Special Considerations in Pediatric Endoscopic Skull Base Surgery
Abstract
1. Introduction
2. Sinonasal Embryology
2.1. Nasal and Pyriform Apertures
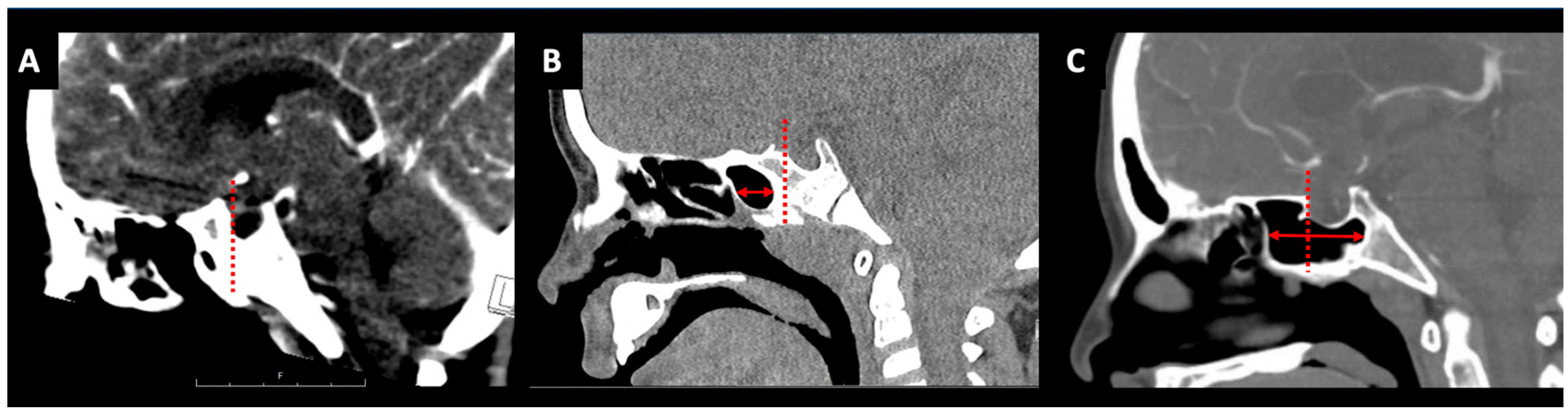
2.2. Sphenoid Sinus Pneumatization
2.3. Intercarotid Distance
3. Diagnostics
General Workup
4. Pediatric Skull Base Pathology (Figure 2)
4.1. Anterior Cranial Fossa
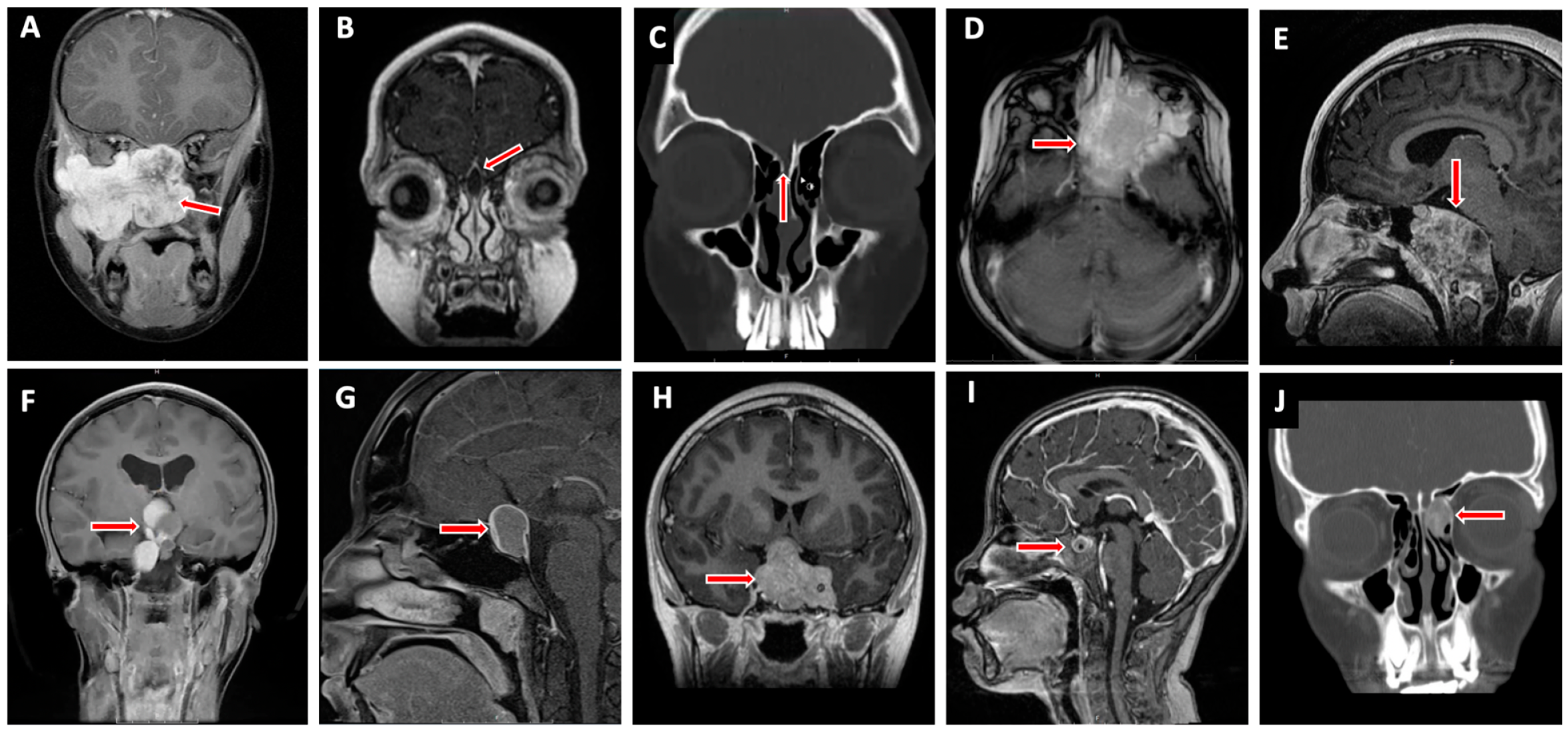
4.1.1. Nasal Dermoid Cyst
4.1.2. Encephalocele
4.1.3. Juvenile Nasopharyngeal Angiofibroma
4.1.4. Esthesioneuroblastoma
4.1.5. Rhabdomyosarcoma
4.1.6. Fibrous Dysplasia
4.2. Middle Cranial Fossa
4.2.1. Rathke’s Cleft Cyst
4.2.2. Craniopharyngioma
4.2.3. Pituitary Adenoma
4.2.4. Germ Cell Tumor (GCT)
4.2.5. Langerhans Cell Histiocytosis/Eosinophilic Granuloma
4.3. Posterior Cranial Fossa
4.3.1. Chordoma/Benign Notochord Cell Tumor
4.3.2. Chondrosarcoma
4.3.3. Other Skull Base Lesions
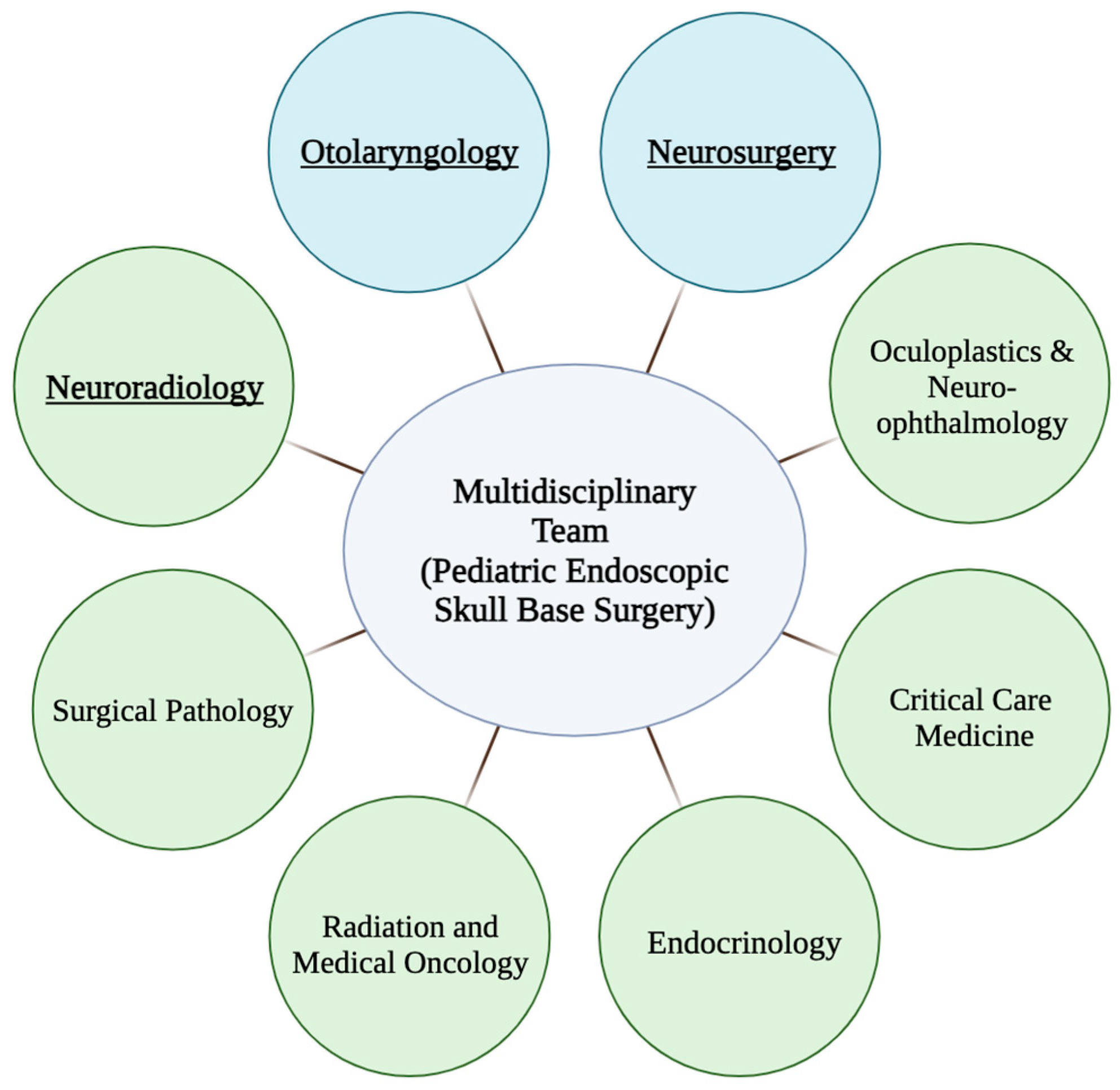
5. Multidisciplinary Treatment Planning
6. Endoscopic Endonasal Approach
Technical Considerations
7. Skull Base Reconstruction
8. Surgical Complications
9. Postoperative Care
10. Strengths and Limitations
11. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- McLaughlin, N.; Carrau, R.L.; Kelly, D.F.; Prevedello, D.M.; Kassam, A.B. Teamwork in Skull Base Surgery: An Avenue for Improvement in Patient Care. Surg. Neurol. Int. 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Leonard, J.; Walz, P. The Development of a Pediatric Skull Base Team: How, Where and Why? Curr. Opin. Otolaryngol. Head Neck Surg. 2023, 31, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Banu, M.A.; Guerrero-Maldonado, A.; McCrea, H.J.; Garcia-Navarro, V.; Souweidane, M.M.; Anand, V.K.; Heier, L.; Schwartz, T.H.; Greenfield, J.P. Impact of Skull Base Development on Endonasal Endoscopic Surgical Corridors. J. Neurosurg. Pediatr. 2014, 13, 155–169. [Google Scholar] [CrossRef]
- Rastatter, J.C.; Snyderman, C.H.; Gardner, P.A.; Alden, T.D.; Tyler-Kabara, E. Endoscopic Endonasal Surgery for Sinonasal and Skull Base Lesions in the Pediatric Population. Otolaryngol. Clin. N. Am. 2015, 48, 79–99. [Google Scholar] [CrossRef]
- Tatreau, J.R.; Patel, M.R.; Shah, R.N.; McKinney, K.A.; Wheless, S.A.; Senior, B.A.; Ewend, M.G.; Germanwala, A.V.; Ebert, C.S.; Zanation, A.M. Anatomical Considerations for Endoscopic Endonasal Skull Base Surgery in Pediatric Patients. Laryngoscope 2010, 120, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Likus, W.; Gruszczyńska, K.; Markowski, J.; Machnikowska-Sokołowska, M.; Olczak, Z.; Bajor, G.; Los, M.J.; Baron, J. Correlations between Selected Parameters of Nasal Cavity in Neonates and Young Infants—Computed Tomography Study. Folia Morphol. 2016, 75, 334–340. [Google Scholar] [CrossRef][Green Version]
- Likus, W.; Bajor, G.; Gruszczyńska, K.; Baron, J.; Markowski, J. Nasal Region Dimensions in Children: A CT Study and Clinical Implications. BioMed Res. Int. 2014, 2014, 125810. [Google Scholar] [CrossRef] [PubMed]
- London, N.R.; Rangel, G.G.; Walz, P.C. The Expanded Endonasal Approach in Pediatric Skull Base Surgery: A Review. Laryngoscope Investig. Otolaryngol. 2020, 5, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.J.; Lang, S.-S.; Adappa, N.D.; Palmer, J.N.; Storm, P.B. Pediatric Pituitary Surgery. Otolaryngol. Clin. N. Am. 2022, 55, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Kobets, A.; Ammar, A.; Dowling, K.; Cohen, A.; Goodrich, J. The Limits of Endoscopic Endonasal Approaches in Young Children: A Review. Childs Nerv. Syst. 2020, 36, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Duek, I.; Pener-Tessler, A.; Yanko-Arzi, R.; Zaretski, A.; Abergel, A.; Safadi, A.; Fliss, D.M. Skull Base Reconstruction in the Pediatric Patient. J. Neurol. Surg. Part B Skull Base 2018, 79, 81–90. [Google Scholar] [CrossRef]
- Walz, P.C.; Drapeau, A.; Shaikhouni, A.; Eide, J.; Rugino, A.J.; Mohyeldin, A.; Carrau, R.; Prevedello, D. Pediatric Pituitary Adenomas. Childs Nerv. Syst. 2019, 35, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Raverot, V.; Perrin, P.; Chanson, P.; Jouanneau, E.; Brue, T.; Raverot, G. Prolactin Immunoassay: Does the High-Dose Hook Effect Still Exist? Pituitary 2022, 25, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Book, L.; Sudar, K. Serum Alpha Fetoprotein (AFP) Levels in Normal Infants. Pediatr. Res. 1981, 15, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Echevarría, M.E.; Fangusaro, J.; Goldman, S. Pediatric Central Nervous System Germ Cell Tumors: A Review. Oncologist 2008, 13, 690–699. [Google Scholar] [CrossRef]
- Adil, E.; Rahbar, R. Paediatric Nasal Dermoid: Evaluation and Management. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Zapata, S.; Kearns, D.B. Nasal Dermoids. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.C.; Bloom, D.C.; Perkins, J.A.; Gruss, J.S.; Inglis, A. Diagnostic and Surgical Challenges in the Pediatric Skull Base. Otolaryngol. Clin. N. Am. 2005, 38, 773–794. [Google Scholar] [CrossRef] [PubMed]
- Szeremeta, W.; Parikh, T.D.; Widelitz, J.S. Congenital Nasal Malformations. Otolaryngol. Clin. N. Am. 2007, 40, 97–112. [Google Scholar] [CrossRef]
- Riley, C.A.; Soneru, C.P.; Overdevest, J.B.; Otten, M.L.; Gudis, D.A. Pediatric Sinonasal and Skull Base Lesions. World J. Otorhinolaryngol.-Head Neck Surg. 2020, 6, 118–124. [Google Scholar] [CrossRef]
- Rahbar, R.; Shah, P.; Mulliken, J.B.; Robson, C.D.; Perez-Atayde, A.R.; Proctor, M.R.; Kenna, M.A.; Scott, M.R.; McGill, T.J.; Healy, G.B. The Presentation and Management of Nasal Dermoid: A 30-Year Experience. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro-Neto, C.D.; Snyderman, C.H.; Fernandez-Miranda, J.; Gardner, P.A. Endoscopic Endonasal Surgery for Nasal Dermoids. Otolaryngol. Clin. N. Am. 2011, 44, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.N.; Siu, J.M.; Purcell, P.L.; Manning, J.P.; Wright, J.; Dahl, J.P.; Hauptman, J.S.; Hopper, R.A.; Lee, A.; Manning, S.C.; et al. Preoperative Imaging and Surgical Findings in Pediatric Frontonasal Dermoids. Laryngoscope 2023, 134, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.D.; Bailey, B.J.; Stiernberg, C.M. Nasal Dermoid with Intracranial Involvement. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 1985, 93, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Fornadley, J.A.; Tami, T.A. The Use of Magnetic Resonance Imaging in the Diagnosis of the Nasal Dermal Sinus-Cyst. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 1989, 101, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.E.; Allen, W.P.; Pai, G.S.; Best, R.; Seaver, L.H.; Dean, J.; Thompson, S. Decline in Prevalence of Neural Tube Defects in a High-Risk Region of the United States. Pediatrics 2000, 106, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.C.; Santoreneos, S.; Rutka, J.T. Tumors of the Skull Base in Children: Review of Tumor Types and Management Strategies. Neurosurg. Focus 2002, 12, e1. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, Q.; Li, X.; Huang, D.; Xian, J.; Cui, S.; Li, Y.; Zhou, B. Endoscopic Transnasal Repair of Cerebrospinal Fluid Leaks with and without an Encephalocele in Pediatric Patients: From Infants to Children. Childs Nerv. Syst. 2015, 31, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.E.J. Imaging of Skull-Base Cephalocoeles and Cerebrospinal Fluid Leaks. Clin. Radiol. 2010, 65, 832–841. [Google Scholar] [CrossRef]
- Riggs, S.; Orlandi, R.R. Juvenile Nasopharyngeal Angiofibroma Recurrence Associated with Exogenous Testosterone Therapy. Head Neck 2010, 32, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.; Kurokawa, R.; Kurokawa, M.; Srinivasan, A. MRI Features of Sinonasal Tract Angiofibroma/Juvenile Nasopharyngeal Angiofibroma: Case Series and Systematic Review. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2023, 33, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Shay, S.G.; Valika, T.; Chun, R.; Rastatter, J. Innovations in Endonasal Sinus Surgery in Children. Otolaryngol. Clin. N. Am. 2019, 52, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Snyderman, C.H.; Pant, H.; Carrau, R.L.; Gardner, P. A New Endoscopic Staging System for Angiofibromas. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Forst, D.A.; Jones, P.S. Skull Base Tumors. Contin. Minneap. Minn. 2023, 29, 1752–1778. [Google Scholar] [CrossRef] [PubMed]
- Kadish, S.; Goodman, M.; Wang, C.C. Olfactory Neuroblastoma. A Clinical Analysis of 17 Cases. Cancer 1976, 37, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Ebersold, M.J.; Olsen, K.D.; Foote, R.L.; Lewis, J.E.; Quast, L.M. Esthesioneuroblastoma: Prognosis and Management. Neurosurgery 1993, 32, 706–714, discussion 714–715. [Google Scholar] [CrossRef] [PubMed]
- Reilly, B.K.; Kim, A.; Peña, M.T.; Dong, T.A.; Rossi, C.; Murnick, J.G.; Choi, S.S. Rhabdomyosarcoma of the Head and Neck in Children: Review and Update. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, E.M.; Potter, B.O. Sarcomas of the Head and Neck Region. Curr. Opin. Oncol. 2003, 15, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.W.; Skapek, S.X. Childhood Rhabdomyosarcoma: New Insight on Biology and Treatment. Curr. Oncol. Rep. 2010, 12, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Darwish, C.; Shim, T.; Sparks, A.D.; Chillakuru, Y.; Strum, D.; Benito, D.A.; Monfared, A. Pediatric Head and Neck Rhabdomyosarcoma: An Analysis of Treatment and Survival in the United States (1975–2016). Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110403. [Google Scholar] [CrossRef] [PubMed]
- Freling, N.J.M.; Merks, J.H.M.; Saeed, P.; Balm, A.J.M.; Bras, J.; Pieters, B.R.; Adam, J.A.; van Rijn, R.R. Imaging Findings in Craniofacial Childhood Rhabdomyosarcoma. Pediatr. Radiol. 2010, 40, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Kana, L.A.; Smith, J.D.; Bellile, E.L.; Chugh, R.; McKean, E.L. Surgical Management of Rhabdomyosarcoma of the Nasal Cavity and Paranasal Sinuses: Analysis of Operative Indications, Settings, and Outcomes. J. Neurol. Surg. Part B Skull Base 2022, 83, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lustig, L.R.; Holliday, M.J.; McCarthy, E.F.; Nager, G.T. Fibrous Dysplasia Involving the Skull Base and Temporal Bone. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.L.; Tartarini, R.J.; Glass, C.H.; De Girolami, U.; Zamani, A.A.; Dunn, I.F. Natural History of Cranial Fibrous Dysplasia Revealed during Long-Term Follow-up: Case Report and Literature Review. Surg. Neurol. Int. 2017, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Rotter, N.; Bonassar, L.J.; Tobias, G.; Lebl, M.; Roy, A.K.; Vacanti, C.A. Age Dependence of Cellular Properties of Human Septal Cartilage: Implications for Tissue Engineering. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1248–1252. [Google Scholar] [CrossRef][Green Version]
- Watley, D.C.; Mong, E.R.; Rana, N.A.; Illing, E.A.; Chaaban, M.R. Surgical Approach to Frontal Sinus Osteoma: A Systematic Review. Am. J. Rhinol. Allergy 2019, 33, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2022, 24, iii1–iii38. [Google Scholar] [CrossRef] [PubMed]
- Delman, B.N. Imaging of Pediatric Pituitary Abnormalities. Endocrinol. Metab. Clin. N. Am. 2009, 38, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Voelker, J.L.; Campbell, R.L.; Muller, J. Clinical, Radiographic, and Pathological Features of Symptomatic Rathke’s Cleft Cysts. J. Neurosurg. 1991, 74, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Byun, W.M.; Kim, O.L.; Kim, D. MR Imaging Findings of Rathke’s Cleft Cysts: Significance of Intracystic Nodules. AJNR Am. J. Neuroradiol. 2000, 21, 485–488. [Google Scholar] [PubMed]
- Graffeo, C.S.; Perry, A.; Link, M.J.; Daniels, D.J. Pediatric Craniopharyngiomas: A Primer for the Skull Base Surgeon. J. Neurol. Surg. Part B Skull Base 2018, 79, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, A.; Walz, P.C.; Eide, J.G.; Rugino, A.J.; Shaikhouni, A.; Mohyeldin, A.; Carrau, R.L.; Prevedello, D.M. Pediatric Craniopharyngioma. Childs Nerv. Syst. 2019, 35, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.N.; Wang, Z.J.; Wehrli, S.L.; Marwaha, S.; Molloy, P.; Phillips, P.C.; Zimmerman, R.A. Proton Spectroscopy of Suprasellar Tumors in Pediatric Patients. Neurosurgery 1997, 41, 388–394, discussion 394–395. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L.; Bueb, K.; Bartels, U.; Roth, C.; Harz, K.; Graf, N.; Korinthenberg, R.; Bettendorf, M.; Kühl, J.; Gutjahr, P.; et al. Obesity after Childhood Craniopharyngioma—German Multicenter Study on Pre-Operative Risk Factors and Quality of Life. Klin. Padiatr. 2001, 213, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Twohy, E.; Geyer, S.; Gerstner, E.R.; Kaufmann, T.J.; Tabrizi, S.; Kabat, B.; Thierauf, J.; Ruff, M.W.; Bota, D.A.; et al. BRAF-MEK Inhibition in Newly Diagnosed Papillary Craniopharyngiomas. N. Engl. J. Med. 2023, 389, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Graffeo, C.S.; Marcellino, C.; Pollock, B.E.; Wetjen, N.M.; Meyer, F.B. Pediatric Pituitary Adenoma: Case Series, Review of the Literature, and a Skull Base Treatment Paradigm. J. Neurol. Surg. Part B Skull Base 2018, 79, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, E.; Pereira, J.; Vaz, R.; Lena, G.; Triglia, J.-M.; Nicollas, R. Combined Endonasal and Neurosurgical Resection of a Congenital Teratoma with Pharyngeal, Intracranial and Orbital Extension: Case Report, Surgical Technique and Review of the Literature. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1991–1994. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Zacharin, M. Endocrine Features of Langerhans Cell Histiocytosis in Paediatric Patients: A 30-Year Review. J. Paediatr. Child. Health 2024, 60, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F. Childhood Langerhans Cell Histiocytosis: A Disease with Many Faces. World J. Pediatr. WJP 2019, 15, 536–545. [Google Scholar] [CrossRef]
- Schmitt, S.; Wichmann, W.; Martin, E.; Zachmann, M.; Schoenle, E.J. Pituitary Stalk Thickening with Diabetes Insipidus Preceding Typical Manifestations of Langerhans Cell Histiocytosis in Children. Eur. J. Pediatr. 1993, 152, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Benato, A.; Riva, G.; Raneri, F. Eosinophilic Granuloma of the Calvarium: Is Conservative Management a Valid Option? Illustrative Case and Systematic Review of the Literature. Childs Nerv. Syst. 2023, 39, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Pour-Rashidi, A.; Asem, P.; Abbasioun, K.; Amirjamshidi, A. Primary Isolated Skull Base Eosinophilic Granuloma Confined to the Anterior Clinoid Process: Illustrative Case. J. Neurosurg. Case Lessons 2022, 4, CASE22178. [Google Scholar] [CrossRef] [PubMed]
- Prevedello, D.M.; Ditzel Filho, L.F.S.; Solari, D.; Carrau, R.L.; Kassam, A.B. Expanded Endonasal Approaches to Middle Cranial Fossa and Posterior Fossa Tumors. Neurosurg. Clin. N. Am. 2010, 21, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ulloa, R.; Soffer, J.; Alcazar-Felix, R.J.; Snyderman, C.H.; Gardner, P.A.; Patel, V.A.; Polster, S.P. Chordoma: A Comprehensive Systematic Review of Clinical Trials. Cancers 2023, 15, 5800. [Google Scholar] [CrossRef] [PubMed]
- Hoch, B.L.; Nielsen, G.P.; Liebsch, N.J.; Rosenberg, A.E. Base of Skull Chordomas in Children and Adolescents: A Clinicopathologic Study of 73 Cases. Am. J. Surg. Pathol. 2006, 30, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Kremenevski, N.; Schlaffer, S.-M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, A.K.; Verma, M. Cranionasal Fistula in Pediatric Population: Is Endonasal Endoscopic Approach Effective? Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Y. A Rare Case of Benign Long-Standing Ecchordosis Physaliphora. Cureus 2023, 15, e49490. [Google Scholar] [CrossRef] [PubMed]
- Korten, A.G.; ter Berg, H.J.; Spincemaille, G.H.; van der Laan, R.T.; Van de Wel, A.M. Intracranial Chondrosarcoma: Review of the Literature and Report of 15 Cases. J. Neurol. Neurosurg. Psychiatry 1998, 65, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; McDowell, M.M.; Goldschmidt, E.; Wang, E.W.; Snyderman, C.H.; Gardner, P.A. A Preoperative Risk Classifier That Predicts Tumor Progression in Patients with Cranial Base Chondrosarcomas. J. Neurosurg. 2020, 134, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.B. Ohmeda Oxicap 4700. Anaesthesia 1990, 45, 1093. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Shin, M.; Niwa, R.; Koizumi, S.; Yoshimoto, S.; Shono, N.; Shinya, Y.; Takami, H.; Tanaka, S.; Umekawa, M.; et al. Revisitation of Imaging Features of Skull Base Chondrosarcoma in Comparison to Chordoma. J. Neurooncol. 2022, 159, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Mertiri, L.; Freiling, J.T.; Desai, N.K.; Kralik, S.F.; Huisman, T.A.G.M. Pediatric and Adult Meningeal, Parenchymal, and Spinal Tuberculosis: A Neuroimaging Review. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2023, 34, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Jamil, O.A.; Lechpammer, M.; Prasad, S.; Litvack, Z.; Dunn, I.F. Giant Cell Reparative Granuloma of the Sphenoid: Case Report and Review of the Literature. Surg. Neurol. Int. 2012, 3, 140. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Morisako, H.; Ohata, H.; Kuwae, Y.; Teranishi, Y.; Goto, T. Pediatric Giant Cell Reparative Granuloma of the Lower Clivus: A Case Report and Review of the Literature. J. Craniovertebral Junction Spine 2021, 12, 86–90. [Google Scholar] [CrossRef]
- Snyderman, C.H.; Wang, E.W.; Fernandez-Miranda, J.C.; Gardner, P.A. The Making of a Skull Base Team and the Value of Multidisciplinary Approach in the Management of Sinonasal and Ventral Skull Base Malignancies. Otolaryngol. Clin. N. Am. 2017, 50, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Bly, R.A.; Berens, A.M.; Perkins, J.A.; Miller, C.; Ferreira, M.; Hauptman, J.S.; Moe, K.S. Surgical Planning in Pediatric Skull Base Surgery. Oper. Tech. Otolaryngol.-Head Neck Surg. 2019, 30, 9–15. [Google Scholar] [CrossRef]
- Franz, L.; Zanoletti, E.; Nicolai, P.; Ferrari, M. Treatment of Skull Base Diseases: A Multidisciplinary Challenge. J. Clin. Med. 2023, 12, 1492. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.J.A.; Sherer, M.; Anetakis, K.; Crammond, D.J.; Balzer, J.R.; Thirumala, P.D. Neurophysiological Characteristics of Cranial Nerves V- and VII-Triggered EMG in Endoscopic Endonasal Approach Skull Base Surgery. J. Neurol. Surg. Part B Skull Base 2021, 82, e342–e348. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.; Hinojosa-Bernal, J.; Munuera, J.; Gomez-Chiari, M.; Haag, O.; Veneri, A.; Valldeperes, A.; Valls, A.; Adell, N.; Santamaria, V.; et al. 3D Printing as Surgical Planning and Training in Pediatric Endoscopic Skull Base Surgery—Systematic Review and Practical Example. Int. J. Pediatr. Otorhinolaryngol. 2023, 168, 111543. [Google Scholar] [CrossRef] [PubMed]
- Munson, P.D.; Moore, E.J. Pediatric Endoscopic Skull Base Surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pool, C.; Slonimsky, E.; King, T.S.; Pradhan, S.; Wilson, M.N. Anatomical Parameters and Growth of the Pediatric Skull Base: Endonasal Access Implications. J. Neurol. Surg. Part B Skull Base 2023, 84, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Young, L.W. The Sphenoidal Sinuses: Radiographic Patterns of Normal Development and Abnormal Findings in Infants and Children. Radiology 1978, 129, 133. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.C.; Kaufman, A.C.; Lerner, D.; Kohanski, M.A.; Tong, C.C.L.; Tajudeen, B.A.; Parasher, A.K.; Lee, J.Y.K.; Storm, P.B.; Palmer, J.N.; et al. Lack of Sphenoid Pneumatization Does Not Affect Endoscopic Endonasal Pediatric Skull Base Surgery Outcomes. Laryngoscope 2019, 129, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Oviedo, P.; Levy, M.L.; Nation, J. Approaching the Sella through the Nonpneumatized Sphenoid in Pediatric Patients. J. Neurol. Surg. Part B Skull Base 2020, 81, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Palmer, J.N.; Adappa, N.D. The Expanded Endonasal Approach for the Treatment of Intracranial Skull Base Disease in the Pediatric Population. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 65–70. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, M.A.; Sellin, J.N.; DeMonte, F. Developmental Considerations in Pediatric Skull Base Surgery. J. Neurol. Surg. Part B Skull Base 2018, 79, 3–12. [Google Scholar] [CrossRef]
- Jellish, W.S.; Murdoch, J.; Leonetti, J.P. Perioperative Management of Complex Skull Base Surgery: The Anesthesiologist’s Point of View. Neurosurg. Focus 2002, 12, e5. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.L.; Tyler-Kabara, E.C.; Gardner, P.A.; Snyderman, C.H.; Wang, E.W. Risk Factors for Cerebrospinal Fluid Leak in Pediatric Patients Undergoing Endoscopic Endonasal Skull Base Surgery. Int. J. Pediatr. Otorhinolaryngol. 2017, 93, 163–166. [Google Scholar] [CrossRef]
- Banu, M.A.; Rathman, A.; Patel, K.S.; Souweidane, M.M.; Anand, V.K.; Greenfield, J.P.; Schwartz, T.H. Corridor-Based Endonasal Endoscopic Surgery for Pediatric Skull Base Pathology with Detailed Radioanatomic Measurements. Neurosurgery 2014, 10 (Suppl. S2), 273–293, discussion 293. [Google Scholar] [CrossRef]
- Giannoni, C.M.; Whitehead, W.E. Use of Endoscopic Vascularized Nasoseptal Flap in Children. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2013, 148, 344–346. [Google Scholar] [CrossRef]
- Ghosh, A.; Hatten, K.; Learned, K.O.; Rizzi, M.D.; Lee, J.Y.; Storm, P.B.; Palmer, J.N.; Adappa, N.D. Pediatric Nasoseptal Flap Reconstruction for Suprasellar Approaches. Laryngoscope 2015, 125, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.N.; Surowitz, J.B.; Patel, M.R.; Huang, B.Y.; Snyderman, C.H.; Carrau, R.L.; Kassam, A.B.; Germanwala, A.V.; Zanation, A.M. Endoscopic Pedicled Nasoseptal Flap Reconstruction for Pediatric Skull Base Defects. Laryngoscope 2009, 119, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Deopujari, C.; Bommakanti, V. The Reconstruction of Skull Base Defects in Infants Using Pedicled Nasoseptal Flap-a Review of Four Cases. Childs Nerv. Syst. 2019, 35, 2157–2162. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, O.; Wengier, A.; Ringel, B.; Carmel Neiderman, N.N.; Ram, Z.; Margalit, N.; Fliss, D.M.; Abergel, A. Nasoseptal Flap for Skull Base Reconstruction in Children. J. Neurol. Surg. Part B Skull Base 2018, 79, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, P.; Vega, M.B.; Ahmed, O.H.; Gardner, P.A.; Snyderman, C.H.; Wang, E.W. Lateral Nasal Wall Flap for Endoscopic Reconstruction of the Skull Base: Anatomical Study and Clinical Series. Int. Forum Allergy Rhinol. 2020, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Prevedello, D.M.; Barges-Coll, J.; Fernandez-Miranda, J.C.; Morera, V.; Jacobson, D.; Madhok, R.; dos Santos, M.C.J.; Zanation, A.; Snyderman, C.H.; Gardner, P.; et al. Middle Turbinate Flap for Skull Base Reconstruction: Cadaveric Feasibility Study. Laryngoscope 2009, 119, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Asmaro, K.; Mohyeldin, A.; Nunez, M.A.; Mao, Y.; Cohen-Gadol, A.A.; Nayak, J.; Fernandez-Miranda, J.C. The Temporoparietal Fascia Flap Transposition Technique for Ventral Skull Base Reconstruction: Anatomic Analysis and Surgical Application. Oper. Neurosurg. Hagerstown Md. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zanation, A.M.; Thorp, B.D.; Parmar, P.; Harvey, R.J. Reconstructive Options for Endoscopic Skull Base Surgery. Otolaryngol. Clin. N. Am. 2011, 44, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- Rastatter, J.C.; Walz, P.C.; Alden, T.D. Pediatric Skull Base Reconstruction: Case Report of a Tunneled Temporoparietal Fascia Flap. J. Neurosurg. Pediatr. 2016, 17, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Fortes, F.S.G.; Carrau, R.L.; Snyderman, C.H.; Kassam, A.; Prevedello, D.; Vescan, A.; Mintz, A.; Gardner, P. Transpterygoid Transposition of a Temporoparietal Fascia Flap: A New Method for Skull Base Reconstruction after Endoscopic Expanded Endonasal Approaches. Laryngoscope 2007, 117, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.-O.; Zenonos, G.A.; Wang, E.W.; Snyderman, C.H.; Gardner, P.A. The Rhinopharyngeal Flap for Reconstruction of Lower Clival and Craniovertebral Junction Defects. J. Neurosurg. 2021, 135, 1319–1327. [Google Scholar] [CrossRef]
- Koutourousiou, M.; Filho, F.V.G.; Costacou, T.; Fernandez-Miranda, J.C.; Wang, E.W.; Snyderman, C.H.; Rothfus, W.E.; Gardner, P.A. Pontine Encephalocele and Abnormalities of the Posterior Fossa Following Transclival Endoscopic Endonasal Surgery. J. Neurosurg. 2014, 121, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Gil, Z.; Abergel, A.; Leider-Trejo, L.; Khafif, A.; Margalit, N.; Amir, A.; Gur, E.; Fliss, D.M. A Comprehensive Algorithm for Anterior Skull Base Reconstruction after Oncological Resections. Skull Base Off. J. N. Am. Skull Base Soc. Al 2007, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Tien, D.A.; Stokken, J.K.; Recinos, P.F.; Woodard, T.D.; Sindwani, R. Cerebrospinal Fluid Diversion in Endoscopic Skull Base Reconstruction: An Evidence-Based Approach to the Use of Lumbar Drains. Otolaryngol. Clin. N. Am. 2016, 49, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Nation, J.; Schupper, A.J.; Deconde, A.; Levy, M. Pediatric Endoscopic Endonasal Approaches for Skull Base Lesions in the Very Young: Is It Safe and Effective? J. Neurol. Surg. Part B Skull Base 2018, 79, 574–579. [Google Scholar] [CrossRef]
- London, N.R.; Rangel, G.G.; Onwuka, A.; Carrau, R.L.; Prevedello, D.M.; Leonard, J.A.; Walz, P.C. Reconstruction of Pediatric Skull Base Defects: A Retrospective Analysis Emphasizing the Very Young. Int. J. Pediatr. Otorhinolaryngol. 2020, 133, 109962. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.S.; Bailey, R.L.; Daniels, L.B.; Vakhshori, V.; Lewis, D.J.; Hossain, A.T.; Sitterley, K.Y.; Lee, J.Y.K.; Storm, P.B.; Heuer, G.G.; et al. Comparative Effectiveness of Treatment Options for Pediatric Craniopharyngiomas. J. Neurosurg. Pediatr. 2014, 13, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Puget, S.; Garnett, M.; Wray, A.; Grill, J.; Habrand, J.-L.; Bodaert, N.; Zerah, M.; Bezerra, M.; Renier, D.; Pierre-Kahn, A.; et al. Pediatric Craniopharyngiomas: Classification and Treatment According to the Degree of Hypothalamic Involvement. J. Neurosurg. 2007, 106, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Kudo, M.; Hide, T.; Shinojima, N.; Makino, K.; Nakamura, H.; Kuratsu, J.-I. Quality of Life and Clinical Features of Long-Term Survivors Surgically Treated for Pediatric Craniopharyngioma. World Neurosurg. 2016, 85, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.A.; Polster, S.P.; Abou-Al-Shaar, H.; Kalmar, C.L.; Zenonos, G.A.; Wang, E.W.; Gardner, P.A.; Snyderman, C.H. Trigeminal Schwannoma: A Retrospective Analysis of Endoscopic Endonasal Management, Treatment Outcomes, and Neuropathic Sequelae. J. Neurol. Surg. Part B Skull Base 2023, 84, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.W.; Gardner, P.A.; Fraser, S.; Stefko, S.T.; Fernandez-Miranda, J.C.; Snyderman, C.H. Reduced Tearing with Stable Quality of Life After Vidian Neurectomy: A Prospective Controlled Trial. Laryngoscope 2021, 131, 1487–1491. [Google Scholar] [CrossRef]
- Stapleton, A.L.; Tyler-Kabara, E.C.; Gardner, P.A.; Snyderman, C.H. The Costs of Skull Base Surgery in the Pediatric Population. J. Neurol. Surg. Part B Skull Base 2015, 76, 39–42. [Google Scholar] [CrossRef][Green Version]
- Snyderman, C.H.; Gardner, P.A.; Tyler-Kabara, E.C. Surgical Management of Clival Chordomas in Children. Oper. Tech. Otolaryngol.-Head Neck Surg. 2019, 30, 63–72. [Google Scholar] [CrossRef]
- Carrau, R.L.; Weissman, J.L.; Janecka, I.P.; Snyderman, C.H.; Curtin, H.D.; Sekhar, L.; Lee, H.S. Computerized Tomography and Magnetic Resonance Imaging Following Cranial Base Surgery. Laryngoscope 1991, 101, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Tien, D.A.; Stokken, J.K.; Recinos, P.F.; Woodard, T.D.; Sindwani, R. Comprehensive Postoperative Management after Endoscopic Skull Base Surgery. Otolaryngol. Clin. N. Am. 2016, 49, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, K.W.; Governale, L.S.; Leonard, J.R.; Elmaraghy, C.A.; Walz, P.C. Recurrent Cerebrospinal Leak after Endonasal Cranial Base Surgery in a 4-Year-Old Male: Challenges for Postoperative Management. Ear. Nose. Throat J. 2021, 100, 472S–474S. [Google Scholar] [CrossRef]
- Lopez, E.M.; Farzal, Z.; Dean, K.M.; Miller, C.; Morse, J.C.; Ebert, C.S.; Kimple, A.J.; Thorp, B.D.; Zanation, A.M. Outcomes in Pediatric Endoscopic Skull Base Surgery: A Systematic Review. J. Neurol. Surg. Part B Skull Base 2022, 84, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Wasserzug, O.; DeRowe, A.; Ringel, B.; Fishman, G.; Fliss, D.M. Open Approaches to the Anterior Skull Base in Children: Review of the Literature. J. Neurol. Surg. Part B Skull Base 2018, 79, 42–46. [Google Scholar] [CrossRef]
- Ballestero, M.F.M.; de Souza, S.N.F.; Pacheco Neto, R.C.; Gondim, G.G.P.; Valera, E.T.; Dos Reis, M.B.F.; Colli, B.O.; de Oliveira, R.S. Pediatric Skull Base Tumors: A Management Challenge. J. Pediatr. Neurosci. 2021, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Sanchez, B.A.; Kim, J.D.; Zhou, S.; Chen, S.; Levy, M.L.; Roxbury, C.; Patel, V.A.; Polster, S.P. Special Considerations in Pediatric Endoscopic Skull Base Surgery. J. Clin. Med. 2024, 13, 1924. https://doi.org/10.3390/jcm13071924
Valencia-Sanchez BA, Kim JD, Zhou S, Chen S, Levy ML, Roxbury C, Patel VA, Polster SP. Special Considerations in Pediatric Endoscopic Skull Base Surgery. Journal of Clinical Medicine. 2024; 13(7):1924. https://doi.org/10.3390/jcm13071924
Chicago/Turabian StyleValencia-Sanchez, Bastien A., Jeeho D. Kim, Sheng Zhou, Sonja Chen, Michael L. Levy, Christopher Roxbury, Vijay A. Patel, and Sean P. Polster. 2024. "Special Considerations in Pediatric Endoscopic Skull Base Surgery" Journal of Clinical Medicine 13, no. 7: 1924. https://doi.org/10.3390/jcm13071924
APA StyleValencia-Sanchez, B. A., Kim, J. D., Zhou, S., Chen, S., Levy, M. L., Roxbury, C., Patel, V. A., & Polster, S. P. (2024). Special Considerations in Pediatric Endoscopic Skull Base Surgery. Journal of Clinical Medicine, 13(7), 1924. https://doi.org/10.3390/jcm13071924





