Abstract
Background: Cardiac resynchronization therapy (CRT) has evolved into an established therapy for patients with chronic heart failure and a wide QRS complex. Data on long-term outcomes over time are scarce and the criteria for implantation remain a subject of investigation. Methods: An international, multicenter, retrospective registry includes 2275 patients who received CRT between 30 November 2000 and 31 December 2019, with a mean follow-up of 3.6 ± 2.7 years. Four time periods were defined, based on landmark trials and guidelines. The combined endpoint was a composite of all-cause mortality, heart transplantation, or left ventricular assist device implantation. Results: The composite endpoint occurred in 656 patients (29.2%). The mean annual implantation rate tripled from 31.5 ± 17.4/year in the first period to 107.4 ± 62.4/year in the last period. In the adjusted Cox regression analysis, the hazard ratio for the composite endpoint was not statistically different between time periods. When compared to sinus rhythm with left bundle branch block (LBBB), a non-LBBB conduction pattern (sinus rhythm: HR 1.51, 95% CI 1.12–2.03; atrial fibrillation: HR 2.08, 95% CI 1.30–3.33) and a QRS duration below 130 ms (HR 1.64, 95% CI 1.29–2.09) were associated with a higher hazard ratio. Conclusions: Despite innovations, an adjusted regression analysis revealed stable overall survival over time, which can at least partially be explained by a shift in patient characteristics.
1. Introduction
Over the past twenty years, cardiac resynchronization therapy (CRT) has evolved as one of the standards of care for patients with heart failure (HF), with a reduced ejection fraction (HFrEF) and a wide QRS complex [1,2]. In the early 2000s, several landmark trials demonstrated the clinical benefit of CRT in patients with HFrEF [3]. COMPANION [4], CARE-HF [5], and RAFT [6] proved the efficacy of CRT in patients with NYHA class III and IV. In 2008 and 2009, REVERSE [7] and MADIT-CRT [8] also revealed that patients with NYHA class I and II were likely to benefit from CRT. Since then, the integration of CRT into numerous clinical practice guidelines, both for pacing and for heart failure, has further underscored its significance.
In parallel, substantial progress has been made both in regard to technical aspects of CRT and pharmacological treatments for heart failure. Likewise, patient selection and implantation indications have evolved over time as new studies have been conducted. Nevertheless, data on how this historical evolution has affected patient selection, CRT implantation rates, and long-term outcomes are scarce.
Therefore, we aim to describe the evolution of patient characteristics, indications, and long-term outcomes in CRT patients over two decades of change and development, using a large, multicenter European CRT registry [9].
2. Materials and Methods
2.1. Study Population
All patients aged ≥18 years who had a CRT implantation in one of 3 participating tertiary care centers, University Hospital Zurich (Zurich, Switzerland), Ziekenhuis Oost-Limburg (Genk, Belgium), and University Hospitals Leuven (Leuven, Belgium), were included in a retrospective registry. The registry has been described previously [9]. In brief, indications for CRT implantation followed the latest literature or ESC guidelines available at the time [1,10]. Accordingly, ischemic, as well as non-ischemic, cardiomyopathies were included and the devices implanted had a cardioverter–defibrillation function, if indicated. Likewise, devices were implanted de novo, or were upgraded from a pacemaker or implantable cardioverter defibrillator (ICD). Optimization of the guideline-directed medical therapy and CRT programming were left to the discretion of the treating physicians. In general, each center had a routine follow-up routine, which included in-person visits and remote monitoring. Ethical approval was granted by the ethical committee at each individual institution. Given the retrospective nature of the study, the requirement for written informed consent was exempted.
2.2. Retrospective Registries
Every patient implanted with a CRT device between 30 November 2000 and 31 December 2019 was included in the registry. Inclusion dates and follow-up times differed between the three hospitals. Demographic data, clinical characteristics, and the baseline pharmaceutical regimen, alongside the biochemical, electrocardiographic, and echocardiographic information prior to CRT implantation were extracted from the electronic medical records. The left ventricular ejection fraction (LVEF) before CRT implantation was acquired from cardiac magnetic resonance imaging or echocardiography. For the latter, measurements were obtained using the modified Simpson’s biplane method or by visual assessment. The consolidation of registries was executed under the oversight of two investigators (B.V. and S.T.). In this study, exclusively shared variables were selected for subsequent analysis.
2.3. Arrangement by Time Periods and Indications
Four distinct time periods were identified, and each patient was allocated to one period according to their implantation date. The demarcation of these periods was chosen according to publication of landmark trials and guidelines. The first period (P1) ranged from the start of the registry (30 November 2000) to the publication of the MADIT-CRT findings on 1 October 2009. The subsequent period (P2) extended from the release of MADIT-CRT until the 2013 ESC pacing and CRT guidelines (25 June 2013). The third period (P3) covered the period from the publication of the 2013 ESC guidelines to the publication of the 2016 ESC guidelines on heart failure on 20 May 2016. The fourth, and last period, (P4) encompassed the time span from the 2016 ESC guidelines to the last patient included on 31 December 2019.
Additionally, analysis was performed according to the rhythm and QRS morphology in patients with a LVEF ≤ 35%. For this analysis, baseline data was arranged according to the presence of sinus rhythm or atrial fibrillation, related to distinctive conduction patterns, specifically left bundle branch block (LBBB) and non-left bundle branch block, and QRS duration > 150 milliseconds (ms), 150 ms to 130 ms and ≤130 ms.
2.4. Endpoints
The study endpoint was a composite of left ventricular assist device implantation, heart transplantation, or all-cause mortality. Specific endpoint occurrences were documented alongside the corresponding dates. Patients who did not experience the composite endpoint were included in the analysis from the date of implantation until the most recent available follow-up, defined as the last clinical contact. If the patient experienced the composite endpoint, the date of decease, heart transplantation, or left ventricular assist device implantation, was considered the last follow-up date. Patients from Ziekenhuis Oost-Limburg who required transplantation or ventricular assist device implantation were referred to University Hospitals Leuven.
2.5. Statistical Analysis
Categorical variables were presented as numbers and percentages. The normal distribution of continuous variables was verified using the Kolmogorov–Smirnov test. Since all continuous variables showed non-normal distribution, these were presented as median and interquartile ranges. A comparison of the parameters between groups was performed using the Kruskal–Wallis test, the Mann–Whitney U test, and the chi-square test. Given the differences in inclusion between the centers, implant rates were adjusted according to the available time in each period and expressed as the mean annual implant rate. Implant rates were calculated separately for each center and analyzed using the chi-square test for any trends. Kaplan–Meier analysis was performed to calculate incidence rates for the composite endpoint, including the log-rank test for comparison by group. Univariable and multivariable Cox proportional hazards regression modelling was performed for the combined endpoint. A multivariable model for the combined endpoint was constructed by including all variables with a p-value < 0.100 in the univariable Cox regression, in a stepwise multivariable model with forward parameter selection (entry p < 0.050). The proportional hazard assumptions were assessed using the Schoenfeld residuals test and proportional hazard plots, while multicollinearity was assessed using covariance matrices. In case of violation of the proportional hazard assumptions, stratification was applied. Next, for the adjusted analysis according to the implant period and rhythm and QRS morphology, these variables were added to the previously developed multivariable Cox regression model. For the analysis according to the rhythm and QRS morphology, variables which included information about the QRS duration and QRS morphology were removed from the previously developed model. For each model, the p-value corresponding to the global Schoenfeld residuals test and Harrell’s C-index are reported either in the manuscript or in the Supplementary Materials. An overview of the data availability is presented in Supplementary Table S1. Missing values were handled by listwise deletion. The statistical analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA) and GraphPad Prism version 9.00 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. Demographics
A total of 2275 patients were enrolled in the registry and the data availability was excellent, with a maximum of 2.3% missing data for all variables (Supplementary Table S1). The baseline demographic characteristics and a comparison between the four time periods are shown in Table 1. In general, the median age at implantation was 70.3 years and 26.4% were female. In 63.9% of patients, an implantable cardioverter defibrillator was implanted and, in 42.8%, the underlying cause of heart failure was an ischemic cardiomyopathy (ICMP). Overall, two-thirds (66.8%) of cases showed left bundle branch block (LBBB) at the time of device implantation, while the remaining portion exhibited non-LBBB conduction patterns. Notably, in 18.9% of implantations, the QRS complex was below 130 ms.

Table 1.
Baseline demographics and clinical characteristics by implant period.
3.2. Endpoint Analysis
The primary composite endpoint occurred in 656 patients (29.2%). Of these, 34 underwent ventricular assist device implantation, 37 underwent heart transplantation, and 585 patients died. The mean follow-up was 3.6 ± 2.7 years. The Kaplan–Meier analysis conducted for the composite endpoint in the overall population is shown in Figure 1A.
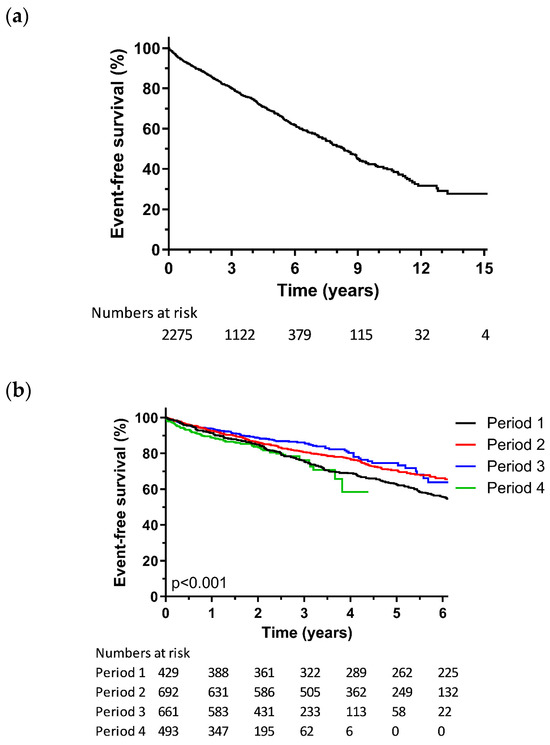
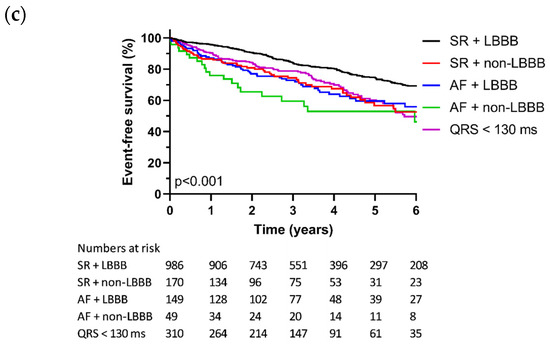
Figure 1.
Kaplan–Meier analysis for all-cause mortality, heart transplantation, or ventricular assist device; (a) Kaplan–Meier analysis for the overall population; (b) Kaplan–Meier analysis according to implant period; (c) Kaplan–Meier analysis according to rhythm and QRS morphology.
Table 2 shows the multivariable Cox proportional hazards regression model for endpoint occurrences in the overall registry. Independent predictors for the composite endpoint were male sex, no ICD, a QRS duration below 130 ms, a non-LBBB conduction pattern, and the absence of pharmacological RAAS inhibition. On the other hand, a lower NYHA functional class, better renal function, the absence of diabetes mellitus, and no history of stroke, or transient ischemic attack, were independently associated with a protective effect with regard to the composite endpoint.

Table 2.
Multivariable Cox proportional hazards regression model for all-cause mortality, heart transplantation, or ventricular assist device.
3.3. Evolution in Time
Progression of the mean annual implant rate across the four distinct periods is demonstrated in Figure 2. Notably, there was a significant and consistent upward trajectory in the number of annual CRT implantations (p = 0.026). Table 1 provides an overview of the evolution of the patient characteristics throughout the four periods, with group-to-group comparisons available in Supplementary Table S2. Over the course of the last two decades, patients who underwent CRT implantation have shown a trend toward older ages. The implantation of combined CRT–ICD devices (CRT-D) was higher in the first period compared to subsequent periods. Likewise, the implantation rates have shifted to lower NYHA functional classes in the latter three periods compared to the initial period. In addition, there has been a trend toward decreased implantation of epicardial leads over time.
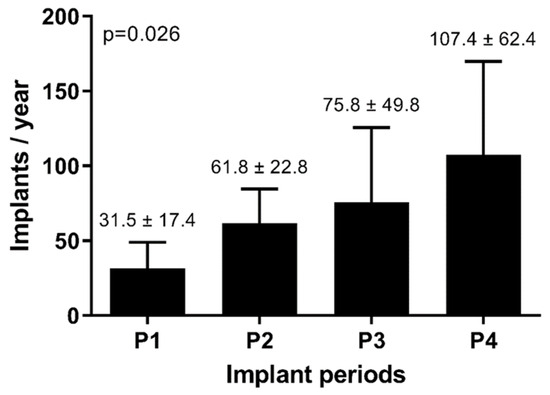
Figure 2.
Evolution of mean implant rate.
In our registry, conduction patterns varied as the indication was not confined solely to left bundle branch block patterns. Right bundle branch block patterns, non-specific patterns, and paced QRS patterns were observed with varying frequency over the distinct periods. In the final period, a lower proportion of patients were on renin–angiotensin–aldosterone system (RAAS) inhibitors before implantation than in the three first periods. The rate of patients taking loop diuretics displayed an initial downward trend, but increased significantly in the last period.
3.4. Endpoint Prediction over Time
The Kaplan–Meier survival curves for the composite endpoint across consecutive time periods are demonstrated in Figure 1B. As shown in Table 3, the cumulative event rates at the 1-year follow-up for P1, P2, P3, and P4 were 8.5%, 7.5%, 6.1%, and 11%, respectively. Over a 3-year span, the cumulative event rates of 24.5%, 19.4%, 13.8%, and 23.8% were observed, and 5-year cumulative event rates of 37.6%, 29.3%, and 25.4% were observed, with 5-year follow-up data not available for P4. In an adjusted Cox proportional hazards regression analysis, the hazard ratio for the composite endpoint was not statistically different between the four periods (Table 3). The final Cox proportional hazards regression model, including analysis by implant period, is presented in Supplementary Table S3.

Table 3.
Adjusted Cox proportional hazards regression model for all-cause mortality, heart transplantation, or ventricular assist device, by implant period.
3.5. Endpoint Prediction According to Rhythm and QRS Morphology
The baseline demographics and clinical characteristics arranged according to the rhythm and QRS morphology are shown in Table 4. Sinus rhythm in combination with an LBBB conduction pattern was significantly more prevalent in women compared to men, whereas the prevalence of sinus rhythm with different conduction patterns or atrial fibrillation with LBBB, non-LBBB, or a QRS below 130 ms, displayed a more balanced distribution between the sexes. Other differences are described in Supplementary Table S4, but they lack meaningful correlations.

Table 4.
Baseline demographics and clinical characteristics according to rhythm and QRS morphology in patients with LVEF ≤ 35%.
The Kaplan–Meier analysis for the composite endpoint, broken down by rhythm and QRS morphology, is displayed in Figure 1C. The cumulative event rate after 5 years was 25.6% for sinus rhythm with an LBBB conduction pattern, while it ranged from 39.8% to 47.1% for patients who had atrial fibrillation and/or non-LBBB conduction patterns (Table 5, Supplementary Table S5). In an adjusted Cox proportional hazards regression model (Table 5), the presence of a non-LBBB conduction pattern was associated with a higher hazard ratio in regard to both sinus rhythm (HR 1.51, 95% CI 1.12–2.03) and atrial fibrillation (HR 2.08, 95% CI 1.30–3.33) for the composite endpoint, compared to an LBBB conduction pattern and sinus rhythm (reference group). Likewise, a QRS duration below 130 ms exhibited a higher hazard ratio (HR 1.64, 95% CI 1.29–2.09) compared to an LBBB conduction pattern and sinus rhythm. There was no significant difference between the patients with LBBB in regard to sinus rhythm and patients with LBBB in regard to AF (HR 1.33, 95% CI 0.97–1.80). The final Cox regression model is presented in Supplementary Table S6.

Table 5.
Adjusted Cox proportional hazards regression model for all-cause mortality, heart transplantation, or ventricular assist device, by indication.
4. Discussion
In this large, multicenter, retrospective CRT registry, representing the real-world experience of three tertiary care centers, we examined the evolution of CRT implantations over time and explored the different implant indications, patient populations, and outcomes.
4.1. Evolution over Time
Over the last two decades, CRT has become one of the cornerstones of treatment in HFrEF, specifically in patients with conduction delays. This is illustrated by the increment in implantations over the years, as the total number of annual CRT implantations has more than tripled from the first period to the fourth period. This increase reflects a combination of the increasing prevalence of heart failure and the evolving indications for CRT. The temporal shift in patient selection criteria is reflected in the baseline clinical characteristics. For example, the observed shift in annual implant rates and NYHA class, with a higher prevalence of NYHA class III in P1, reflects the findings in the MADIT-CRT study, which primarily focused on patients with moderate-to-severe heart failure [8]. This expanded the potential pool of CRT candidates to a different subset of the patient population, with potentially different disease characteristics. Further, CRT devices have been increasingly implanted in older patients, leading to a higher burden from comorbidities and, consequently, an elevated likelihood of reaching the composite endpoint, irrespective of their comparable improvement in left ventricular function, as a recent retrospective analysis in 2656 geriatric patients showed [11]. Also, the rate of implanted epicardial leads declined over time. This decline may be attributed to technological evolutions, such as quadripolar leads [12,13] and active fixation techniques [14], which have resulted in more stable transvenous pacing. Additionally, the decline in the implantation of a device with a defibrillator function over time, might be attributed to the more stringent criteria for ICD implantation in increasing age, as in our registry almost 25% of patients were 77 years or older, as well as the lack of randomized data indicating a clear benefit of CRT-D over CRT-P [15].
While unadjusted cumulative event rates in our registry declined over time, apart from P4 which extends over the COVID-19 era, these findings did not persist in the adjusted regression analysis. The overall event-free rate aligns with the recent long-term follow-up of the RAFT study, which included patients in NYHA class II and III, who also constitute the predominant portion of our registry [16]. The 5-year cumulative event-rate for the similar composite endpoint in RAFT, based on a visual assessment of the Kaplan–Meier graphs, was approximately 30% and is, therefore, comparable with our multicenter real-world experience of 31.8% [16]. Real-world data on the evolution of mortality and the outcome of CRT are scarce so far, with only two recent analyses of retrospective registries. Darden et al. described a United States registry, from 2011 to 2015, of patients aged ≥65 years implanted with a CRT-D, which disclosed a lower mortality at the 2-year follow-up [17]. Similarly, another retrospective registry, by Leyva et al., encompassing data from 2010 to 2019 from the United Kingdom, also showed decreased mortality after the 2-year follow-up period, with a hazard ratio of 0.72 (95% CI 0.69–0.76) when comparing 2010–2011 to 2018–2019 [18], despite demonstrating increasing comorbidities. While our analysis may be prone to the effect of unknown or unavailable confounders, as are all retrospective studies, there are relevant differences between our registry and previous registries, which may in part be responsible for the difference in outcome. Besides the age cut-off, the study by Darden et al. only included patients who underwent de novo CRT-D implantations and the use of ACE inhibitors and angiotensin-receptor blockers was approximately 76%. Also, the study by Leyva et al. did not include patients who underwent a CRT upgrade [9] and the data availability was at a different level of granularity (e.g., chronic kidney disease as a binary variable versus different chronic kidney disease stages in our study). On the other hand, we did not have detailed information available on the peri- or postprocedural complications, which may have had an influence given the increasing frailty of the patients. Of note, data from our registry predates the widespread use of sodium–glucose cotransporter 2 inhibitors for heart failure, while angiotensin receptor neprilysin inhibitors [19] were only introduced briefly before the inclusion of the last patient.
4.2. Implant Indication
The current cardiac pacing and CRT guidelines [1] suggest the strongest response as CRT for patients with a QRS duration exceeding 150ms and an LBBB conduction pattern. However, the guidelines also advocate, albeit with a less robust recommendation, for the implantation of CRT devices in patients with a QRS duration ≥ 150 ms and a non-LBBB conduction pattern, and for a QRS between 130–150 ms and an LBBB conduction pattern. An even weaker recommendation for CRT is formulated for cases with a QRS duration between 130–150 ms and a non-LBBB conduction pattern.
Electrophysiological properties that predict a favorable response to CRT remain a topic of ongoing debate. No randomized controlled trials (RCTs) have been performed specifically to assess disease modification by CRT in different QRS morphologies. Yet a substudy of RAFT [20], showed a benefit from CRT in patients with an LBBB conduction pattern and a QRS ≥ 160 ms, which appeared to be absent in patients with a non-LBBB conduction pattern, especially if the QRS was below 160 ms. A similar conclusion was drawn from a post hoc analysis of MADIT-CRT [21]. A post hoc meta-analysis of five RCTs confirmed this correlation between an LBBB conduction pattern and a more beneficial response to CRT [22], while there remains more ambiguity regarding patients with non-LBBB conduction patterns [23]. Other meta-analyses place greater emphasis on the QRS duration, suggesting patients with a QRS duration below 150 ms appear to derive less benefits from CRT implantation, irrespective of the conduction pattern [24,25].
Within our multicenter real-world registry, although the non-adjusted incidence rates for the composite endpoint were comparable between patients with a QRS duration of ≥150 ms and patients with a QRS duration between 130 and 150 ms (Supplementary Table S5), the final adjusted model revealed a significant difference only in regard to the conduction pattern. Specifically, patients with an LBBB conduction pattern had a lower chance of reaching the composite endpoint when compared to patients with a non-LBBB conduction pattern, irrespective of the QRS duration at the time of implantation (Supplementary Table S5). Considering the absence of true control groups without CRT, we are restricted to reporting this observation only. Early retrospective studies have also indicated increased event rates [26,27] or less echocardiographic and symptomatic benefit [28] after a 3-year follow-up period among patients with a non-LBBB conduction pattern. Importantly, our study does not indicate a non-response in regard to CRT among patients with a non-LBBB conduction pattern.
The inquiry into which patients, specifically which QRS duration and conduction pattern, benefit the most from CRT implantation remains a subject of research, partly because in most meta-analyses non-LBBB conduction patterns are mostly aggregated into one group. A recent meta-analysis advocates subdivision within the non-LBBB category into more specific groups, such as intraventricular conduction delay (IVCD) and right bundle branch block (RBBB) [29]. It suggests that patients with a QRS duration of longer than 150 ms and IVCD benefit more from CRT than patients with a QRS ≥ 150 ms and RBBB. Our registry recorded 18.9% of implantations in patients with a QRS duration below 130 ms, despite findings from EchoCRT [30] indicating no benefit, and possibly even harm, from CRT in this population. This number is comparable to the number of guideline-discordant implantations found in the registry by Darden et al., but may reflect true BLOCK-HF patients [17,31].
Additionally, it is important to acknowledge that our registry is influenced by temporal changes in the guidelines. This, in particular, has an effect on the interpretation of implant indication. For instance, the definition of left bundle branch block, according to the ESC guidelines, was subject to change over the years, leading to different patient selection and potentially effecting the interpretation of clinical endpoints in disease modification by CRT [32].
4.3. Limitations
The retrospective study design is associated with the inherent limitations of retrospective research, including the potential impact of missing data. Given the absence of a true control population, we are unable to define absolute clinical benefits and we are limited to indirect observations only. As such, our study only reports associations without implying causality for the observed associations. The analysis was constrained by the restricted set of biochemical and echocardiographic variables, encompassing only those available for all patients among the participating centers. Therefore, several variables of interest were not available for analysis, including QRS duration after CRT implantation, atrioventricular conduction delays, and left ventricular remodeling variables, such as the left ventricular end-diastolic diameter. Lastly, the primary endpoint was a composite of endpoints encompassing all-cause mortality, heart transplantation, and implantation of ventricular assist device. As such, it does not include data about changes in quality of life, heart failure admission rates, or functional improvement. Retrospective ascertainment of these potential endpoints imposes a risk of bias to the analysis.
5. Conclusions
This real-world registry of 2275 patients presents the evolution of CRT implantations over almost two decades, showing a notable increase in procedures. Despite pharmaceutical and technological innovations, an adjusted regression analysis revealed stable overall survival over time, at least partially explained by the shift in patient characteristics. These findings highlight the challenges to the ongoing quest to improve clinical outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13071903/s1, Table S1: Data availability; Table S2: Between group comparison of demographics and clinical characteristics for implant period; Table S3: Final Cox proportional hazard regression model for the combined endpoint by implant period; Table S4: Between group comparison of demographics and clinical characteristics by rhythm and QRS morphology; Table S5: Detailed incidence and cumulative event rates for the combined endpoint by rhythm and QRS morphology and duration in patients with LVEF ≤ 35%; Table S6: Final Cox proportional hazard regression model for the combined endpoint by rhythm and QRS morphology; Table S7: QRS duration categorized.
Author Contributions
Conceptualization, J.B., S.T., G.V., J.-U.V., M.D., J.S., R.W., F.R., W.M., S.W. and B.V.; methodology, J.B., S.T., G.V., S.W. and B.V.; formal analysis, J.B., S.T. and B.V.; data curation, J.B., S.T., G.V., P.M., S.I., P.B., A.B. and B.V.; writing—J.B., S.T., S.W. and B.V.; writing—review and editing, G.V., P.M., S.I., P.B., J.-U.V., M.D., A.B., J.S., R.W., F.R. and W.M. All authors have read and agreed to the published version of the manuscript.
Funding
B.V., P.M. and S.T. are supported by a research grant from the Frans Van de Werf Fund for Clinical Cardiovascular Research (Leuven, Belgium). J.-U.V. hold a personal research mandate from the FWO (1832922N). R.W. is supported as a postdoctoral clinical researcher by the Fund for Scientific Research Flanders.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the respective Ethics Committees at University Hospitals Leuven (protocol code S64276, on 11 August 2020), Ziekenhuis Oost-Limburg (protocol code b371201627103, on 13 January 2013), and University Hospital Zurich (protocol code KEK-ZH-NR: 2011-0304, on 13 August 2014).
Informed Consent Statement
Patient consent was waived given the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (accurately indicate status).
Conflicts of Interest
Sander Trenson: advisory board Medtronic, Vifor Pharma, AstraZeneca, Novartis; speaker fees from AstraZeneca, Bayer, Boehringer Ingelheim, and Novartis. Jan Steffel: consultant and/or speaker fees from Abbott, Alexion, AstraZeneca, Bayer, Berlin-Chemie, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, BMS, Daiichi Sankyo, Medscape, Medtronic, Menarini, Merck/MSD, Organon, Pfizer, Saja, Servier, and WebMD; ownership of Swiss EP and CorXL. Stephan Winnik: educational grant support through his institution and/or travel support and/or consulting/speaker fees from Abbott, Bayer, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston-Scientific, Cardinal Health, Daichi-Sankyo, Fehling Instruments, Medtronic, and Servier. RW: research funding from Abbott, Biotronik, Boston Scientific, Medtronic; speaker and consultancy fees from Medtronic, Boston Scientific, Biotronik, and Abbott. FR: has not received personal payments from pharmaceutical companies or device manufacturers in the last 3 years (remuneration for the time spent on activities, such as participation as a steering committee member in clinical trials and a member of the Pfizer Research Award selection committee in Switzerland, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research, educational and/or travel grants from Abbott, Amgen, Astra Zeneca, Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Corteria, Daiichi, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Kantar, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, Trama Solutions, V-Wave, Vascular Medical, Vifor, Wissens Plus, and ZOLL. The research and educational grants do not impact Prof. Ruschitzka’s personal remuneration. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, B.A.; Chew, D.S.; Vandenberk, B. Paradigm Shifts in Cardiac Pacing: Where Have We Been and What Lies Ahead? J. Clin. Med. 2023, 12, 2938. (In English) [Google Scholar] [CrossRef] [PubMed]
- Leyva, F.; Nisam, S.; Auricchio, A. 20 years of cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2014, 64, 1047–1058. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Feldman, A.M.; Saxon, L.A. Heart failure management using implantable devices for ventricular resynchronization: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial. COMPANION Steering Committee and COMPANION Clinical Investigators. J. Card. Fail. 2000, 6, 276–285. (In English) [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef]
- Linde, C.; Abraham, W.T.; Gold, M.R.; Sutton, M.S.J.; Ghio, S.; Daubert, C.; REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. (In English) [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M., III; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009, 361, 1329–1338. (In English) [Google Scholar] [CrossRef] [PubMed]
- Trenson, S.; Voros, G.; Martens, P.; Ingelaere, S.; Betschart, P.; Voigt, J.; Dupont, M.; Breitenstein, A.; Steffel, J.; Willems, R.; et al. Long-term outcome after upgrade to cardiac resynchronization therapy: A propensity score-matched analysis. Eur. J. Heart Fail. 2023. (In English) [Google Scholar] [CrossRef]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.G.F.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. (In English) [Google Scholar] [CrossRef]
- Behon, A.; Merkel, E.D.; Schwertner, W.R.; Kuthi, L.K.; Veres, B.; Masszi, R.; Kovács, A.; Lakatos, B.K.; Zima, E.; Gellér, L.; et al. Long-term outcome of cardiac resynchronization therapy patients in the elderly. Geroscience 2023, 45, 2289–2301. (In English) [Google Scholar] [CrossRef]
- Rijal, S.; Wolfe, J.; Rattan, R.; Durrani, A.; Althouse, A.D.; Marroquin, O.C.; Jain, S.; Mulukutla, S.; Saba, S. Lead related complications in quadripolar versus bipolar left ventricular leads. Indian Pacing Electrophysiol. J. 2017, 17, 3–7. (In English) [Google Scholar] [CrossRef]
- Boriani, G.; Connors, S.; Kalarus, Z.; Lemke, B.; Mullens, W.; Asensi, J.O.; Raatikainen, P.; Gazzola, C.; Farazi, T.G.; Leclercq, C. Cardiac Resynchronization Therapy with a Quadripolar Electrode Lead Decreases Complications at 6 Months: Results of the MORE-CRT Randomized Trial. JACC Clin. Electrophysiol. 2016, 2, 212–220. (In English) [Google Scholar] [CrossRef]
- Robertson, C.; Duffey, O.; Tang, P.; Fairhurst, N.; Monteiro, C.; Green, P.; Grogono, J.; Davies, M.; Lewis, A.; Wijesurendra, R.; et al. An active fixation quadripolar left ventricular lead for cardiac resynchronization therapy with reduced postoperative complication rates. J. Cardiovasc. Electrophysiol. 2022, 33, 458–463. (In English) [Google Scholar] [CrossRef]
- Pauwelyn, M.; Ingelaere, S.; Hoffmann, R.; Vijgen, J.; Mairesse, G.H.; Blankoff, I.; Vandekerckhove, Y.; Waroux, J.-B.I.P.d.; Vandenberk, B.; Willems, R. Implantable cardiac defibrillators in octogenarians. J. Geriatr. Cardiol. 2023, 20, 23–31. (In English) [Google Scholar] [CrossRef]
- Sapp, J.L.; Sivakumaran, S.; Redpath, C.J.; Khan, H.; Parkash, R.; Exner, D.V.; Healey, J.S.; Thibault, B.; Sterns, L.D.; Lam, N.H.N.; et al. Long-Term Outcomes of Resynchronization-Defibrillation for Heart Failure. N. Engl. J. Med. 2024, 390, 212–220. [Google Scholar] [CrossRef]
- Darden, D.; Peterson, P.N.; Xin, X.; Munir, M.B.; Minges, K.E.; Goldenberg, I.; Poole, J.E.; Feld, G.K.; Birgersdotter-Green, U.; Curtis, J.P.; et al. Temporal trends and long-term outcomes among recipients of cardiac resynchronization therapy with defibrillator in the United States, 2011-2015: Insights from the National Cardiovascular Data Registry. Heart Rhythm O2 2022, 3, 405–414. (In English) [Google Scholar] [CrossRef]
- Leyva, F.; Zegard, A.; Patel, P.; Stegemann, B.; Marshall, H.; Ludman, P.; de Bono, J.; Boriani, G.; Qiu, T. Improved prognosis after cardiac resynchronization therapy over a decade. Europace 2023, 25, euad141. (In English) [Google Scholar] [CrossRef]
- Mcmurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. (In English) [Google Scholar] [CrossRef]
- Birnie, D.H.; Ha, A.; Higginson, L.; Sidhu, K.; Green, M.; Philippon, F.; Thibault, B.; Wells, G.; Tang, A. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: Results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Circ. Heart Fail. 2013, 6, 1190–1198. (In English) [Google Scholar] [CrossRef]
- Zareba, W.; Klein, H.; Cygankiewicz, I.; Hall, W.J.; McNitt, S.; Brown, M.; Cannom, D.; Daubert, J.P.; Eldar, M.; Gold, M.R.; et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 123, 1061–1072. (In English) [Google Scholar] [CrossRef]
- Sipahi, I.; Chou, J.C.; Hyden, M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: Meta-analysis of randomized controlled trials. Am. Heart. J. 2012, 163, 260–267.e3. (In English) [Google Scholar] [CrossRef]
- Cunnington, C.; Kwok, C.S.; Satchithananda, D.K.; Patwala, A.; Khan, M.A.; Zaidi, A.; Ahmed, F.Z.; Mamas, M.A. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology: Meta-analysis of randomised controlled trials. Heart 2015, 101, 1456–1462. (In English) [Google Scholar] [CrossRef]
- Sipahi, I.; Carrigan, T.P.; Rowland, D.Y.; Stambler, B.S.; Fang, J.C. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: Meta-analysis of randomized controlled trials. Arch. Intern. Med. 2011, 171, 1454–1462. (In English) [Google Scholar] [CrossRef]
- Cleland, J.G.; Abraham, W.T.; Linde, C.; Gold, M.R.; Young, J.B.; Daubert, J.C.; Sherfesee, L.; Wells, G.A.; Tang, A.S. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur. Heart J. 2013, 34, 3547–3556. (In English) [Google Scholar] [CrossRef]
- Bilchick, K.C.; Kamath, S.; DiMarco, J.P.; Stukenborg, G.J. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation 2010, 122, 2022–2030. (In English) [Google Scholar] [CrossRef]
- Adelstein, E.C.; Saba, S. Usefulness of baseline electrocardiographic QRS complex pattern to predict response to cardiac resynchronization. Am. J. Cardiol. 2009, 103, 238–242. (In English) [Google Scholar] [CrossRef]
- Rickard, J.; Kumbhani, D.J.; Gorodeski, E.Z.; Baranowski, B.; Wazni, O.; Martin, D.O.; Grimm, R.; Wilkoff, B.L. Cardiac resynchronization therapy in non-left bundle branch block morphologies. Pacing Clin. Electrophysiol. 2010, 33, 590–595. (In English) [Google Scholar] [CrossRef]
- Friedman, D.J.; Al-Khatib, S.M.; Dalgaard, F.; Fudim, M.; Abraham, W.T.; Cleland, J.G.; Curtis, A.B.; Gold, M.R.; Kutyifa, V.; Linde, C.; et al. Cardiac Resynchronization Therapy Improves Outcomes in Patients with Intraventricular Conduction Delay But Not Right Bundle Branch Block: A Patient-Level Meta-Analysis of Randomized Controlled Trials. Circulation 2023, 147, 812–823. (In English) [Google Scholar] [CrossRef]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J., III; Gras, D. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N. Engl. J. Med. 2013, 369, 1395–1405. (In English) [Google Scholar] [CrossRef]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; Sutton, M.S.J. Biventricular pacing for atrioventricular block and systolic dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef]
- Caputo, M.L.; van Stipdonk, A.; Illner, A.; D’Ambrosio, G.; Regoli, F.; Conte, G.; Moccetti, T.; Klersy, C.; Prinzen, F.W.; Vernooy, K.; et al. The definition of left bundle branch block influences the response to cardiac resynchronization therapy. Int. J. Cardiol. 2018, 269, 165–169. (In English) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).