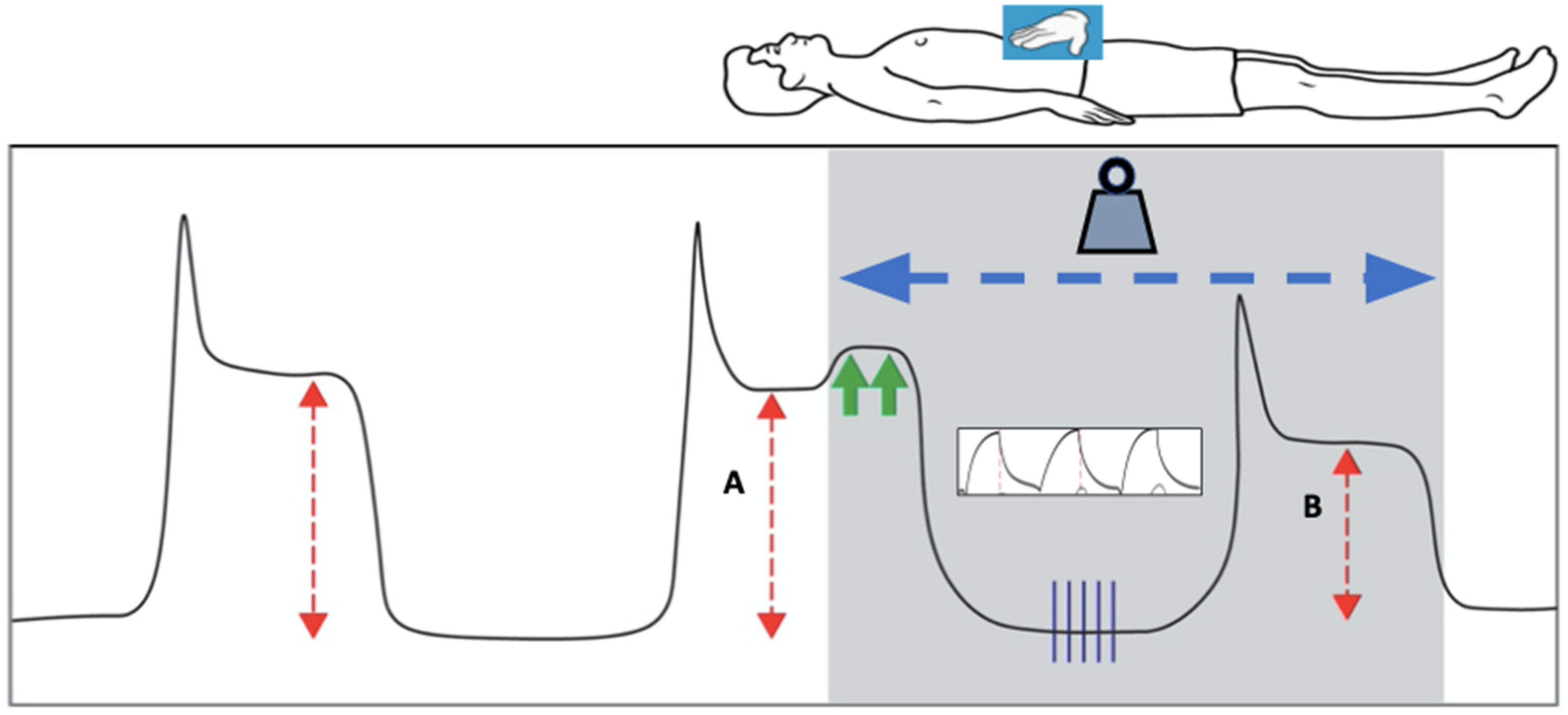
9.4. Challenge Traditional Assumptions Regarding Lung Protection
9.5. Prone Positioning May Forestall or Negate the Need for Intubation
9.6. The COVID-19 Pandemic Expanded Our Experience with Venovenous ECMO for ARDS Treatment
Funding
Conflicts of Interest
References
- WHO COVID-19 Dashboard 31 December 2023–15 January 2024. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 15 January 2024).
- Ganesh, B.; Rajakumar, T.; Malathi, M.; Manikandan, N.; Nagaraj, J.; Santhakumar, A.; Elangovan, A.; Malik, Y.S. Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: An updated overview of current knowledge and future perspectives. Clin. Epidemiol. Glob. Health 2021, 10, 100694. [Google Scholar] [CrossRef] [PubMed]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 516. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 209, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Dries, D.J. Critical Care Medicine: The Essentials and More, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Baindara, P.; Roy, D.; Mandal, S.M.; Schrum, A.G. Conservation and Enhanced Binding of SARS-CoV-2 Omicron Spike Protein to Coreceptor Neuropilin-1 Predicted by Docking Analysis. Infect. Dis. Rep. 2022, 14, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Copin, M.C.; Parmentier, E.; Duburcq, T.; Poissy, J.; Mathieu, D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020, 46, 1124–1126. [Google Scholar] [CrossRef]
- Hariri, L.P.; North, C.M.; Shih, A.R.; Israel, R.A.; Maley, J.H.; Villalba, J.A.; Vinarsky, V.; Rubin, J.; Okin, D.A.; Sclafani, A.; et al. Lung Histopathology in Coronavirus Disease 2019 as Compared with Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review. Chest 2021, 159, 73–84. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Rossi, S. COVID-19 pneumonia: ARDS or not? Crit. Care 2020, 24, 154. [Google Scholar] [CrossRef]
- Chiumello, D.; Busana, M.; Coppola, S.; Romitti, F.; Formenti, P.; Bonifazi, M.; Pozzi, T.; Palumbo, M.M.; Cressoni, M.; Herrmann, P.; et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: A matched cohort study. Intensive Care Med. 2020, 46, 2187–2196. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Marini, J.J.; Rocco, P.R.M.; Gattinoni, L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am. J. Respir. Crit. Care Med. 2020, 201, 767–774. [Google Scholar] [CrossRef]
- López-Aguilar, J.; Piacentini, E.; Villagrá, A.; Murias, G.; Pascotto, S.; Saenz-Valiente, A.; Fernández-Segoviano, P.; Hotchkiss, J.R.; Blanch, L. Contributions of vascular flow and pulmonary capillary pressure to ventilator-induced lung injury. Crit. Care Med. 2006, 34, 1106–1112. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Mascheroni, D.; Kolobow, T.; Fumagalli, R.; Moretti, M.P.; Chen, V.; Buckhold, D. Acute respiratory failure following pharmacologically induced hyperventilation: An experimental animal study. Intensive Care Med. 1988, 15, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; Fantini, R.; Tabbì, L.; Castaniere, I.; Pisani, L.; Pellegrino, M.R.; Della Casa, G.; D’Amico, R.; Girardis, M.; Nava, S.; et al. Early Inspiratory Effort Assessment by Esophageal Manometry Predicts Noninvasive Ventilation Outcome in De Novo Respiratory Failure. A Pilot Study. Am. J. Respir. Crit. Care Med. 2020, 202, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Jubran, A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care 2020, 10, 78. [Google Scholar] [CrossRef]
- Manrique, S.; Claverias, L.; Magret, M.; Masclans, J.R.; Bodi, M.; Trefler, S.; Canadell, L.; Díaz, E.; Sole-Violan, J.; Bisbal-Andrés, E.; et al. Timing of intubation and ICU mortality in COVID-19 patients: A retrospective analysis of 4198 critically ill patients during the first and second waves. BMC Anesthesiol. 2023, 23, 140. [Google Scholar] [CrossRef]
- Yamamoto, R.; Kaito, D.; Homma, K.; Endo, A.; Tagami, T.; Suzuki, M.; Umetani, N.; Yagi, M.; Nashiki, E.; Suhara, T.; et al. Early intubation and decreased in-hospital mortality in patients with coronavirus disease 2019. Crit. Care 2022, 26, 124. [Google Scholar] [CrossRef]
- Green, A.; Rachoin, J.S.; Schorr, C.; Dellinger, P.; Casey, J.D.; Park, I.; Gupta, S.; Baron, R.M.; Shaefi, S.; Hunter, K.; et al. Timing of invasive mechanical ventilation and death in critically ill adults with COVID-19: A multicenter cohort study. PLoS ONE 2023, 18, e0285748. [Google Scholar] [CrossRef]
- Grotberg, J.C.; Kraft, B.D. Timing of Intubation in COVID-19: When It Is Too Early and When It Is Too Late. Crit. Care Explor. 2023, 5, e0863. [Google Scholar] [CrossRef]
- Camous, L.; Pommier, J.D.; Martino, F.; Tressieres, B.; Demoule, A.; Valette, M. Very late intubation in COVID-19 patients: A forgotten prognosis factor? Crit. Care 2022, 26, 89. [Google Scholar] [CrossRef]
- González, J.; Benítez, I.D.; de Gonzalo-Calvo, D.; Torres, G.; de Batlle, J.; Gómez, S.; Moncusí-Moix, A.; Carmona, P.; Santisteve, S.; Monge, A.; et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: A prospective cohort study. Crit. Care 2022, 26, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.; Choi, M.; Choi, W.I.; Joh, J.; Park, J.; Kim, J. Early intubation and clinical outcomes in patients with severe COVID-19: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 226. [Google Scholar] [CrossRef]
- Papoutsi, E.; Giannakoulis, V.G.; Xourgia, E.; Routsi, C.; Kotanidou, A.; Siempos, I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. Crit. Care 2021, 25, 121. [Google Scholar] [CrossRef] [PubMed]
- Roca, O.; Messika, J.; Caralt, B.; García-de-Acilu, M.; Sztrymf, B.; Ricard, J.D.; Masclans, J.R. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J. Crit. Care 2016, 35, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Scaravilli, V.; Grasselli, G.; Castagna, L.; Zanella, A.; Isgrò, S.; Lucchini, A.; Patroniti, N.; Bellani, G.; Pesenti, A. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: A retrospective study. J. Crit. Care 2015, 30, 1390–1394. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Ma, W.; He, H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: A multi-center prospective cohort study. Crit. Care 2020, 24, 28. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Pavlov, I.; Perez, Y.; Tan, W.; Roca, O.; Tavernier, E.; Kharat, A.; McNicholas, B.; Ibarra-Estrada, M.; et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 573–583. [Google Scholar] [CrossRef]
- Liu, L.; Xie, J.; Wang, C.; Zhao, Z.; Chong, Y.; Yuan, X.; Qiu, H.; Zhao, M.; Yang, Y.; Slutsky, A.S. Prone position improves lung ventilation-perfusion matching in non-intubated COVID-19 patients: A prospective physiologic study. Crit. Care 2022, 26, 193. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Cardoso, S.O.; Manetta, J.A.; Pereira, V.G.; Espósito, D.C.; Pasqualucci Mde, O.; Damasceno, M.C.; Schultz, M.J. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA 2012, 308, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Gattinoni, L. Time Course of Evolving Ventilator-Induced Lung Injury: The “Shrinking Baby Lung”. Crit. Care Med. 2020, 48, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- ARDSnet. NIH NHLBI ARDS Clinical Network Mechanical Ventilation Protocol Summary. 2008. Available online: http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf (accessed on 12 December 2023).
- Barthélémy, R.; Beaucoté, V.; Bordier, R.; Collet, M.; Le Gall, A.; Hong, A.; de Roquetaillade, C.; Gayat, E.; Mebazaa, A.; Chousterman, B.G. Haemodynamic impact of positive end-expiratory pressure in SARS-CoV-2 acute respiratory distress syndrome: Oxygenation versus oxygen delivery. Br. J. Anaesth. 2021, 126, e70–e72. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Bonifazi, M.; Pozzi, T.; Formenti, P.; Papa, G.F.S.; Zuanetti, G.; Coppola, S. Positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome: The heterogeneous effects. Crit. Care 2021, 25, 431. [Google Scholar] [CrossRef] [PubMed]
- Cove, M.E.; Pinsky, M.R.; Marini, J.J. Are we ready to think differently about setting PEEP? Crit. Care 2022, 26, 222. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Fauvel, N.J.; Singh, S.; Soni, N. Ventilatory ratio: A simple bedside measure of ventilation. Br. J. Anaesth. 2009, 102, 692–697. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Taccone, P.; Carlesso, E.; Marini, J.J. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am. J. Respir. Crit. Care Med. 2013, 188, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Hubmayr, R.D. The prone position eliminates compression of the lungs by the heart. Am. J. Respir. Crit. Care Med. 2000, 161, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Albert, R.K.; Beitler, J.; Gattinoni, L.; Jaber, S.; Marini, J.J.; Munshi, L.; Papazian, L.; Pesenti, A.; Vieillard-Baron, A.; et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020, 46, 2385–2396. [Google Scholar] [CrossRef]
- Skarpsno, E.S.; Mork, P.J.; Nilsen, T.I.L.; Holtermann, A. Sleep positions and nocturnal body movements based on free-living accelerometer recordings: Association with demographics, lifestyle, and insomnia symptoms. Nat. Sci. Sleep 2017, 9, 267–275. [Google Scholar] [CrossRef]
- Langer, T.; Brioni, M.; Guzzardella, A.; Carlesso, E.; Cabrini, L.; Castelli, G.; Dalla Corte, F.; De Robertis, E.; Favarato, M.; Forastieri, A.; et al. Prone position in intubated, mechanically ventilated patients with COVID-19: A multi-centric study of more than 1000 patients. Crit. Care 2021, 25, 128. [Google Scholar] [CrossRef]
- Fossali, T.; Pavlovsky, B.; Ottolina, D.; Colombo, R.; Basile, M.C.; Castelli, A.; Rech, R.; Borghi, B.; Ianniello, A.; Flor, N.; et al. Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients with COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit. Care Med. 2022, 50, 723–732. [Google Scholar] [CrossRef]
- Camporota, L.; Sanderson, B.; Chiumello, D.; Terzi, N.; Argaud, L.; Rimmelé, T.; Metuor, R.; Verstraete, A.; Cour, M.; Bohé, J.; et al. Prone Position in COVID-19 and -COVID-19 Acute Respiratory Distress Syndrome: An International Multicenter Observational Comparative Study. Crit. Care Med. 2022, 50, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Palumbo, M.M.; Sverzellati, N.; Busana, M.; Malchiodi, L.; Bresciani, P.; Ceccarelli, P.; Sani, E.; Romitti, F.; Bonifazi, M.; et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022, 48, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R. Recruitment Maneuvers and PEEP Titration. Respir. Care 2015, 60, 1688–1704. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Terragni, P.; Mascia, L.; Fanelli, V.; Quintel, M.; Herrmann, P.; Hedenstierna, G.; Slutsky, A.S.; Ranieri, V.M. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit. Care Med. 2004, 32, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Selickman, J.; Marini, J.J. Chest wall loading in the ICU: Pushes, weights, and positions. Ann. Intensive Care 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Samanta, S.; Soni, K.D. Supine chest compression: Alternative to prone ventilation in acute respiratory distress syndrome. Am. J. Emerg. Med. 2014, 32, e489.e5–e489.e6. [Google Scholar] [CrossRef] [PubMed]
- Kummer, R.L.; Shapiro, R.S.; Marini, J.J.; Huelster, J.S.; Leatherman, J.W. Paradoxically Improved Respiratory Compliance with Abdominal Compression in COVID-19 ARDS. Chest 2021, 160, 1739–1742. [Google Scholar] [CrossRef]
- Elmufdi, F.S.; Marini, J.J. Dorsal Push and Abdominal Binding Improve Respiratory Compliance and Driving Pressure in Proned Coronavirus Disease 2019 Acute Respiratory Distress Syndrome. Crit. Care Explor. 2021, 3, e0593. [Google Scholar] [CrossRef]
- Lassola, S.; Miori, S.; Sanna, A.; Pace, R.; Magnoni, S.; Vetrugno, L.; Umbrello, M. Effect of chest wall loading during supine and prone position in a critically ill COVID-19 patient: A new strategy for ARDS? Crit. Care 2021, 25, 442. [Google Scholar] [CrossRef]
- Rezoagli, E.; Bastia, L.; Grassi, A.; Chieregato, A.; Langer, T.; Grasselli, G.; Caironi, P.; Pradella, A.; Santini, A.; Protti, A.; et al. Paradoxical Effect of Chest Wall Compression on Respiratory System Compliance: A Multicenter Case Series of Patients with ARDS, with Multimodal Assessment. Chest 2021, 160, 1335–1339. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Improving lung compliance by external compression of the chest wall. Crit. Care 2021, 25, 264. [Google Scholar] [CrossRef]
- Selickman, J.; Tawfik, P.; Crooke, P.S.; Dries, D.J.; Shelver, J.; Gattinoni, L.; Marini, J.J. Paradoxical response to chest wall loading predicts a favorable mechanical response to reduction in tidal volume or PEEP. Crit. Care 2022, 26, 201. [Google Scholar] [CrossRef]
- Selickman, J.; Crooke, P.S.; Tawfik, P.; Dries, D.J.; Gattinoni, L.; Marini, J.J. Paradoxical Positioning: Does “Head Up” Always Improve Mechanics and Lung Protection? Crit. Care Med. 2022, 50, 1599–1606. [Google Scholar] [CrossRef]
- Bastia, L.; Rezoagli, E.; Guarnieri, M.; Engelberts, D.; Forlini, C.; Marrazzo, F.; Spina, S.; Bassi, G.; Giudici, R.; Post, M.; et al. External chest-wall compression in prolonged COVID-19 ARDS with low-compliance: A physiological study. Ann. Intensive Care 2022, 12, 35. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Gajkowski, E.F.; Herrera, G.; Hatton, L.; Velia Antonini, M.; Vercaemst, L.; Cooley, E. ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygenation Circuits. ASAIO J. 2022, 68, 133–152. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Nesseler, N.; Fadel, G.; Mansour, A.; Para, M.; Falcoz, P.E.; Mongardon, N.; Porto, A.; Bertier, A.; Levy, B.; Cadoz, C.; et al. Extracorporeal Membrane Oxygenation for Respiratory Failure Related to COVID-19: A Nationwide Cohort Study. Anesthesiology 2022, 136, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Lippi, G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J. Crit. Care 2020, 58, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Shekar, K.; Ling, R.R.; Barbaro, R.P.; Wong, S.N.; Tan, C.S.; Rochwerg, B.; Fernando, S.M.; Takeda, S.; MacLaren, G.; et al. Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit. Care 2021, 25, 211. [Google Scholar] [CrossRef] [PubMed]
- Ling, R.R.; Ramanathan, K.; Sim, J.J.L.; Wong, S.N.; Chen, Y.; Amin, F.; Fernando, S.M.; Rochwerg, B.; Fan, E.; Barbaro, R.P.; et al. Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: A systematic review and meta-analysis. Crit. Care 2022, 26, 147. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lotz, C.; Karagiannidis, C.; Weber-Carstens, S.; Kluge, S.; Putensen, C.; Wehrfritz, A.; Schmidt, K.; Ellerkmann, R.K.; Oswald, D.; et al. Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation. Crit. Care 2022, 26, 190. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kummer, R.L.; Marini, J.J. The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome—Lessons Learned? J. Clin. Med. 2024, 13, 1833. https://doi.org/10.3390/jcm13071833
Kummer RL, Marini JJ. The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome—Lessons Learned? Journal of Clinical Medicine. 2024; 13(7):1833. https://doi.org/10.3390/jcm13071833
Chicago/Turabian StyleKummer, Rebecca L., and John J. Marini. 2024. "The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome—Lessons Learned?" Journal of Clinical Medicine 13, no. 7: 1833. https://doi.org/10.3390/jcm13071833
APA StyleKummer, R. L., & Marini, J. J. (2024). The Respiratory Mechanics of COVID-19 Acute Respiratory Distress Syndrome—Lessons Learned? Journal of Clinical Medicine, 13(7), 1833. https://doi.org/10.3390/jcm13071833





