Thoughts on the Etiology of Cherubism
Abstract
:1. Background
2. Origin of the Name, Symptoms, and Pathogenesis of Cherubism
3. Molecular Biology
4. Relation to the Extracellular Matrix and Immunology
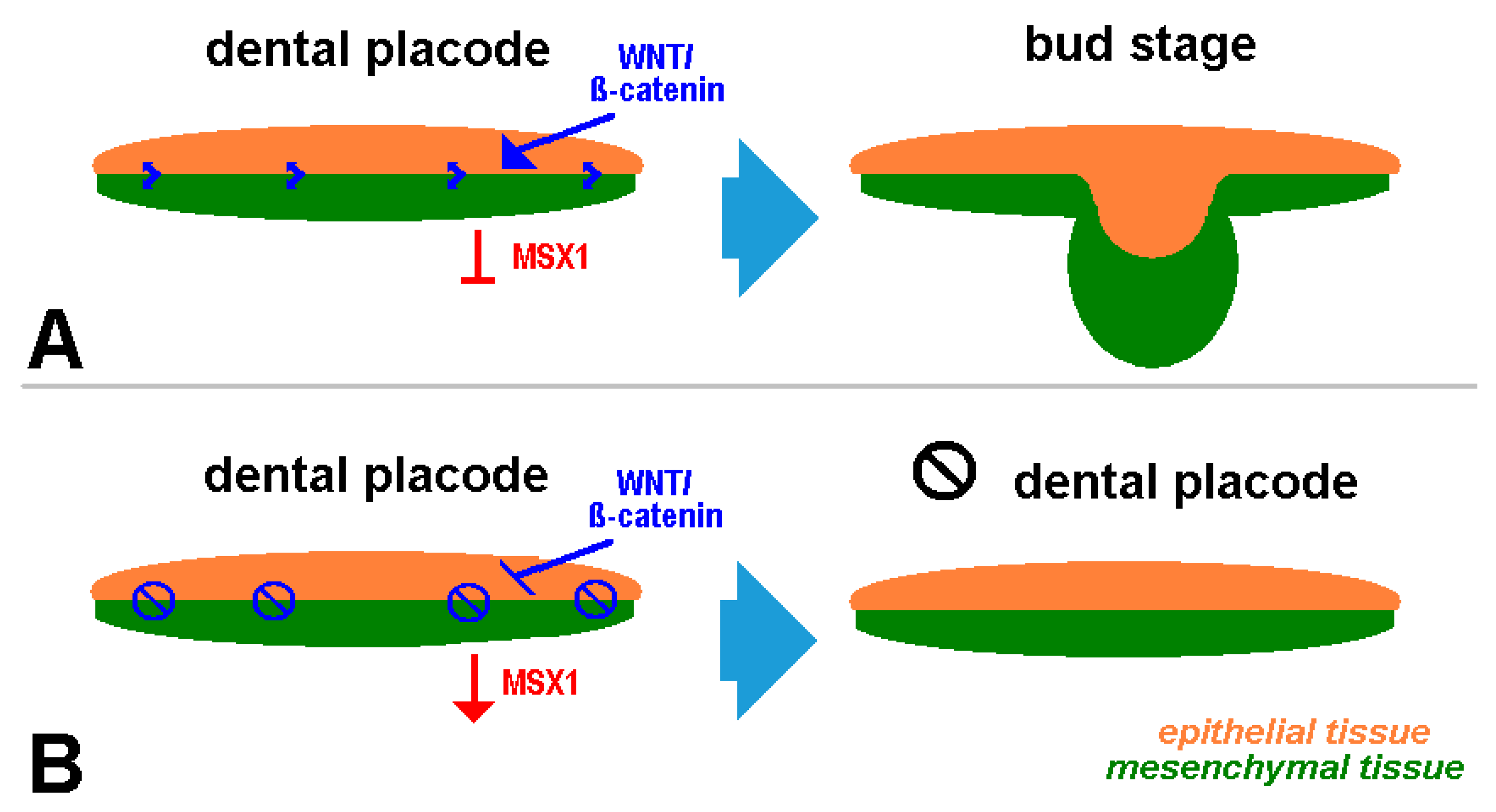
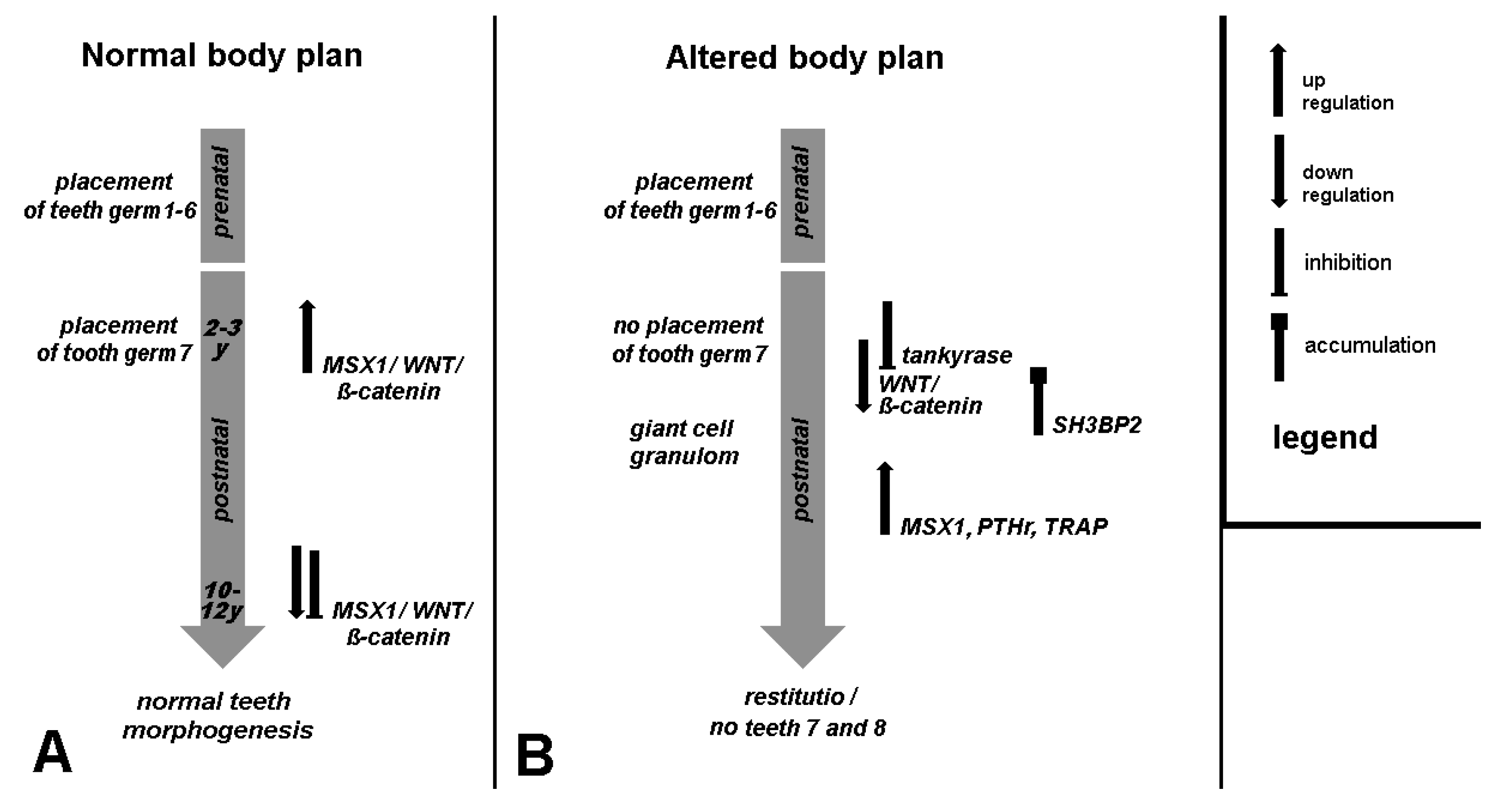
5. Clinical Signs of Cherubism Related to WNT/ß-Catenin/MSX1 Expression
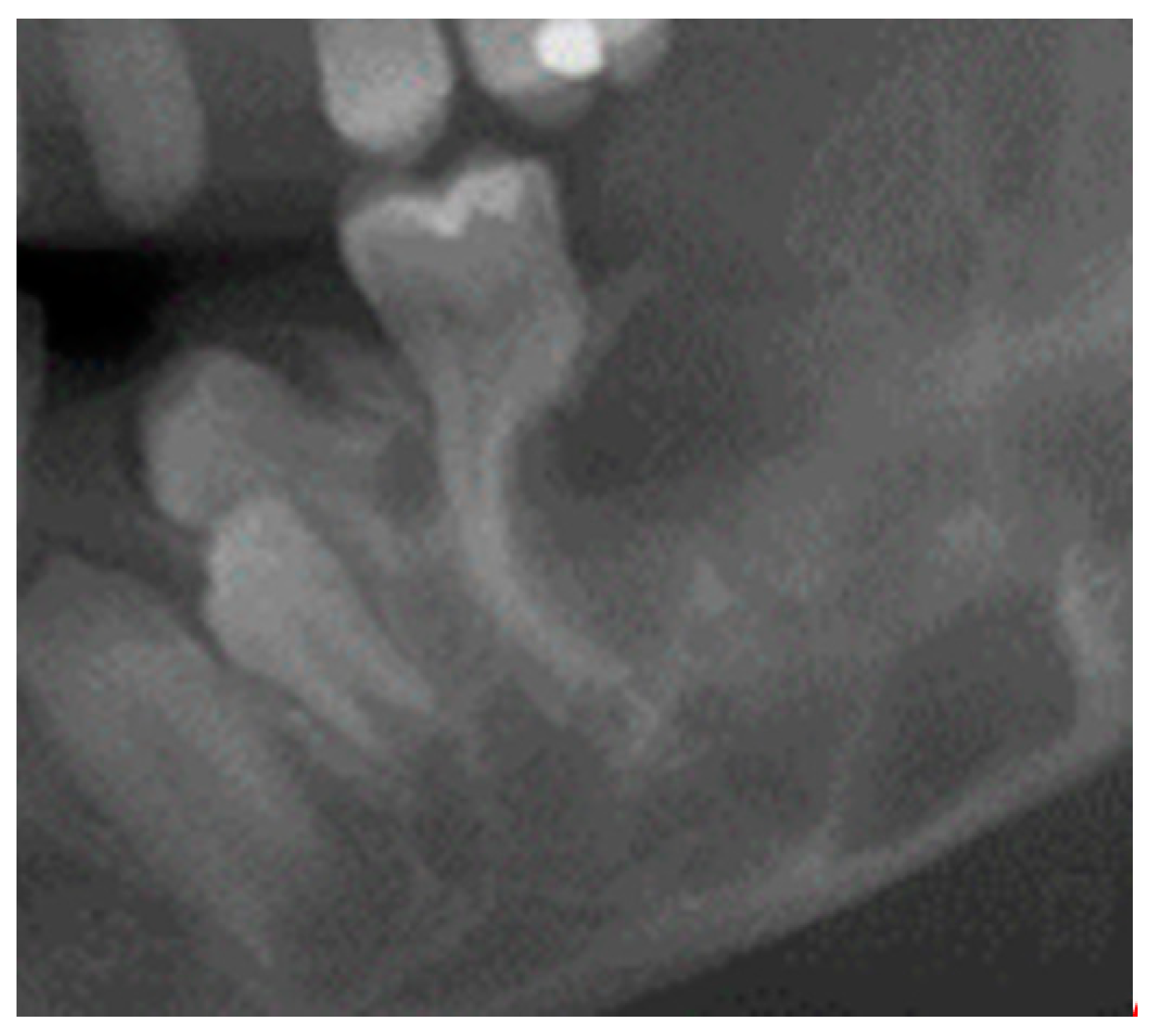
6. Relation to the Periodontium
7. Summarized Evaluation from a Semantic, Animal Experiment, and Study Perspective
- -
- Mice have a different dental development and signaling pathways than humans: while all teeth are laid down prenatally in mice, in humans, the formation of the second and third molars only occurs postnatally via the replacement dentition, parallel to the formation of the first symptoms of classic cherubism.
- -
- While the mandible originates from the cranial neural crest, the bones of the limbs are of mesodermal origin [59]. Because tubular bone is formed mesenchymally, “neural crest cells (NCCs)” derive from dorsal side of the neural tube; they proliferate rapidly and migrate along predetermined, well-characterized routes into developing frontonasal plate and gill arches. This is the reason for the contribution of NCCs to many craniofacial structures [60].
- -
- Accordingly, the alveolar process has a different immunology than long bones in the sense that, under pathologic conditions, the differentiation of immature myeloid cells generated in the bone marrow is partially blocked into mature myeloid cells. This results in MDSCs [25]. The mechanism of the tankyrase inhibition of PARsylation-mediated ubiquitylation can be connected with cherubism [61].
8. Therapy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, W.A. Familial multilocular cystic disease of the jaws. Am. J. Cancer 1933, 17, 946–950. [Google Scholar] [CrossRef]
- Hyckel, P.; Berndt, A.; Schleier, P.; Clement, J.H.; Beensen, V.; Peters, H.; Kosmehl, H. Cherubism-new hypotheses on pathogenesis and therapeutic consequences. J. Craniomaxillofac Surg. 2005, 33, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hyckel, P.; Schleier, P.; Wehrhan, F. Cherubism–Pathogenesis still uncertain? Trends Dentist 2018, 1, 133–135. [Google Scholar]
- Pispa, J.; Thesleff, I. Mechanisms of ectodermal organogenesis. Dev. Biol. 2003, 262, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Fleischmannova, J.; Matalova, E.; Tucker, A.S.; Sharpe, P.T. Mouse models of tooth abnormalities. Eur. J. Oral Sci. 2008, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Qin, C.; Chai, O.H.; Lan, Y.; Jiang, R.; Kwon, H.E. MSX1 drives tooth morphogenesis through controlling Wnt signaling activity. J. Dent. Res. 2022, 101, 832–839. [Google Scholar] [CrossRef]
- Sollazzo, V.; Pezzetti, F.; Massari, L.; Palmieri, A.; Brunelli, G.; Zollino, I.; Lucchese, A.; Caruso, G.; Carinci, F. Evaluation of gene expression in MG63 human osteoblastlike cells exposed to tantalum powder by microarray technology. Int. J. Periodontics Restor. Dent. 2011, 31, e17–e28. [Google Scholar]
- Bei, M.; Maas, R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 1998, 125, 4325–4333. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.A.; Gerrie, J.; Pritchard, J. Cherubism--familial fibrous dysplasia of the jaws. J. Bone Jt. Surg. Br. 1950, 32-B, 334–347. [Google Scholar] [CrossRef]
- Jones, W.A.; Gerrie, J.; Pritchard, J. Cherubism—A familial fibrous dysplasia of the jaws. Oral Surg. Oral Med. Oral Pathol. 1952, 5, 292–305. [Google Scholar] [CrossRef]
- Jones, W.A. Cherubism. A thumbnail sketch of its diagnosis and a conservative method of treatment. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 648–653. [Google Scholar] [CrossRef]
- Biernat, B. Medizinische und Molekulargenetische Untersuchung von Cherubismuspatienten. Dissertation 2013. Freie Universität Berlin. Available online: https://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjoseDnnr-EAxWJiv0HHcSiAPIQFnoECBYQAQ&url=https%3A%2F%2Frefubium.fu-berlin.de%2Fbitstream%2Ffub188%2F9118%2F1%2FDissertation-Beatrice_Biernat_2.pdf&usg=AOvVaw1XYNd4ELY86VDdhgmOx3oC&opi=89978449 (accessed on 4 March 2024).
- Ouyang, W.; Goh, C.E.; Ng, W.B.; Chew, F.T.; Yap, E.P.H.; Hsu, C.S. Genetic/protein association of atopic dermatitis and tooth agenesis. Int. J. Mol. Sci. 2023, 24, 5754. [Google Scholar] [CrossRef] [PubMed]
- Tiziani, V.; Reichenberger, E.; Buzzo, C.L.; Niazi, S.; Fukai, N.; Stiller, M.; Peters, H.; Salzano, F.M.; Raposo do Amaral, C.M.; Olsen, B.R. The gene for cherubism maps to chromosome 4p16. Am. J. Hum. Genet. 1999, 65, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mangion, J.; Rahman, N.; Edkins, S.; Barfoot, R.; Nguyen, T.; Sigurdsson, A.; Townend, J.V.; Fitzpatrick, D.R.; Flanagan, A.M.; Stratton, M.R. The gene for cherubism maps to chromosome 4p16.3. Am. J. Hum. Genet. 1999, 65, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Tiziani, V.; Santanna, C.; Fukai, N.; Maulik, C.; Garfinkle, J.; Ninomiya, C.; doAmaral, C.; Peters, H.; Habal, M.; et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat. Genet. 2001, 28, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Kalantar Motamedi, M.H. Treatment of cherubism with locally aggressive behavior presenting in adulthood: Report of four cases and a proposed new grading system. J. Oral Maxillofac. Surg. 1998, 56, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, E.J.; Levine, M.A.; Olsen, B.R.; Papadaki, M.E.; Lietman, S.A. The role of SH3BP2 in the pathophysiology of cherubism. Orphanet J. Rare Dis. 2012, 7, S5. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Guimarães, L.M.; Gomes, C.C.; Gomez, R.S. Cherubism: A systematic literature review of clinical and molecular aspects. Int. J. Oral Maxillofac. Surg. 2021, 50, 43–53. [Google Scholar] [CrossRef]
- Pubmed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=Cherubism (accessed on 4 March 2024).
- Levaot, N.; Voytyuk, O.; Dimitriou, I.; Sircoulomb, F.; Chandrakumar, A.; Deckert, M.; Krzyzanowski, P.M.; Scotter, A.; Gu, S.; Janmohamed, S.; et al. Loss of tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism. Cell 2011, 147, 1324–1339. [Google Scholar] [CrossRef]
- Mukai, T.; Fujita, S.; Morita, Y. Tankyrase (PARP5) Inhibition induces bone loss through accumulation of its substrate SH3BP2. Cells 2019, 8, 195. [Google Scholar] [CrossRef]
- Medio, M.; Yeh, E.; Popelut, A.; Babajko, S.; Berdal, A.; Helms, J.A. Wnt/β-catenin signaling and Msx1 promote outgrowth of the maxillary prominences. Front. Physiol. 2012, 3, 375. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Wu, X.S.; Wang, J.S.; Zhang, C.M.; Wang, S.L. Homeobox protein MSX-1 inhibits expression of bone morphogenetic protein 2.; bone morphogenetic protein 4.; and lymphoid enhancer-binding factor 1 via Wnt/β-catenin signaling to prevent differentiation of dental mesenchymal cells during the late bell stage. Eur. J. Oral Sci. 2018, 126, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Prokop, S.; Heppner, F.L.; Goebel, H.H.; Stenzel, W. M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis. Am. J. Pathol. 2011, 178, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Kwack, K.H.; Lamb, N.A.; Bard, J.E.; Kramer, E.D.; Zhang, L.; Abrams, S.I.; Kirkwood, K.L. Discovering myeloid cell heterogeneity in mandibular bone-Cell by cell analysis. Front. Physiol. 2021, 12, 731549. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.E.; Scheuer, H.A.; Zustin, J.; Grob, T. Cherubism: A case report with surgical intervention. Anticancer. Res. 2016, 36, 3109–3115. [Google Scholar] [PubMed]
- Wehrhan, F.; Büttner-Herold, M.; Hyckel, P.; Moebius, P.; Preidl, R.; Distel, L.; Ries, J.; Amann, K.; Schmitt, C.; Neukam, F.W.; et al. Increased malignancy of oral squamous cell carcinomas (OSCC) is associated with macrophage polarization in regional lymph nodes-an immunohistochemical study. BMC Cancer 2014, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, D.; Hooda, A.; Sharma, V.K.; Kamboj, M. Unravelling the role of immunohistochemistry in giant cell lesions of jaws: A systematic review. J. Oral Maxillofac. Pathol. 2023, 27, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Borsi, L.; Hyckel, P.; Kosmehl, H. Fibrillary co-deposition of laminin-5 and large unspliced tenascin-C in the invasive front of oral squamous cell carcinoma in vivo and in vitro. J. Cancer Res. Clin. Oncol. 2001, 127, 286–292. [Google Scholar] [CrossRef]
- Haas, M.; Berndt, A.; Hyckel, P.; Stiller, K.J.; Kosmehl, H. Laminin-5 bei Erkrankungen der Mundhöhle [Laminin-5 in diseases of the oral cavity]. Mund. Kiefer Gesichtschir 2000, 4, 25–29. [Google Scholar] [CrossRef]
- Abedsaeidi, M.; Hojjat, F.; Tavassoli, A.; Sahebkar, A. Biology of tenascin C and its role in physiology and pathology. Curr. Med. Chem. 2024; in press. [Google Scholar]
- Berendsen, A.D.; Olsen, B.R. Tankyrase loses its grip on SH3BP2 in cherubism. Cell 2011, 147, 1222–1223. [Google Scholar] [CrossRef]
- Nassif, A.; Senussi, I.; Meary, F.; Loiodice, S.; Hotton, D.; Robert, B.; Bensidhoum, M.; Berdal, A.; Babajko, S. Msx1 role in craniofacial bone morphogenesis. Bone 2014, 66, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Houpis, C.H.; Tosios, K.I.; Papavasileiou, D.; Christopoulos, P.G.; Koutlas, I.G.; Sklavounou, A.; Alexandridis, C. Parathyroid hormone-related peptide (PTHrP).; parathyroid hormone/parathyroid hormone-related peptide receptor 1 (PTHR1).; and MSX1 protein are expressed in central and peripheral giant cell granulomas of the jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 415–424. [Google Scholar] [CrossRef]
- Kitahara, Y.; Suda, N.; Kuroda, T.; Beck, F.; Hammond, V.E.; Takano, Y. Disturbed tooth development in parathyroid hormone-related protein (PTHrP)-gene knockout mice. Bone 2002, 30, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chu, E.Y.; Watt, B.; Zhang, Y.; Gallant, N.M.; Andl, T.; Yang, S.H.; Lu, M.M.; Piccolo, S.; Schmidt-Ullrich, R.; et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008, 313, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Spodzieja, K.; Olczak-Kowalczyk, D. Premature loss of deciduous teeth as a symptom of systemic disease: A narrative literature review. Int. J. Environ. Res. Public Health 2022, 19, 3386. [Google Scholar] [CrossRef] [PubMed]
- Jowett, A.K.; Vainio, S.; Ferguson, M.W.; Sharpe, P.T.; Thesleff, I. Epithelial-mesenchymal interactions are required for msx 1 and msx 2 gene expression in the developing murine molar tooth. Development 1993, 117, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, H.; Mues, G.; D’Souza, R. Genes affecting tooth morphogenesis. Orthod. Craniofac Res. 2007, 10, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.T.; Scanlon, C.S.; Soehren, S.; Matsuo, M.; Kapila, Y.L. Implications of cultured periodontal ligament cells for the clinical and experimental setting: A review. Arch. Oral Biol. 2011, 56, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Fleischmannova, J.; Matalova, E.; Sharpe, P.T.; Misek, I.; Radlanski, R.J. Formation of the tooth-bone interface. J. Dent. Res. 2010, 89, 108–115. [Google Scholar] [CrossRef]
- Tang, R.; Wei, F.; Wei, L.; Wang, S.; Ding, G. Osteogenic differentiated periodontal ligament stem cells maintain their immunomodulatory capacity. J. Tissue Eng. Regen. Med. 2014, 8, 226–232. [Google Scholar] [CrossRef]
- Schiraldi, C.; Stellavato, A.; D’Agostino, A.; Tirino, V.; d’Aquino, R.; Woloszyk, A.; De Rosa, A.; Laino, L.; Papaccio, G.; Mitsiadis, T.A. Fighting for territories: Time-lapse analysis of dental pulp and dental follicle stem cells in co-culture reveals specific migratory capabilities. Eur. Cell Mater. 2012, 24, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.E.; Giese, M.; Schmelzle, R.; Mautner, V.F.; Scheuer, H.A. Jaw malformations plus displacement and numerical aberrations of teeth in neurofibromatosis type 1: A descriptive analysis of 48 patients based on panoramic radiographs and oral findings. J. Craniomaxillofac. Surg. 2003, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tosios, K.I.; Gopalakrishnan, R.; Koutlas, I.G. So-called hybrid central odontogenic fibroma/central giant cell lesion of the jaws. A report on seven additional cases, including an example in a patient with cherubism, and hypotheses on the pathogenesis. Head Neck Pathol. 2008, 2, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.B. Central cemento-ossifying fibroma: Primary odontogenic or osseous neoplasm? J. Oral Maxillofac. Surg. 2015, 73, S87–S93. [Google Scholar] [CrossRef]
- Luscan, A.; Shackleford, G.; Masliah-Planchon, J.; Laurendeau, I.; Ortonne, N.; Varin, J.; Lallemand, F.; Leroy, K.; Dumaine, V.; Hivelin, M.; et al. The activation of the WNT signaling pathway is a Hallmark in neurofibromatosis type 1 tumorigenesis. Clin. Cancer Res. 2014, 20, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Groeger, S.; Meyle, J.; Ruf, S. MAPK and β-Catenin signaling: Implication and interplay in orthodontic tooth movement. Front. Biosci. 2022, 27, 54. [Google Scholar] [CrossRef] [PubMed]
- Hermans, F.; Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Intertwined signaling pathways governing tooth development: A give-and-take between canonical Wnt and Shh. Front. Cell Dev. Biol. 2021, 9, 758203. [Google Scholar] [CrossRef]
- Tobón-Arroyave, S.I.; Isaza-Guzmán, D.M.; Flórez-Moreno, G.A. Immunohistochemical comparative study of aggressive and non-aggressive central giant cell lesions of the jaws based on the tenascin-C expression profile. J. Histochem. Cytochem. 2021, 69, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Tongiorgi, E.; Bernhardt, R.R.; Zinn, K.; Schachner, M. Tenascin-C mRNA is expressed in cranial neural crest cells.; in some placodal derivatives.; and in discrete domains of the embryonic zebrafish brain. J. Neurobiol. 1995, 28, 391–407. [Google Scholar] [CrossRef]
- Schroll, I. Genexpression Während der Zahnentwicklung der Maus (Mus musculus). Doctoral Dissertation, Ludwigs Maximillian University, Munich, Germany, 2004. Available online: https://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjW7L-rpL-EAxVH4AIHHcqoAYEQFnoECBAQAQ&url=https%3A%2F%2Fedoc.ub.uni-muenchen.de%2F1842%2F1%2FSchroll_Ilse.pdf&usg=AOvVaw2Ki8xdStJuZex5fc4PaMrx&opi=89978449 (accessed on 4 March 2024).
- Tokavanich, N.; Wein, M.N.; English, J.D.; Ono, N.; Ono, W. The role of Wnt signaling in postnatal tooth root development. Front. Dent. Med. 2021, 2, 769134. [Google Scholar] [CrossRef]
- Schleier, P.; Neumann, U.; Müller, A.; Hyckel, P. Computertomographische Aspekte der Schädelentwicklung eines Kindes mit Cherubismus [Computed tomographic aspects of skull development in a child with cherubism]. HNO 2003, 51, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Seward, G.R.; Hankey, G.T. Cherubism. Oral Surg. Oral Med. Oral Pathol. 1957, 10, 952–974. [Google Scholar] [CrossRef] [PubMed]
- Chen Wongworawat, Y.; Jack, D.; Inman, J.C.; Abdelhalim, F.; Cobb, C.; Zuppan, C.W.; Raza, A. Regional lymph node enlargement in clinically severe cherubism. Clin. Pathol. 2019, 12, 2632010X19861107. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Monteiro, N.; Sah, S.K.; Javaheri, H.; Ueki, Y.; Fan, Z.; Reichenberger, E.J.; Chen, I.P. Tlr2/4-mediated hyperinflammation promotes cherubism-like jawbone expansion in Sh3bp2 (P416R) knockin mice. JBMR Plus 2021, 6, e10562. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.P.; Fischer, H.; Aydin, S.; Steffen, C.; Schmidt-Bleek, K.; Rendenbach, C. Uncovering the unique characteristics of the mandible to improve clinical approaches to mandibular regeneration. Front. Physiol. 2023, 14, 1152301. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.M.; Bayona, F.; Baddam, P.; Graf, D. Craniofacial development: Neural crest in molecular embryology. Head Neck Pathol. 2021, 15, 1–15. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Rottapel, R. PARsylation-mediated ubiquitylation: Lessons from rare hereditary disease Cherubism. Trends Mol. Med. 2023, 29, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, W.H.; van der Wal, J.E.; de Lange, J.; van den Berg, H. Multiple versus solitary giant cell lesions of the jaw: Similar or distinct entities? Bone 2021, 149, 115935. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, P.E.; Porporatti, A.L.; Cohen-Solal, M.; Kadlub, N.; Coudert, A.E. Pharmacological management of cherubism: A systematic review. Front. Endocrinol. 2023, 14, 1104025. [Google Scholar] [CrossRef]
- Ricalde, P.; Ahson, I.; Schaefer, S.T. A paradigm shift in the management of cherubism? A preliminary report using imatinib. J. Oral Maxillofac. Surg. 2019, 77, 1278.e1–1278.e7. [Google Scholar] [CrossRef]
- Tickenbrock, L.; Hehn, S.; Sargin, B.; Evers, G.; Ng, P.R.; Choudhary, C.; Berdel, W.E.; Müller-Tidow, C.; Serve, H. Activation of Wnt signaling in cKit-ITD mediated transformation and imatinib sensitivity in acute myeloid leukemia. Int. J. Hematol. 2008, 88, 174–180. [Google Scholar] [CrossRef]
- Ghandadi, M.; Valadan, R.; Mohammadi, H.; Akhtari, J.; Khodashenas, S.; Ashari, S. Wnt-β-catenin signaling pathway.; the Achilles’ heels of cancer multidrug resistance. Curr. Pharm. Des. 2019, 25, 4192–4207. [Google Scholar] [CrossRef] [PubMed]
- Wehrhan, F.; Hyckel, P.; Amann, K.; Ries, J.; Stockmann, P.; Schlegel, K.; Neukam, F.; Nkenke, E. Msx-1 is suppressed in bisphosphonate-exposed jaw bone analysis of bone turnover-related cell signalling after bisphosphonate treatment. Oral Dis. 2011, 17, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Wehrhan, F.; Moebius, P.; Amann, K.; Ries, J.; Preidl, R.; Neukam, F.W.; Weber, M. Macrophage and osteoclast polarization in bisphosphonate associated necrosis and osteoradionecrosis. J. Craniomaxillofac. Surg. 2017, 45, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Homm, A.; Müller, S.; Frey, S.; Amann, K.; Ries, J.; Geppert, C.; Preidl, R.; Möst, T.; Kämmerer, P.W.; et al. Zoledronate causes a systemic shift of macrophage polarization towards M1 in vivo. Int. J. Mol. Sci. 2021, 22, 1323. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Ries, J.; Büttner-Herold, M.; Geppert, C.I.; Kesting, M.; Wehrhan, F. Differences in inflammation and bone resorption between apical granulomas.; radicular cysts.; and dentigerous cysts. J. Endod. 2019, 45, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Meijer, E.; van den Berg, H.; Cleven, A.H.G.; Edelenbos, E.; Schreuder, W.H. Treatment of progressive cherubism during the second dental transitional phase with calcitonin. Case Rep. Dent. 2023, 2023, 2347855. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Ng, C.; Slattery, A.; Nair, P.; Eisman, J.A.; Center, J.R. Preadmission bisphosphonate and mortality in critically ill patients. J. Clin. Endocrinol. Metab. 2016, 101, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Damale, M.G.; Pathan, S.K.; Shinde, D.B.; Patil, R.H.; Arote, R.B.; Sangshetti, J.N. Insights of tankyrases: A novel target for drug discovery. Eur. J. Med. Chem. 2020, 207, 112712. [Google Scholar] [CrossRef]
- Liu, F.; Millar, S.E. Wnt/beta-catenin signaling in oral tissue development and disease. J. Dent. Res. 2010, 89, 318–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyckel, P.; Liehr, T. Thoughts on the Etiology of Cherubism. J. Clin. Med. 2024, 13, 2082. https://doi.org/10.3390/jcm13072082
Hyckel P, Liehr T. Thoughts on the Etiology of Cherubism. Journal of Clinical Medicine. 2024; 13(7):2082. https://doi.org/10.3390/jcm13072082
Chicago/Turabian StyleHyckel, Peter, and Thomas Liehr. 2024. "Thoughts on the Etiology of Cherubism" Journal of Clinical Medicine 13, no. 7: 2082. https://doi.org/10.3390/jcm13072082
APA StyleHyckel, P., & Liehr, T. (2024). Thoughts on the Etiology of Cherubism. Journal of Clinical Medicine, 13(7), 2082. https://doi.org/10.3390/jcm13072082






