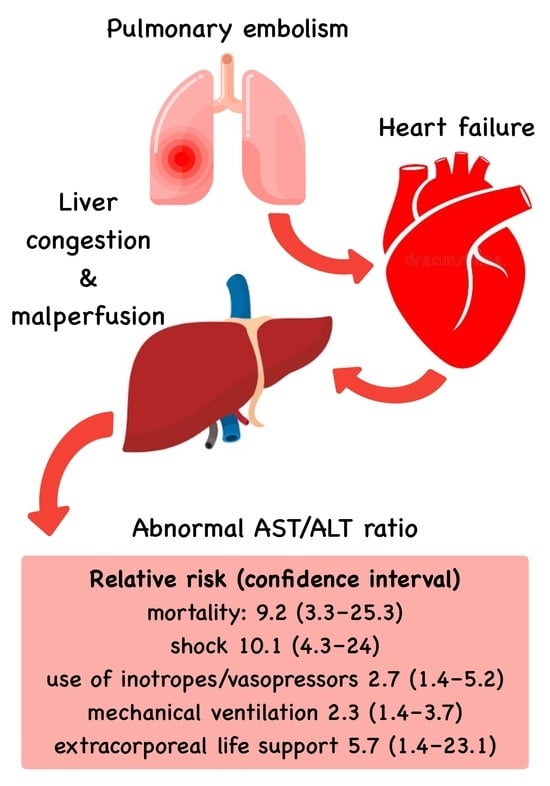
De Ritis Ratio to Predict Clinical Outcomes of Intermediate- and High-Risk Pulmonary Embolisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Participants
3.2. Normal Versus Abnormal AST/ALT Ratio before IPW
3.3. Normal Versus Abnormal AST/ALT Ratio after IPW
3.4. Causal Relative Risk of Primary and Secondary Outcomes after IPW
4. Discussion
4.1. Main Findings
4.2. Previous Studies on the Role of Transaminase Levels in PE
4.3. Risk Stratification, Echocardiography, and Biomarkers in Patients with PEs
4.4. Bleeding and Haemorrhagic Shock Complications
4.5. Implications
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.; Ammari, Z.; Dasa, O.; Ruzieh, M.; Burlen, J.J.; Shunnar, K.M.; Nguyen, H.T.; Xie, Y.; Brewster, P.; Chen, T.; et al. Long-term mortality after massive, and low-risk pulmonary embolism. Vasc. Med. 2020, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 543–603. [Google Scholar]
- Jiang, C.; Xie, M. Clinical outcomes of intermediate-risk pulmonary embolism across a Northeastern health system: A multi-center retrospective cohort study. Cureus 2021, 13, e15888. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.-F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.-F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Gheorghiade, M.; Bubenek, S.; Vinereanu, D.; Vaduganathan, M.; Macarie, C.; Chioncel, O.; on behalf of the Romanian Acute Heart Failure Syndromes (RO-AHFS) study investigators. The predictive value of transaminases at admission in patients hospitalized for heart failure: Findings from the RO-AHFS registry. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 99–108. [Google Scholar] [CrossRef]
- Arcidi, J.M., Jr.; Moore, G.W.; Hutchins, G.M. Hepatic morphology in cardiac dysfunction: A clinicopathologic study of 1000 subjects at autopsy. Am. J. Pathol. 1981, 104, 159–166. [Google Scholar] [PubMed]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK stage classification expert consensus update: A review and incorporation of validation studies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100008. [Google Scholar]
- Zweck, E.; Thayer, K.L.; Helgestad, O.K.L.; Kanwar, M.; Ayouty, M.; Garan, A.R.; Hernandez-Montfort, J.; Mahr, C.; Wencker, D.; Sinha, S.S.; et al. Phenotyping cardiogenic shock. J. Am. Heart Assoc. 2021, 10, e020085. [Google Scholar] [CrossRef] [PubMed]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Durak, K.; Kalverkamp, S.; Zayat, R.; Alizai, P.H.; Spillner, J.; Kersten, A. Emergency colectomy during mechanical circulatory support for septic cardiomyopathy. JTCVS Technol. 2022, 14, 122–124. [Google Scholar] [CrossRef]
- Cohen, J.A.; Kaplan, M.M. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig. Dis. Sci. 1979, 24, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Bell, S.C.; Branson, R.; Chalmers, J.D.; Marshall, R.; Maslove, D.M.; Ost, D.E.; Punjabi, N.M.; Schatz, M.; Smyth, A.R.; et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann. Am. Thorac. Soc. 2019, 16, 22–28. [Google Scholar] [CrossRef]
- Moons, P. Propensity weighting: How to minimise comparative bias in non-randomised studies? Eur. J. Cardiovasc. Nurs. 2020, 19, 83–88. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 1 April 2022).
- Aksoy, M.N.M.; Turna, F.; Sahin, I.; Agac, S. Is AST/ALT ratio a predictor of in-hospital mortality in pulmonary embolism patients? J. Coll. Physicians Surg. Pak. 2022, 32, 171–176. [Google Scholar] [PubMed]
- Aslan, S.; Meral, M.; Akgun, M.; Acemoglu, H.; Ucar, E.Y.; Gorguner, M.; Mirici, A. Liver dysfunction in patients with acute pulmonary embolism. Hepatol. Res. 2007, 37, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Leeuwenkamp, O.; Grobara, P.; Johnston, M.; Söhne, M.; Ten Wolde, M.; Büller, H. The incidence and prognostic significance of elevated cardiac troponins in patients with pulmonary embolism. J. Thromb. Haemost. 2005, 3, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.J.; Fraga, D.N.; Belyshev, V.; Bost, W.; Gardner, C.A.; O’connell, N.S. Intermediate-risk pulmonary embolism: Echocardiography predictors of clinical deterioration. Crit. Care 2022, 26, 160. [Google Scholar] [CrossRef] [PubMed]
- Dahhan, T.; Siddiqui, I.; Tapson, V.F.; Velazquez, E.J.; Sun, S.; Davenport, C.A.; Samad, Z.; Rajagopal, S. Clinical and echocardiographic predictors of mortality in acute pulmonary embolism. Cardiovasc. Ultrasound 2016, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, S.; Olivotto, I.; Cecchini, P.; Pieralli, F.; Camaiti, A.; Santoro, G.; Conti, A.; Agnelli, G.; Berni, G. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 2000, 101, 2817–2822. [Google Scholar] [CrossRef]
- Pinedo, M.; Villacorta, E.; Tapia, C.; Arnold, R.; López, J.; Revilla, A.; Gómez, I.; Fulquet, E.; Román, J.A.S. Inter- and intra-observer variability in the echocardiographic evaluation of right ventricular function. Rev. Esp. Cardiol. 2010, 63, 802–809. [Google Scholar] [CrossRef]
- Kostrubiec, M.; Pruszczyk, P.; Kaczynska, A.; Kucher, N. Persistent NT-proBNP elevation in acute pulmonary embolism predicts early death. Clin. Chim. Acta 2007, 382, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Munoz, S.J.; Stravitz, R.T.; Gabriel, D.A. Coagulopathy of acute liver failure. Clin. Liver Dis. 2009, 13, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Vasques, F.; Cavazza, A.; Bernal, W. Acute liver failure. Curr. Opin. Crit. Care 2022, 28, 198–207. [Google Scholar] [CrossRef]
- Craxì, A.; Cammà, C.; Giunta, M. Clinical aspects of bleeding complications in cirrhotic patients. Blood Coagul. Fibrinolysis 2000, 11 (Suppl. S1), S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Kaymaz, C.; Akbal, O.Y.; Tanboga, I.H.; Hakgor, A.; Yilmaz, F.; Ozturk, S.; Poci, N.; Turkday, S.; Ozdemir, N.; Konstantinides, S. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: A meta-analysis. Curr. Vasc. Pharmacol. 2018, 16, 179–189. [Google Scholar] [CrossRef]
- Furfaro, D.; Stephens, R.S.; Streiff, M.B.; Brower, R. Catheter-directed thrombolysis for intermediate-risk pulmonary embolism. Ann. Am. Thorac. Soc. 2018, 15, 134–144. [Google Scholar] [CrossRef]
- Cole, S.R.; Hernán, M.A. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 2008, 168, 656–664. [Google Scholar] [CrossRef]


| Total Number of Participants (n = 230) | Patients with Normal AST/ALT Ratio (n = 178) | Patients with Abnormal AST/ALT Ratio (n = 52) | p-Value | |
|---|---|---|---|---|
| Age (years) | 67 (55–77) | 68.5 (57–77) | 61.5 (52–73) | 0.023 * |
| Female sex, n (%) | 97 (42.1) | 75 (42.1) | 22 (42.3) | 1.000 |
| Total LOS (days) | 10 (6–16) | 10 (6–15) | 11.5 (5–25) | 0.234 |
| Coronary artery disease, n (%) | 9 (3.9) | 4 (2.2) | 5 (9.6) | 0.045 * |
| Prior CABG surgery, n (%) | 5 (2.2) | 3 (1.7) | 2 (3.8) | 0.690 |
| Arterial hypertension, n (%) | 33 (14.3) | 24 (13.5) | 9 (17.3) | 0.640 |
| COPD, n (%) | 1 (0.4) | 2 (1.1) | 0 (0) | 0.834 |
| Diabetes mellitus type 2, n (%) | 40 (17.4) | 34 (19.1) | 6 (11.5) | 0.290 |
| Chronic kidney disease, n (%) † | 31 (13.5) | 24 (13.5) | 7 (13.5) | 1.000 |
| Chronic left heart failure, n (%) | 39 (17) | 21 (11.8) | 18 (34.6) | <0.001 * |
| Liver cirrhosis, n (%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Obesity, n (%) | 44 (19.1) | 36 (20.2) | 8 (15.4) | 0.562 |
| Anticoagulation before PE, n (%) | 16 (7) | 12 (6.7) | 4 (7.7) | 1.000 |
| LVAD for chronic heart failure, n (%) | 1 (0.4) | 0 (0) | 1 (1.9) | 0.512 |
| Deep venous thrombosis, n (%) | 104 (45.2) | 86 (48.3) | 18 (34.6) | 0.112 |
| Right heart systolic dysfunction, n (%) § | 143 (62.2) | 105 (59) | 38 (73.1) | 0.093 |
| Shock, n (%) §§ | 32 (13.9) | 13 (7.3) | 19 (36.5) | <0.001 * |
| obstructive, n (%) | 32 (13.9) | 13 (7.3) | 19 (36.5) | <0.001 * |
| septic, n (%) | 2 (0.9) | 0 (0) | 2 (3.8) | 0.075 |
| hemorrhagic, n (%) | 3 (1.3) | 1 (0.6) | 2 (3.8) | 0.254 |
| Pleural effusion, n (%) | 39 (17) | 29 (16.3) | 10 (19.2) | 0.774 |
| Infarction pneumonia, n (%) †† | 28 (12.1) | 22 (12.4) | 6 (11.5) | 1.000 |
| Baseline troponin T (µg/L) | 56 (27–138) | 52 (26–117) | 83.5 (33.3–241) | 0.061 |
| USAT for PE, n (%) | 128 (55.7) | 111 (62.4) | 17 (32.7) | <0.001 * |
| Intermediate low-risk, n (%) | 106 (46.1) | 90 (50.6) | 16 (30.8) | 0.018 * |
| Intermediate high-risk, n (%) | 92 (40) | 75 (42.1) | 17 (32.7) | 0.288 |
| High-risk, n (%) | 32 (13.9) | 13 (7.3) | 19 (36.5) | <0.001 * |
| Total Number of Participants (n = 230) | Patients with Normal AST/ALT Ratio (n = 178) | Patients with Abnormal AST/ALT Ratio (n = 52) | p-Value | |
|---|---|---|---|---|
| Mortality, n (%) † | 25 (10.9) | 5 (2.8) | 20 (38.5) | <0.001 * |
| Maximum NT-proBNP level (pg/mL) | 2800 (1200–6875) | 2550 (1146–5625) | 3700 (1750–9891) | 0.023 * |
| Shock, n (%) | 24 (10.4) | 7 (3.9) | 17 (32.7) | <0.001 * |
| obstructive, n (%) | 14 (6.1) | 1 (0.6) | 13 (25) | <0.001 * |
| septic, n (%) | 3 (1.3) | 2 (1.1) | 1 (1.9) | 1.000 |
| haemorrhagic, n (%) | 9 (3.9) | 5 (2.8) | 4 (7.8) | 0.222 |
| Acute bleeding-related anaemia, n (%) | 54 (23.5) | 32 (18) | 22 (42.3) | 0.001 * |
| Acute kidney failure, n (%) § | 50 (21.7) | 29 (16.3) | 21 (40.4) | <0.001 * |
| Cardiac arrest, n (%) | 29 (12.6) | 14 (7.9) | 15 (28.8) | <0.001 * |
| Treatment with inotropes or vasopressors, n (%) | 41 (17.8) | 20 (11.2) | 21 (40.4) | <0.001 * |
| Treatment with mechanical ventilation, n (%) | 62 (27) | 30 (16.9) | 32 (61.5) | <0.001 * |
| Duration of mechanical ventilation (h) | 0 (0–2) | 0 (0–0) | 55 (0–195) | <0.001 * |
| Treatment with VA-ECMO, n (%) | 10 (4.3) | 3 (1.7) | 7 (13.5) | 0.001 * |
| Treatment with CRRT, n (%) | 17 (7.4) | 7 (3.9) | 10 (19.2) | 0.001 * |
| Patients with Normal AST/ALT Ratio (n = 230.4) | Patients with Abnormal AST/ALT Ratio (n = 234.34) | p-Value | |
|---|---|---|---|
| Age (years) | 67 (55–77) | 61 (53.3–71) | 0.076 |
| Female sex, n (%) | 93.5 (40.6) | 76.3 (32.6) | 0.373 |
| Total LOS (days) | 10 (7–17) | 12 (7.4–33.4) | 0.218 |
| Coronary artery disease, n (%) | 11.7 (5.1) | 11 (4.7) | 0.915 |
| Prior CABG surgery, n (%) | 8.9 (3.9) | 7.1 (3) | 0.800 |
| Arterial hypertension, n (%) | 30.3 (13.1) | 24.7 (10.5) | 0.610 |
| COPD, n (%) | 2.3 (1) | 0 (0) | 0.159 |
| Diabetes mellitus type 2, n (%) | 38.7 (16.8) | 44.1 (18.8) | 0.819 |
| Chronic kidney disease, n (%) † | 28.8 (12.5) | 27.9 (11.9) | 0.927 |
| Chronic left heart failure, n (%) | 28.1 (12.2) | 55 (23.5) | 0.070 |
| Liver cirrhosis, n (%) | 0 (0) | 0 (0) | NA |
| Obesity, n (%) | 43.1 (18.7) | 42.1 (18) | 0.925 |
| Anticoagulation before PE, n (%) | 15.6 (6.8) | 14.8 (6.3) | 0.916 |
| LVAD for chronic heart failure, n (%) | 0 (0) | 4.1 (1.7) | 0.327 |
| Deep venous thrombosis, n (%) | 107.2 (46.5) | 94.9 (40.5) | 0.545 |
| Right heart systolic dysfunction, n (%) § | 136.4 (59.2) | 148 (63.2) | 0.697 |
| Shock, n (%) §§ | 32.5 (14.1) | 31.2 (13.3) | 0.876 |
| obstructive, n (%) | 32.5 (14.1) | 31.2 (13.3) | 0.876 |
| septic, n (%) | 0 (0) | 4.7 (2) | 0.180 |
| hemorrhagic, n (%) | 2.9 (1.2) | 3.1 (1.3) | 0.957 |
| Pleural effusion, n (%) | 38.3 (16.6) | 36.6 (15.6) | 0.880 |
| Infarction pneumonia, n (%) †† | 107.2 (46.5) | 94.9 (40.5) | 0.545 |
| Baseline troponin T (µg/l) | 53 (26-122) | 72 (35-239) | 0.81 |
| USAT for PE, n (%) | 128.9 (55.9) | 139 (59.3) | 0.712 |
| Intermediate low-risk, n (%) | 109.1 (47.3) | 102.7 (43.8) | 0.725 |
| Intermediate high-risk, n (%) | 88.8 (38.5) | 100.4 (42.9) | 0.652 |
| High-risk, n (%) | 32.5 (14.1) | 31.2 (13.3) | 0.876 |
| Patients with Normal AST/ALT Ratio (n = 230.4) | Patients with Abnormal AST/ALT Ratio (n = 234.34) | p-Value | |
|---|---|---|---|
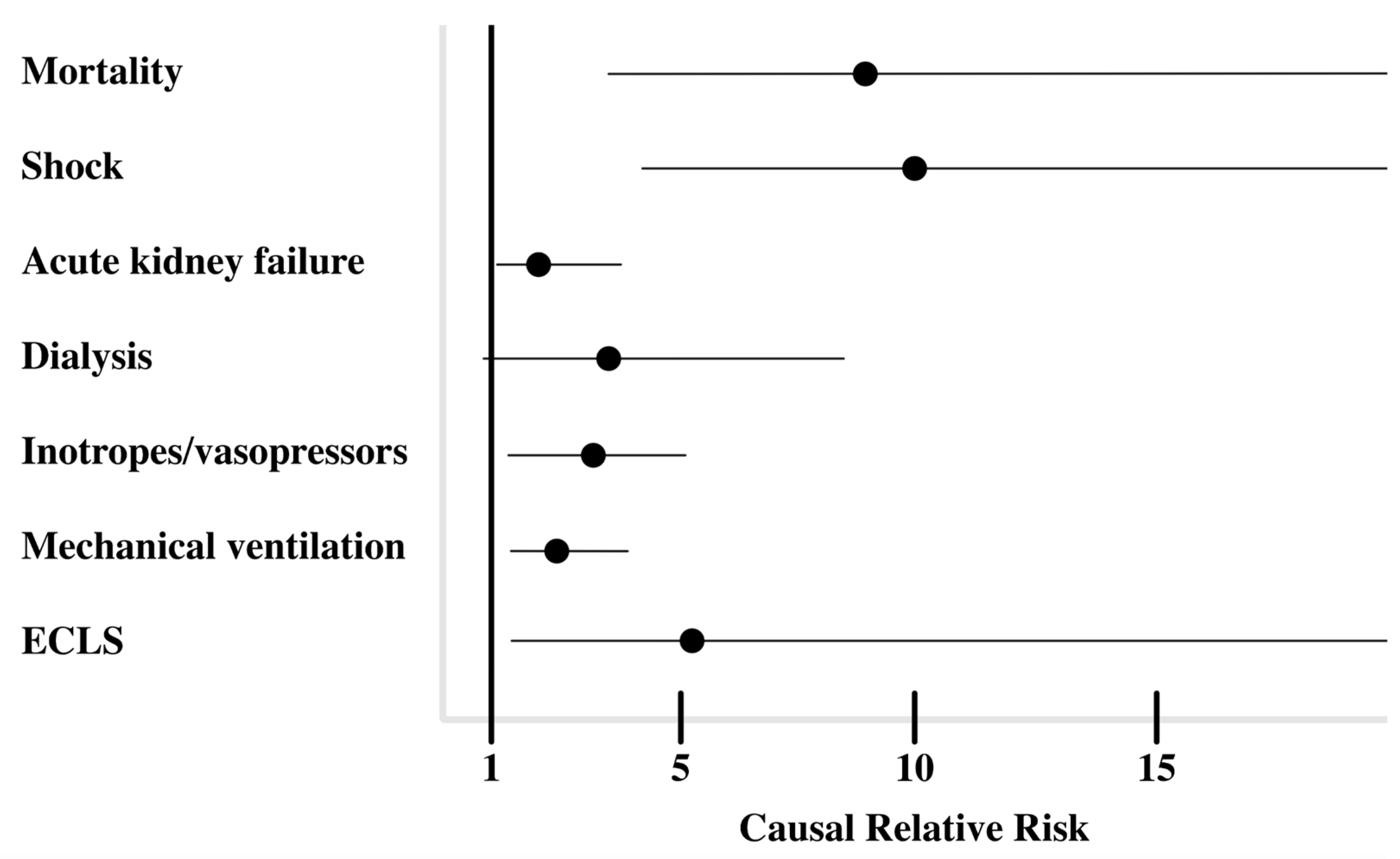
| Mortality, n (%) † | 6.4 (2.8) | 60.1 (25.7) | <0.001 * |
| Maximum NT-proBNP level (pg/mL) | 2400 (982–5067) | 3909 (1590–9528) | 0.021 * |
| Shock, n (%) | 8.6 (3.7) | 88.6 (37.8) | <0.001 * |
| obstructive, n (%) | 1.3 (0.6) | 59.5 (25.4) | <0.001 * |
| septic, n (%) | 2.7 (1.2) | 4 (1.7) | 0.774 |
| haemorrhagic, n (%) | 5.6 (2.4) | 28 (12) | 0.013 * |
| Acute bleeding-related anaemia, n (%) | 49.8 (21.6) | 91.7 (39.1) | 0.044 * |
| Acute kidney failure, n (%) § | 47.8 (20.8) | 94.6 (40.4) | 0.027 * |
| Cardiac arrest, n (%) | 21.3 (9.3) | 45 (19.2) | 0.082 |
| Treatment with inotropes or vasopressors, n (%) | 25.7 (11.1) | 71.1 (30.3) | 0.003 * |
| Treatment with mechanical ventilation, n (%) | 52.6 (22.8) | 122.3 (52.2) | 0.002 * |
| Duration of mechanical ventilation (h) | 0 (0–0) | 4 (0–91) | 0.004 * |
| Treatment with VA-ECMO, n (%) | 3.9 (1.7) | 22.6 (9.7) | 0.006 * |
| Treatment with CRRT, n (%) | 16.7 (7.2) | 49.1 (21) | 0.048 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, K.; Nubbemeyer, K.; Zayat, R.; Spillner, J.; Dineva, S.; Kalverkamp, S.; Kersten, A. De Ritis Ratio to Predict Clinical Outcomes of Intermediate- and High-Risk Pulmonary Embolisms. J. Clin. Med. 2024, 13, 2104. https://doi.org/10.3390/jcm13072104
Durak K, Nubbemeyer K, Zayat R, Spillner J, Dineva S, Kalverkamp S, Kersten A. De Ritis Ratio to Predict Clinical Outcomes of Intermediate- and High-Risk Pulmonary Embolisms. Journal of Clinical Medicine. 2024; 13(7):2104. https://doi.org/10.3390/jcm13072104
Chicago/Turabian StyleDurak, Koray, Katharina Nubbemeyer, Rashad Zayat, Jan Spillner, Slavena Dineva, Sebastian Kalverkamp, and Alexander Kersten. 2024. "De Ritis Ratio to Predict Clinical Outcomes of Intermediate- and High-Risk Pulmonary Embolisms" Journal of Clinical Medicine 13, no. 7: 2104. https://doi.org/10.3390/jcm13072104
APA StyleDurak, K., Nubbemeyer, K., Zayat, R., Spillner, J., Dineva, S., Kalverkamp, S., & Kersten, A. (2024). De Ritis Ratio to Predict Clinical Outcomes of Intermediate- and High-Risk Pulmonary Embolisms. Journal of Clinical Medicine, 13(7), 2104. https://doi.org/10.3390/jcm13072104