Plug-Based Embolization Techniques of Aortic Side Branches during Standard and Complex Endovascular Aortic Repair
Abstract
1. Introduction
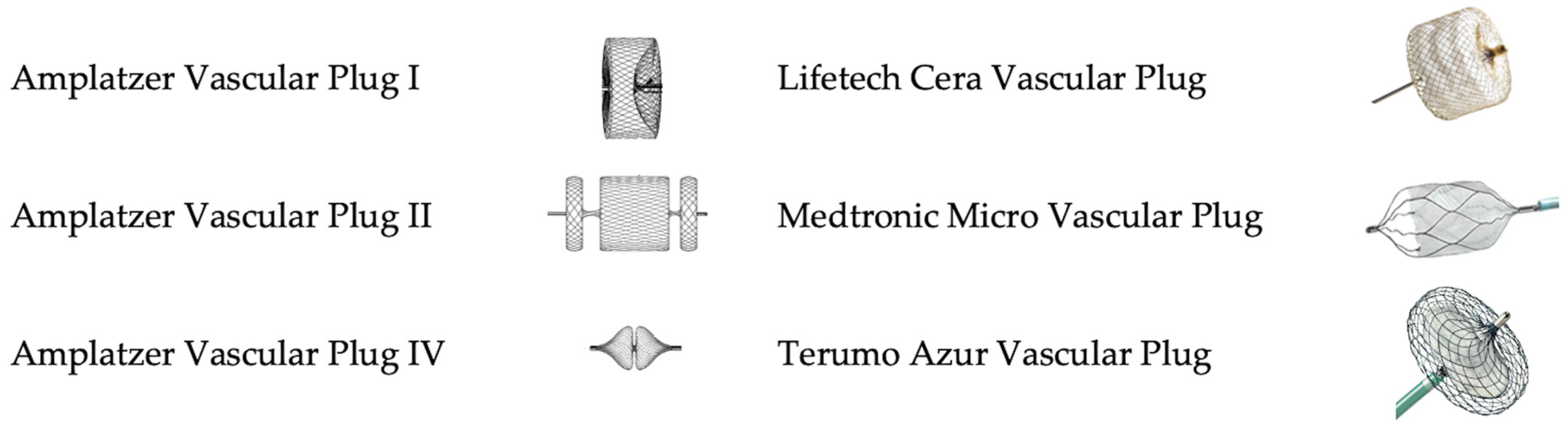
2. Vascular Plugs: Overview of Available Devices
2.1. Amplatzer Vascular Plugs
2.2. Cera Vascular Plug
2.3. Micro Vascular Plug
2.4. Azur Vascular Plug
3. Clinical Applications of Vascular Plugs during Endovascular Repair of Complex Aortic Aneurysms
3.1. Left Subclavian Artery (LSA)
- -
- -
3.2. Spinal Segmental Artery Embolization for the Prevention of Spinal Cord Ischemia
3.3. Occlusion of Aortic Side Branches for Endoleak Prevention
3.4. Embolization in Aortic Dissection
3.5. Intentional Directional Branch Closure in Complex Aortic Repair
- -
- As a modification of the original technique from Ferreira et al. [44], Tenorio et al. [45] suggested that branch elongation with a balloon-expandable stent before plug release should be the preferred choice in order to extend the sealing zone; the directional branch should be extended at least 20 mm beyond the branch cuff, and the plug should be released entirely within the stent, with no lobes protruding on the outside (Figure 6a). This configuration would limit any plug migration caused by short landing length inside the branch cuff.
- -
- Alternatively, the “dog bone” technique [46] consists of deploying a balloon-expandable stent-graft inside the branch, sizing it 2 mm larger than the branch itself. It should be inflated to 8 mm, and the proximal and distal portions of the stent should be flared with a larger balloon (4 mm more than the branch diameter). An AVP II should then be released at the beginning of the narrowed part, thus creating a bottleneck effect.
- -
- The MVP-7Q (MVP, Medtronic, Minneapolis, MN, USA) has also been used to occlude branches, with promising results in terms of early success and avoiding the use of a further bridging stent–graft, thus reducing overall procedural costs and possibly operating time [47] (Figure 6b). Aside from the economic and technical aspects, the MVP PTFE membrane design guarantees immediate vessel occlusion, which is of paramount importance in symptomatic/ruptured aneurysms, as opposed to the AVP II design which needs time to achieve complete branch thrombosis.
3.6. Internal Iliac Artery (IIA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocha, R.V.; Friedrich, J.O.; Elbatarny, M.; Yanagawa, B.; Al-Omran, M.; Forbes, T.L.; Lindsay, T.F.; Ouzounian, M. A Systematic Review and Meta-Analysis of Early Outcomes after Endovascular versus Open Repair of Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2018, 68, 1936–1945.e5. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Grandi, A.; Carta, N.; Cambiaghi, T.; Bilman, V.; Melissano, G.; Chiesa, R. Comparison of Anatomic Feasibility of Three Different Multibranched Off-the-Shelf Stent-Grafts Designed for Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2021, 74, 1472–1482.e4. [Google Scholar] [CrossRef] [PubMed]
- Leyon, J.J.; Littlehales, T.; Rangarajan, B.; Hoey, E.T.; Ganeshan, A. Endovascular Embolization: Review of Currently Available Embolization Agents. Curr. Probl. Diagn. Radiol. 2014, 43, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Geisel, D.; Gebauer, B.; Malinowski, M.; Stockmann, M.; Denecke, T. Comparison of CT and MRI Artefacts from Coils and Vascular Plugs Used for Portal Vein Embolization. Eur. J. Radiol. 2014, 83, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tam, M.D.B.S.; Pierce, G.; McLennan, G.; Sands, M.J.; Lieber, M.S.; Wang, W. Utility of the Amplatzer Vascular Plug in Splenic Artery Embolization: A Comparison Study with Conventional Coil Technique. Cardiovasc. Intervent. Radiol. 2011, 34, 522–531. [Google Scholar] [CrossRef]
- Batubara, E.A.D.; Nugraha, R.A.; Amshar, M.; Siddiq, T.; Indriani, S.; Adiarto, S. Ischemic Complications Following Thoracic Endovascular Aortic Repair with and without Revascularization of Left Subclavian Artery: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2022, 86, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.G.R.; Woo, K.; Beck, A.W.; Scali, S.T.; Weaver, F.A. Association of Left Subclavian Artery Coverage without Revascularization and Spinal Cord Ischemia in Patients Undergoing Thoracic Endovascular Aortic Repair: A Vascular Quality Initiative® Analysis. Vascular 2017, 25, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Natour, A.K.; Shepard, A.; Onofrey, K.; Peshkepija, A.; Nypaver, T.; Weaver, M.; Lee, A.; Kabbani, L. Left Subclavian Artery Revascularization Is Associated with Less Neurologic Injury after Endovascular Repair of Acute Type B Aortic Dissection. J. Vasc. Surg. 2023, 78, 1170–1179.e2. [Google Scholar] [CrossRef]
- D’Oria, M.; Mani, K.; DeMartino, R.; Czerny, M.; Donas, K.P.; Wanhainen, A.; Lepidi, S. Narrative Review on Endovascular Techniques for Left Subclavian Artery Revascularization during Thoracic Endovascular Aortic Repair and Risk Factors for Postoperative Stroke. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 764–772. [Google Scholar] [CrossRef]
- Madenci, A.L.; Ozaki, C.K.; Belkin, M.; McPhee, J.T. Carotid-Subclavian Bypass and Subclavian-Carotid Transposition in the Thoracic Endovascular Aortic Repair Era. J. Vasc. Surg. 2013, 57, 1275–1282.e2. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Kärkkäinen, J.M.; Tenorio, E.R.; Oderich, G.S.; Mendes, B.C.; Shuja, F.; Colglazier, J.; DeMartino, R.R. Perioperative Outcomes of Carotid-Subclavian Bypass or Transposition versus Endovascular Techniques for Left Subclavian Artery Revascularization during Nontraumatic Zone 2 Thoracic Endovascular Aortic Repair in the Vascular Quality Initiative. Ann. Vasc. Surg. 2020, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.E.; Zhang, L.; Magee, G.A.; Ham, S.W.; Ziegler, K.R.; Weaver, F.A.; Fleischman, F.; Han, S.M. Periscope Sandwich Stenting as an Alternative to Open Cervical Revascularization of Left Subclavian Artery during Zone 2 Thoracic Endovascular Aortic Repair. J. Vasc. Surg. 2021, 73, 466–475.e3. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Huang, M.; Wang, K.; Sun, J.; Guan, W.; Fan, J.; Li, Y. Physician-Modified Endograft with Left Subclavian Artery Fenestration for Ruptured Type B Aortic Dissection. Ann. Vasc. Surg. 2021, 77, 352.e7–352.e11. [Google Scholar] [CrossRef] [PubMed]
- Redlinger, R.E.; Ahanchi, S.S.; Panneton, J.M. In Situ Laser Fenestration during Emergent Thoracic Endovascular Aortic Repair Is an Effective Method for Left Subclavian Artery Revascularization. J. Vasc. Surg. 2013, 58, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Zeng, Q.L.; Xiang, Y.L.; Qiu, C.Y.; Li, Z.J.; He, Y.Y.; Zhu, Q.Q.; Wu, Z.H.; Wang, X.; Zhang, H.K. Experimental Analysis of the Quality of Needle-Assisted Fenestration in Aortic Stent-Grafts and the Differences between Gradual and Rapid Balloon Dilation. J. Endovasc. Ther. 2021, 28, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Ohki, T.; Kanaoka, Y.; Maeda, K. Clinical Outcomes of Left Subclavian Artery Coverage on Morbidity and Mortality during Thoracic Endovascular Aortic Repair for Distal Arch Aneurysms. World J. Surg. 2015, 39, 2812–2822. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ohuchi, Y.; Yata, S.; Adachi, A.; Endo, M.; Takasugi, S.; Fujii, S.; Hashimoto, M.; Kaminou, T.; Ogawa, T.; et al. Compressed Amplatzer Vascular Plug II Embolization of the Left Subclavian Artery for Thoracic Endovascular Aortic Repair Is Efficient and Safety Method Comparable to Conventional Coil Embolization. Yonago Acta Med. 2019, 62, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Salvati, S.; Fittipaldi, A.; Melloni, A.; Kahlberg, A.; Cambiaghi, T.; Melissano, G.; Chiesa, R. Carotid to Subclavian Bypass and Amplatzer Vascular Plug Subclavian Endovascular Occlusion before Thoracic Open or Endovascular Repair. J. Vasc. Surg. 2020, 71, 1480–1488.e1. [Google Scholar] [CrossRef]
- Matsumura, J.S.; Rizvi, A.Z. Left Subclavian Artery Revascularization: Society for Vascular Surgery® Practice Guidelines. J. Vasc. Surg. 2010, 52, 65S–70S. [Google Scholar] [CrossRef]
- Branzan, D.; Etz, C.D.; Moche, M.; Von Aspern, K.; Staab, H.; Fuchs, J.; Bergh, F.T.; Scheinert, D.; Schmidt, A. Ischaemic Preconditioning of the Spinal Cord to Prevent Spinal Cord Ischaemia during Endovascular Repair of Thoracoabdominal Aortic Aneurysm: First Clinical Experience. EuroIntervention 2018, 14, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Lella, S.K.; Waller, H.D.; Pendleton, A.; Latz, C.A.; Boitano, L.T.; Dua, A. A Systematic Review of Spinal Cord Ischemia Prevention and Management after Open and Endovascular Aortic Repair. J. Vasc. Surg. 2022, 75, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Petroff, D.; Czerny, M.; Kölbel, T.; Melissano, G.; Lonn, L.; Haunschild, J.; Von Aspern, K.; Neuhaus, P.; Pelz, J.; Epstein, D.M.; et al. Paraplegia Prevention in Aortic Aneurysm Repair by Thoracoabdominal Staging with “minimally Invasive Staged Segmental Artery Coil Embolisation” (MIS2ACE): Trial Protocol for a Randomised Controlled Multicentre Trial. BMJ Open 2019, 9, e025488. [Google Scholar] [CrossRef] [PubMed]
- Gallitto, E.; Faggioli, G.L.; Campana, F.; Feroldi, F.M.; Cappiello, A.; Caputo, S.; Pini, R.; Gargiulo, M. Type 2 Endoleaks after Fenestarted/Branched Endografting for Juxta/Para-Renal Aortic Aneurysms. J. Vasc. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Marecki, H.L.; Finnesgard, E.J.; Nuvvula, S.; Nguyen, T.T.; Boitano, L.T.; Jones, D.W.; Schanzer, A.; Simons, J.P. Characterization and Management of Type II and Complex Endoleaks after Fenestrated/Branched Endovascular Aneurysm Repair. J. Vasc. Surg. 2023, 78, 29–37. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Oderich, G.S.; Tenorio, E.R.; Kärkkäinen, J.M.; Mendes, B.C.; Macedo, T.A.; Vrtiska, T.; DeMartino, R.R. Natural History of Isolated Type II Endoleaks in Patients Treated by Fenestrated-Branched Endovascular Repair for Pararenal and Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2020, 72, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Seike, Y.; Matsuda, H.; Shimizu, H.; Ishimaru, S.; Hoshina, K.; Michihata, N.; Yasunaga, H.; Komori, K. Nationwide Analysis of Persistent Type II Endoleak and Late Outcomes of Endovascular Abdominal Aortic Aneurysm Repair in Japan: A Propensity-Matched Analysis. Circulation 2022, 145, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Mastrorilli, D.; Ziani, B. Natural History, Diagnosis, and Management of Type II Endoleaks after Endovascular Aortic Repair: Review and Update. Ann. Vasc. Surg. 2020, 62, 420–431. [Google Scholar] [CrossRef]
- Yu, H.Y.H.; Lindström, D.; Wanhainen, A.; Tegler, G.; Asciutto, G.; Mani, K. An Updated Systematic Review and Meta-Analysis of Pre-Emptive Aortic Side Branch Embolization to Prevent Type II Endoleaks after Endovascular Aneurysm Repair. J. Vasc. Surg. 2023, 77, 1815–1821. [Google Scholar] [CrossRef]
- Müller-Wille, R.; Uller, W.; Gößmann, H.; Heiss, P.; Wiggermann, P.; Dollinger, M.; Kasprzak, P.; Pfister, K.; Stroszczynski, C.; Wohlgemuth, W.A. Inferior Mesenteric Artery Embolization before Endovascular Aortic Aneurysm Repair Using Amplatzer Vascular Plug Type 4. Cardiovasc. Interv. Radiol. 2014, 37, 928–934. [Google Scholar] [CrossRef]
- Morikage, N.; Samura, M.; Takeuchi, Y.; Tanaka, Y.; Ueda, K.; Harada, T.; Yamashita, O.; Suehiro, K.; Hamano, K. Innovative Procedure for Inferior Mesenteric Artery Embolization Using the Amplatzer Vascular Plug 4 during Endovascular Aneurysm Repair. Ann. Vasc. Surg. 2017, 44, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Samura, M.; Morikage, N.; Otsuka, R.; Mizoguchi, T.; Takeuchi, Y.; Nagase, T.; Harada, T.; Yamashita, O.; Suehiro, K.; Hamano, K. Endovascular Aneurysm Repair with Inferior Mesenteric Artery Embolization for Preventing Type II Endoleak: A Prospective Randomized Controlled Trial. Ann. Surg. 2020, 271, 238–244. [Google Scholar] [CrossRef]
- Vargo, P.R.; Maigrot, J.L.; Roselli, E.E. Chronic Thoracoabdominal Aortic Dissection: Endovascular Options to Obliterate the False Lumen. Ann. Cardiothorac. Surg. 2021, 10, 778–783. [Google Scholar] [CrossRef]
- Eleshra, A.; Haulon, S.; Bertoglio, L.; Lindsay, T.; Rohlffs, F.; Dias, N.; Tsilimparis, N.; Panuccio, G.; Kölbel, T.; Mougin, J.; et al. Custom Made Candy Plug for Distal False Lumen Occlusion in Aortic Dissection: International Experience. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 50–56. [Google Scholar] [CrossRef]
- Li, H.L.; Chan, Y.C.; Jia, H.Y.; Cheng, S.W. Methods and Outcomes of Endovascular False Lumen Embolization for Thoracic Aortic Dissection. Ann. Vasc. Surg. 2022, 85, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Arbabi, C.; McMackin, K.; Tjaden, B.; Schonefeld, S.; Baril, D.; Gupta, N.Y.; Gewertz, B.; Azizzadeh, A. Initial Experience with a Modified “Candy-Plug” Technique for False Lumen Embolization in Chronic Type B Aortic Dissection. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101075. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Ohki, T.; Ozawa, H. Repair of Chronic Aneurysmal Aortic Dissection Using a Stent Graft and an Amplatzer® Vascular Plug: A Case Study. Ann. Vasc. Surg. 2017, 39, 288.e5–288.e12. [Google Scholar] [CrossRef] [PubMed]
- Sandström, C.; Roos, H.; Henrikson, O.; Fagman, E.; Johnsson, Å.A.; Jeppsson, A.; Falkenberg, M. Endovascular Plugs to Occlude Proximal Entries in Chronic Aortic Dissection. Interact. Cardiovasc. Thorac. Surg. 2022, 35, 3–10. [Google Scholar] [CrossRef]
- Falkenberg, M.; Roos, H.; Lepore, V.; Svensson, G.; Zachrisson, K.; Henrikson, O. Endovascular Closure of Chronic Dissection Entries in the Aortic Arch Using the Amplatzer Vascular Plug II as a Sealing Button. J. Endovasc. Ther. 2016, 23, 378–383. [Google Scholar] [CrossRef]
- Eleshra, A.; Hatm, M.; Spanos, K.; Panuccio, G.; Rohlffs, F.; Debus, E.S.; Behrendt, C.A.; Tsilimparis, N.; Kölbel, T. Early Outcomes of T-Branch off-the-Shelf Multibranched Stent Graft in Urgent and Emergent Repair of Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2022, 75, 416–424.e2. [Google Scholar] [CrossRef]
- Konstantinou, N.; Antonopoulos, C.N.; Jerkku, T.; Banafsche, R.; Kölbel, T.; Fiorucci, B.; Tsilimparis, N. Systematic Review and Meta-Analysis of Published Studies on Endovascular Repair of Thoracoabdominal Aortic Aneurysms with the t-Branch off-the-Shelf Multibranched Endograft. J. Vasc. Surg. 2020, 72, 716–725.e1. [Google Scholar] [CrossRef] [PubMed]
- Grandi, A.; Carta, N.; Cambiaghi, T.; Bilman, V.; Melissano, G.; Chiesa, R.; Bertoglio, L. Sex-Related Anatomical Feasibility Differences in Endovascular Repair of Thoracoabdominal Aortic Aneurysms With a Multibranched Stent-Graft. J. Endovasc. Ther. 2021, 28, 283–294. [Google Scholar] [CrossRef]
- Hongku, K.; Resch, T.; Sonesson, B.; Kristmundsson, T.; Dias, N.V. Applicability and Midterm Results of Branch Cuff Closure with Vascular Plug in Branched Endovascular Repair for Thoracoabdominal Aortic Aneurysms. J. Vasc. Surg. 2017, 66, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Monteiro, M.; Lanziotti, L. How to Occlude a Side Branch on a Branched Stent-Graft during an Endovascular Thoracoabdominal Aortic Aneurysm Repair. J. Endovasc. Ther. 2009, 16, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, E.R.; Oderich, G.S.; Kölbel, T.; Gargiulo, M.; Timaran, C.H.; Bertoglio, L.; Modarai, B.; Jama, K.; Eleshra, A.; Lima, G.B.B.; et al. Outcomes of Off-the-Shelf Multibranched Stent Grafts with Intentional Occlusion of Directional Branches Using Endovascular Plugs during Endovascular Repair of Complex Aortic Aneurysms. J. Vasc. Surg. 2022, 75, 1142–1150.e4. [Google Scholar] [CrossRef]
- Chisci, E.; Dalla Caneva, P.; Michelagnoli, S. The “Dog Bone” Technique to Occlude a Branch Intentionally. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 1035. [Google Scholar] [CrossRef] [PubMed]
- Xodo, A.; Taglialavoro, J.; Lepidi, S.; Pilon, F.; Calvagna, C.; Griselli, F.; Milite, D.; Badalamenti, G.; Ruaro, B.; D’Oria, M. Novel Technique for Intentional Occlusion of Directional Branches during Complex Endovascular Aortic Repair Using Microvascular Plugs. Vasc. Endovasc. Surg. 2023, 58, 110–114. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Mendes, B.C.; Bews, K.; Hanson, K.; Johnstone, J.; Shuja, F.; Kalra, M.; Bower, T.; Oderich, G.S.; DeMartino, R.R. Perioperative Outcomes after Use of Iliac Branch Devices Compared with Hypogastric Occlusion or Open Surgery for Elective Treatment of Aortoiliac Aneurysms in the NSQIP Database. Ann. Vasc. Surg. 2020, 62, 35–44. [Google Scholar] [CrossRef]
- Warein, E.; Feugier, P.; Chaufour, X.; Molin, V.; Malikov, S.; Bartoli, M.A.; Coscas, R.; Picquet, J.; Peyrot, H.; Favre, J.P.; et al. Amplatzer Plug to Occlude the Internal Iliac Artery during Endovascular Aortic Aneurysm Repair: A Large Multicenter Study. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 641–646. [Google Scholar] [CrossRef]
- Wu, Z.; Raithel, D.; Ritter, W.; Qu, L. Preliminary Embolization of the Hypogastric Artery to Expand the Applicability of Endovascular Aneurysm Repair. J. Endovasc. Ther. 2011, 18, 114–120. [Google Scholar] [CrossRef]
- Wong, K.; Johnson, P.; Chen, Z.; Newsome, J.; Bercu, Z.; Findeiss, L.K.; Dariushnia, S.; Rajani, R.; Kokabi, N. A Meta-Analysis of Comparative Outcome and Cost-Effectiveness of Internal Iliac Artery Embolization with Vascular Plug Versus Coil. Cardiovasc. Interv. Radiol. 2020, 43, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bracale, U.M.; Petrone, A.; Provenzano, M.; Ielapi, N.; Ferrante, L.; Turchino, D.; del Guercio, L.; Pakeliani, D.; Andreucci, M.; Serra, R. The Use of the Amplatzer Vascular Plug in the Prevention of Endoleaks during Abdominal Endovascular Aneurysm Repair: A Systematic Literature Review on Current Applications. Vascular 2022, 30, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Dierks, A.; Sauer, A.; Wolfschmidt, F.; Hassold, N.; Kellersmann, R.; Bley, T.A.; Kickuth, R. Proximal Occlusion of Unaffected Internal Iliac Artery versus Distal Occlusion of Aneurysmatic Internal Iliac Artery Prior to EVAR: A Comparative Evaluation of Efficacy and Clinical Outcome. Br. J. Radiol. 2017, 90, 20160527. [Google Scholar] [CrossRef] [PubMed]
- Spanos, K.; Kölbel, T.; Kubitz, J.C.; Wipper, S.; Konstantinou, N.; Heidemann, F.; Rohlffs, F.; Debus, S.E.; Tsilimparis, N. Risk of Spinal Cord Ischemia after Fenestrated or Branched Endovascular Repair of Complex Aortic Aneurysms. J. Vasc. Surg. 2019, 69, 357–366. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Tenorio, E.R.; Oderich, G.S.; Mendes, B.C.; Kalra, M.; Shuja, F.; Colglazier, J.J.; DeMartino, R.R. Outcomes of the Gore Excluder Iliac Branch Endoprosthesis Using Division Branches of the Internal Iliac Artery as Distal Landing Zones. J. Endovasc. Ther. 2020, 27, 316–327. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, M.; Lima, G.B.B.; Dias, N.; Parlani, G.; Farber, M.; Tsilimparis, N.; DeMartino, R.; Timaran, C.; Kolbel, T.; Gargiulo, M.; et al. Outcomes of “Anterior Versus Posterior Divisional Branches of the Hypogastric Artery as Distal Landing Zone for Iliac Branch Devices”: The International Multicentric R3OYAL Registry. J. Endovasc. Ther. 2022, 31, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Mine, T.; Ikeda, S.; Mizushima, S.; Happoh, S.; Takashi, Y.; Fujitsuna, R.; Ueda, T.; Kawase, Y.; Fujii, M.; Kumita, S.I. Impact of System-F in Delivering Vascular Plugs for Aortic Side Branch Embolization during Endovascular Aneurysm Repair. J. Endovasc. Ther. 2023, 15266028231179422. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.; Kraetsch, A.; Wieners, G.; Redlich, U.; Gaffke, G.; Ricke, J.; Dudeck, O. Embolization of the Gastroduodenal Artery before Selective Internal Radiotherapy: A Prospectively Randomized Trial Comparing Platinum-Fibered Microcoils with the Amplatzer Vascular Plug II. Cardiovasc. Interv. Radiol. 2009, 32, 455–461. [Google Scholar] [CrossRef]
- Guéroult, A.M.; Bashir, A.; Azhar, B.; Budge, J.; Roy, I.; Loftus, I.; Holt, P. Long Term Outcomes and Durability of Fenestrated Endovascular Aneurysm Repair: A Meta-Analysis of Time to Event Data. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 119–129. [Google Scholar] [CrossRef]






| Plug Name | Plug Diameter (mm) | Plug Length (mm) | Delivery System Minimum Internal Diameter (Inches) |
|---|---|---|---|
| AVP I * | 4–6–8–10–12–14–16 | 7–8 | 0.056–0.088 |
| AVP II * | 3–4–6–8–10–12–14–16–18–20–22 | 6–18 | 0.056–0.098 |
| AVP IV * | 4–5–6–7–8 | 10–13.5 | 0.038 (diagnostic catheters) |
| Cera vascular plug | 4–24 | 7–14 | 0.055–0.122 |
| MVP ** | 5.3–6.5–9.2–13 | 12–12–16–18 | 0.021 microcatheter–0.043 |
| Azur vascular plug | 5–8–10 | 13.5–18.5 | 0.027 PG pro peripheral microcatheter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melloni, A.; D’Oria, M.; Dioni, P.; Ongaro, D.; Badalamenti, G.; Lepidi, S.; Bonardelli, S.; Bertoglio, L. Plug-Based Embolization Techniques of Aortic Side Branches during Standard and Complex Endovascular Aortic Repair. J. Clin. Med. 2024, 13, 2084. https://doi.org/10.3390/jcm13072084
Melloni A, D’Oria M, Dioni P, Ongaro D, Badalamenti G, Lepidi S, Bonardelli S, Bertoglio L. Plug-Based Embolization Techniques of Aortic Side Branches during Standard and Complex Endovascular Aortic Repair. Journal of Clinical Medicine. 2024; 13(7):2084. https://doi.org/10.3390/jcm13072084
Chicago/Turabian StyleMelloni, Andrea, Mario D’Oria, Pietro Dioni, Deborah Ongaro, Giovanni Badalamenti, Sandro Lepidi, Stefano Bonardelli, and Luca Bertoglio. 2024. "Plug-Based Embolization Techniques of Aortic Side Branches during Standard and Complex Endovascular Aortic Repair" Journal of Clinical Medicine 13, no. 7: 2084. https://doi.org/10.3390/jcm13072084
APA StyleMelloni, A., D’Oria, M., Dioni, P., Ongaro, D., Badalamenti, G., Lepidi, S., Bonardelli, S., & Bertoglio, L. (2024). Plug-Based Embolization Techniques of Aortic Side Branches during Standard and Complex Endovascular Aortic Repair. Journal of Clinical Medicine, 13(7), 2084. https://doi.org/10.3390/jcm13072084







