Identifying Predictors of Neck Disability in Patients with Cervical Pain Using Machine Learning Algorithms: A Cross-Sectional Correlational Study
Abstract
:1. Introduction
2. Materials and Methods
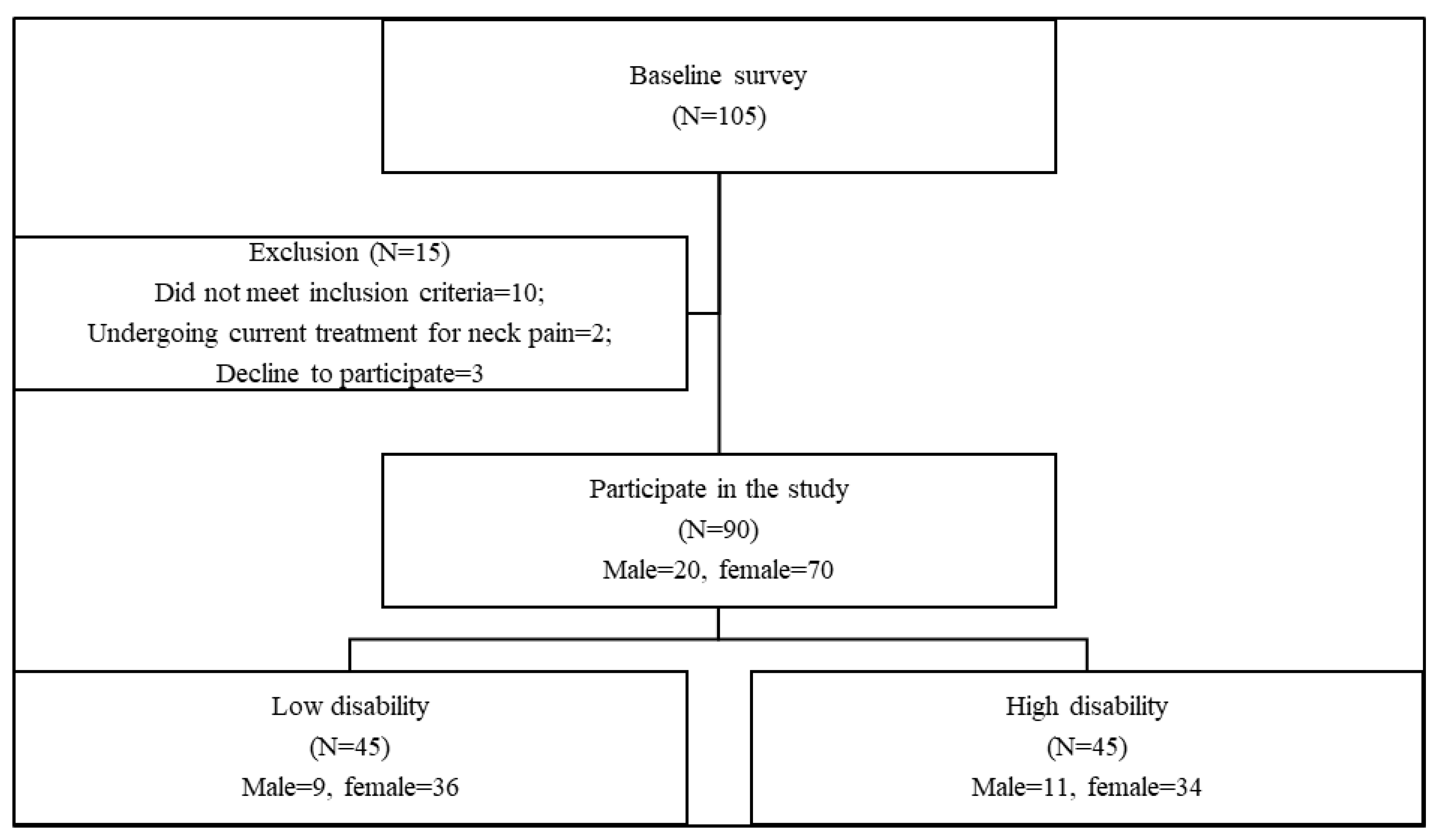
2.1. Population
2.2. Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazeminasab, S.; Nejadghaderi, S.A.; Amiri, P.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Neck pain: Global epidemiology, trends and risk factors. BMC Musculoskelet. Disord. 2022, 23, 26. [Google Scholar] [CrossRef]
- Elabd, A.M.; Ibrahim, A.R.; Elhafez, H.M.; Hussien, H.A.; Elabd, O.M. Efficacy of Kinesio Taping and Postural Correction Exercises on Levator Scapula Electromyographic Activities in Mechanical Cervical Dysfunction: A Randomized Blinded Clinical Trial. J. Manip. Physiol. Ther. 2020, 43, 588–596. [Google Scholar] [CrossRef]
- Jull, G. Cervicogenic headache. Musculoskelet. Sci. Pract. 2023, 66, 102787. [Google Scholar] [CrossRef]
- Ginszt, M.; Szkutnik, J.; Zielinski, G.; Bakalczuk, M.; Stodolkiewicz, M.; Litko-Rola, M.; Ginszt, A.; Rahnama, M.; Majcher, P. Cervical Myofascial Pain Is Associated with an Imbalance of Masticatory Muscle Activity. Int. J. Environ. Res. Public Health 2022, 19, 1577. [Google Scholar] [CrossRef] [PubMed]
- Weigl, M.; Letzel, J.; Angst, F. Prognostic factors for the improvement of pain and disability following multidisciplinary rehabilitation in patients with chronic neck pain. BMC Musculoskelet. Disord. 2021, 22, 330. [Google Scholar] [CrossRef]
- Kanaan, S.; Almhdawi, K.; Khader, Y.; Jain, T.; Jaber, A.; Almomani, F. Predictors of neck disability among undergraduate students: A cross-sectional study. Work 2022, 72, 1119–1128. [Google Scholar] [CrossRef]
- Boolani, A.; Gruber, A.H.; Torad, A.A.; Stamatis, A. Identifying Current Feelings of Mild and Moderate to High Depression in Young, Healthy Individuals Using Gait and Balance: An Exploratory Study. Sensors 2023, 23, 6624. [Google Scholar] [CrossRef]
- Kadry, A.M.; Torad, A.; Elwan, M.A.; Kakar, R.S.; Bradley, D.; Chaudhry, S.; Boolani, A. Using Machine Learning to Identify Feelings of Energy and Fatigue in Single-Task Walking Gait: An Exploratory Study. Appl. Sci. 2022, 12, 3083. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Behravan, H.; Hartikainen, J.M.; Tengstrom, M.; Kosma, V.M.; Mannermaa, A. Predicting breast cancer risk using interacting genetic and demographic factors and machine learning. Sci. Rep. 2020, 10, 11044. [Google Scholar] [CrossRef]
- Bier, J.D.; Scholten-Peeters, W.G.M.; Staal, J.B.; Pool, J.; van Tulder, M.W.; Beekman, E.; Knoop, J.; Meerhoff, G.; Verhagen, A.P. Clinical Practice Guideline for Physical Therapy Assessment and Treatment in Patients with Nonspecific Neck Pain. Phys. Ther. 2018, 98, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Marquié, L.; Duarte, L.R.; Mariné, C.; Lauque, D.; Sorum, P.C. How patients and physicians rate patients’ pain in a French emergency department using a verbally administered numerical rating scale and a visual analog scale. Acute Pain 2008, 10, 31–37. [Google Scholar] [CrossRef]
- Van Roo, J.D.; Lazio, M.P.; Pesce, C.; Malik, S.; Courtney, D.M. Visual analog scale (VAS) for assessment of acute mountain sickness (AMS) on Aconcagua. Wilderness Environ. Med. 2011, 22, 7–14. [Google Scholar] [CrossRef] [PubMed]
- En, M.C.; Clair, D.A.; Edmondston, S.J. Validity of the Neck Disability Index and Neck Pain and Disability Scale for measuring disability associated with chronic, non-traumatic neck pain. Man. Ther. 2009, 14, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Swanenburg, J.; Humphreys, K.; Langenfeld, A.; Brunner, F.; Wirth, B. Validity and reliability of a German version of the Neck Disability Index (NDI-G). Man. Ther. 2014, 19, 52–58. [Google Scholar] [CrossRef]
- Dunleavy, K.; Mariano, H.; Wiater, T.; Goldberg, A. Reliability and minimal detectable change of spinal length and width measurements using the Flexicurve for usual standing posture in healthy young adults. J. Back Musculoskelet. Rehabil. 2010, 23, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rheault, W.; Ferris, S.; Foley, J.A.; Schaffhauser, D.; Smith, R. Intertester reliability of the flexible ruler for the cervical spine. J. Orthop. Sports Phys. Ther. 1989, 10, 254–256. [Google Scholar] [CrossRef]
- Harrison, D.E.; Haas, J.W.; Cailliet, R.; Harrison, D.D.; Holland, B.; Janik, T.J. Concurrent Validity of Flexicurve Instrument Measurements: Sagittal Skin Contour of the Cervical Spine Compared With Lateral Cervical Radiographic Measurements. J. Manip. Physiol. Ther. 2005, 28, 597–603. [Google Scholar] [CrossRef]
- Elabd, A.; Ibrahim, A.; Elhafez, H. Kinesio taping versus postural correction exercises on mechanically triggered neck dysfunction. Int. J. Ther. Rehabil. 2017, 24, 155–162. [Google Scholar] [CrossRef]
- Szeto, G.P.; Straker, L.M.; O’Sullivan, P.B. Examining the low, high and range measures of muscle activity amplitudes in symptomatic and asymptomatic computer users performing typing and mousing tasks. Eur. J. Appl. Physiol. 2009, 106, 243–251. [Google Scholar] [CrossRef]
- Jensen, C.; Vasseljen, O.; Westgaard, R.H. The influence of electrode position on bipolar surface electromyogram recordings of the upper trapezius muscle. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 67, 266–273. [Google Scholar] [CrossRef]
- McLean, L. The effect of postural correction on muscle activation amplitudes recorded from the cervicobrachial region. J. Electromyogr. Kinesiol. 2005, 15, 527–535. [Google Scholar] [CrossRef]
- Nicoletti, C.; Spengler, C.M.; Laubli, T. Physical workload, trapezius muscle activity, and neck pain in nurses’ night and day shifts: A physiological evaluation. Appl. Ergon. 2014, 45, 741–746. [Google Scholar] [CrossRef]
- Bello, B.; Bundey, Y.N.; Bhave, R.; Khotimchenko, M.; Baran, S.W.; Chakravarty, K.; Varshney, J. Integrating AI/ML Models for Patient Stratification Leveraging Omics Dataset and Clinical Biomarkers from COVID-19 Patients: A Promising Approach to Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6250. [Google Scholar] [CrossRef]
- Ying, X. An overview of overfitting and its solutions. J. Phys. Conf. Ser. 2019, 1168, 022022. [Google Scholar] [CrossRef]
- Jiang, N.; Luk, K.D.; Hu, Y. A Machine Learning-based Surface Electromyography Topography Evaluation for Prognostic Prediction of Functional Restoration Rehabilitation in Chronic Low Back Pain. Spine 2017, 42, 1635–1642. [Google Scholar] [CrossRef]
- Shim, J.G.; Ryu, K.H.; Cho, E.A.; Ahn, J.H.; Kim, H.K.; Lee, Y.J.; Lee, S.H. Machine Learning Approaches to Predict Chronic Lower Back Pain in People Aged over 50 Years. Medicina 2021, 57, 1230. [Google Scholar] [CrossRef]
- Ferreira, P.H.; Beckenkamp, P.; Maher, C.G.; Hopper, J.L.; Ferreira, M.L. Nature or nurture in low back pain? Results of a systematic review of studies based on twin samples. Eur. J. Pain 2010, 14, 231–237. [Google Scholar] [CrossRef]
- Pinto, R.Z.; Ferreira, P.H.; Kongsted, A.; Ferreira, M.L.; Maher, C.G.; Kent, P. Self-reported moderate-to-vigorous leisure time physical activity predicts less pain and disability over 12 months in chronic and persistent low back pain. Eur. J. Pain 2014, 18, 1190–1198. [Google Scholar] [CrossRef]
- Young, S.B.; Aprill, C.; Braswell, J.; Ogard, W.K.; Richards, J.S.; McCarthy, J.P. Psychological factors and domains of neck pain disability. Pain Med. 2009, 10, 310–318. [Google Scholar] [CrossRef]
- Czępińska, A.; Zawadka, M.; Wójcik, Z.; Rzezak-Siwiec, A.; Gawda, P. Association between pain intensity, neck disability index, and working conditions among women employed in horticulture. Ann. Agric. Environ. Med. 2023, 30, 531–535. [Google Scholar] [CrossRef]
- Lee, H.; Hubscher, M.; Moseley, G.L.; Kamper, S.J.; Traeger, A.C.; Mansell, G.; McAuley, J.H. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain 2015, 156, 988–997. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- Ali Bhutto, M.; Abdullah, A.; Asadullah Arslan, S.; Sarfraz Khan, M.; Khan Bugti, M.; Jehan Rana, Z. Prevalence of Neck Pain in Relation to Gender, Posture and Ergonomics in Computer Users. Acta Sci. Orthop. 2019, 2, 02–06. [Google Scholar] [CrossRef]
- Straker, L.M.; O’Sullivan, P.B.; Smith, A.J.; Perry, M.C. Relationships between prolonged neck/shoulder pain and sitting spinal posture in male and female adolescents. Man Ther. 2009, 14, 321–329. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci. Rep. 2019, 9, 8515. [Google Scholar] [CrossRef]
- Viester, L.; Verhagen, E.; Hengel, K.M.; Koppes, L.; Beek, A.; Bongers, P. The relation between body mass index and musculoskeletal symptoms in the working population. BMC Musculoskelet. Disord. 2013, 14, 238. [Google Scholar] [CrossRef]
- Veiersted, K.B.; Westgaard, R.H.; Andersen, P. Electromyographic evaluation of muscular work pattern as a predictor of trapezius myalgia. Scand. J. Work Environ. Health 1993, 19, 284–290. [Google Scholar] [CrossRef]
- Mahmoud, N.F.; Hassan, K.A.; Abdelmajeed, S.F.; Moustafa, I.M.; Silva, A.G. The relationship between forward head posture and neck pain: A systematic review and meta-analysis. Curr. Rev. Musculoskelet. Med. 2019, 12, 562–577. [Google Scholar] [CrossRef]

| Low Disability | High Disability | Sig. | |
|---|---|---|---|
| Male/Female * | 9:36 | 11:34 | 0.695 |
| Age (Years) | 26.91 ± 3.99 | 27.48 ± 3.9 | 0.495 |
| Height (m) | 1.64 ± 0.05 | 1.64 ± 0.05 | 0.721 |
| Weight (KG) | 74.66 ± 9.22 | 73.93 ± 9.91 | 0.875 |
| BMI (KG/H2) | 28.06 ± 3.18 | 27.92 ± 3.44 | 0.842 |
| Model | Mean | Lower CI | Upper CI | Min | Q1 | Q2 | Q3 | Max |
|---|---|---|---|---|---|---|---|---|
| MLP | 0.708 | 0.645 | 0.771 | 0.272 | 0.646 | 0.727 | 0.827 | 0.961 |
| RF | 0.583 | 0.528 | 0.638 | 0.243 | 0.49 | 0.576 | 0.71 | 0.848 |
| KN | 0.405 | 0.299 | 0.512 | −0.324 | 0.269 | 0.427 | 0.599 | 0.841 |
| DT | 0.361 | 0.257 | 0.466 | −0.463 | 0.255 | 0.369 | 0.559 | 0.843 |
| Ridge | 0.336 | 0.288 | 0.384 | 0.061 | 0.269 | 0.32 | 0.44 | 0.541 |
| SVR | 0.037 | 0.024 | 0.049 | −0.049 | 0.03 | 0.045 | 0.062 | 0.08 |
| SGD | −0.003 | −0.021 | 0.015 | −0.115 | −0.035 | 0 | 0.028 | 0.085 |
| GB | −0.148 | −0.369 | 0.072 | −1.302 | −0.526 | −0.008 | 0.364 | 0.603 |
| Model | Mean | Lower CI | Upper CI | Min | Q1 | Q2 | Q3 | Max |
|---|---|---|---|---|---|---|---|---|
| LDA | 0.841 | 0.798 | 0.884 | 0.556 | 0.778 | 0.889 | 0.889 | 1 |
| GNB | 0.793 | 0.734 | 0.851 | 0.333 | 0.778 | 0.833 | 0.889 | 1 |
| RF | 0.789 | 0.73 | 0.848 | 0.444 | 0.667 | 0.833 | 0.889 | 1 |
| MLP | 0.759 | 0.718 | 0.8 | 0.444 | 0.667 | 0.778 | 0.778 | 1 |
| DT | 0.748 | 0.691 | 0.806 | 0.444 | 0.667 | 0.778 | 0.861 | 1 |
| KN | 0.711 | 0.654 | 0.768 | 0.333 | 0.667 | 0.722 | 0.778 | 1 |
| Rank | Classifier Variable | Regressor Variable |
|---|---|---|
| 1 | VAS | VAS |
| 2 | Gender * | Gender * |
| 3 | Weight | Weight |
| 4 | UT_MDF | LV_MDF |
| 5 | Age | LV_NOR_RMS |
| 6 | Height | UT_MDF |
| 7 | Surface contour of flexicurve | UT_NOR_RMS |
| 8 | LV_NOR_RMS | Height |
| 9 | UT_NOR_RMS | Surface contour of flexicurve |
| 10 | BMI | BMI |
| 11 | LV_MDF | Age |
| Variable | Low Disability | High Disability | Sig. | Partial Eta Squared |
|---|---|---|---|---|
| VAS | 3.14 ± 1.23 | 5.87 ± 1.41 | <0.001 * | 0.568 |
| Surface contour of flexicurve | 32.48 ± 4.16 | 23.8 ± 6.63 | <0.001 * | 0.410 |
| LV_NOR_RMS | 9.06 ± 9.16 | 15.07 ± 10.31 | 0.002 * | 0.105 |
| UT_MDF | 75.97 ± 18.7 | 61.64 ± 16.38 | <0.001 * | 0.172 |
| UT_NOR_RMS | 4.98 ± 3.8 | 10.74 ± 6 | <0.001 * | 0.298 |
| LV_MDF | 71.78 ± 15.45 | 59.38 ± 13.24 | <0.001 * | 0.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torad, A.A.; Ahmed, M.M.; Elabd, O.M.; El-Shamy, F.F.; Alajam, R.A.; Amin, W.M.; Alfaifi, B.H.; Elabd, A.M. Identifying Predictors of Neck Disability in Patients with Cervical Pain Using Machine Learning Algorithms: A Cross-Sectional Correlational Study. J. Clin. Med. 2024, 13, 1967. https://doi.org/10.3390/jcm13071967
Torad AA, Ahmed MM, Elabd OM, El-Shamy FF, Alajam RA, Amin WM, Alfaifi BH, Elabd AM. Identifying Predictors of Neck Disability in Patients with Cervical Pain Using Machine Learning Algorithms: A Cross-Sectional Correlational Study. Journal of Clinical Medicine. 2024; 13(7):1967. https://doi.org/10.3390/jcm13071967
Chicago/Turabian StyleTorad, Ahmed A., Mohamed M. Ahmed, Omar M. Elabd, Fayiz F. El-Shamy, Ramzi A. Alajam, Wafaa Mahmoud Amin, Bsmah H. Alfaifi, and Aliaa M. Elabd. 2024. "Identifying Predictors of Neck Disability in Patients with Cervical Pain Using Machine Learning Algorithms: A Cross-Sectional Correlational Study" Journal of Clinical Medicine 13, no. 7: 1967. https://doi.org/10.3390/jcm13071967
APA StyleTorad, A. A., Ahmed, M. M., Elabd, O. M., El-Shamy, F. F., Alajam, R. A., Amin, W. M., Alfaifi, B. H., & Elabd, A. M. (2024). Identifying Predictors of Neck Disability in Patients with Cervical Pain Using Machine Learning Algorithms: A Cross-Sectional Correlational Study. Journal of Clinical Medicine, 13(7), 1967. https://doi.org/10.3390/jcm13071967






