Advancements in Laser Therapies for Dermal Hyperpigmentation in Skin of Color: A Comprehensive Literature Review and Experience of Sequential Laser Treatments in a Cohort of 122 Indian Patients
Abstract
1. Introduction
1.1. An Overview of Entities
1.2. Effects on Quality of Life
1.3. Challenges in Treating Dermal Pigmentation
1.4. Mechanisms and Evolution of Pigmentary Laser Systems
2. Methods
3. Discussion
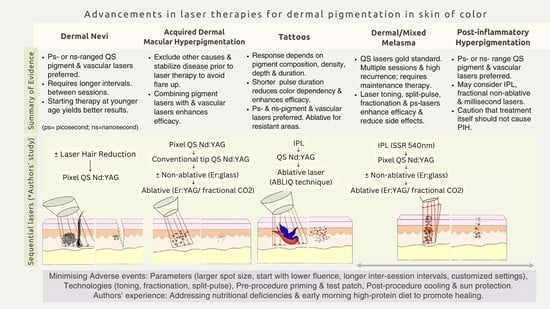
3.1. Individual Entities: Summary of Evidence
3.1.1. Dermal Melanocytosis (Nevus of Ota and Hori’s Nevus)
3.1.2. Acquired Dermal Macular Hyperpigmentation (ADMH)
3.1.3. Tattoos
3.1.4. Post-Inflammatory Hyperpigmentation (PIH)
3.1.5. Mixed-Type/Dermal Melasma
3.2. Authors’ Single-Centre Experience of Sequential Laser Therapy in a Cohort of 122 Indian Patients
3.3. Minimizing Adverse Effects
3.4. Literature Gap and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, J. Differential diagnosis and management of hyperpigmentation. Clin. Exp. Derm. 2022, 47, 251–258. [Google Scholar] [CrossRef]
- Panda, S.; Bandyopadhyay, D. Skin of Colour: The IJDVL View. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 583–585. [Google Scholar] [CrossRef]
- Aurangabadkar, S.J. Optimizing Q-switched lasers for melasma and acquired dermal melanoses. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 10–17. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Wang, R.F.; Ko, D.; Friedman, B.J.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part. I. Pathogenesis and clinical features of common pigmentary disorders. J. Am. Acad. Dermatol. 2023, 88, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Vashisht, K.R.; Makadia, S. A prospective randomized comparative study on 60 Indian patients of melasma, comparing pixel Q-switched NdYAG (1064 nm), super skin rejuvenation (540 nm) and ablative pixel erbium YAG (2940 nm) lasers, with a review of the literature. J. Cosmet. Laser Ther. 2019, 21, 297–307. [Google Scholar] [CrossRef]
- Kapoor, R.; Dhatwalia, S.K.; Kumar, R.; Rani, S.; Parsad, D. Emerging role of dermal compartment in skin pigmentation: Comprehensive review. Acad. Dermatol. Venereol. 2020, 34, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Seité, S.; Deshayes, P.; Dréno, B.; Misery, L.; Reygagne, P.; Saiag, P.; Stengel, F.; Roguedas-Contios, A.M.; Rougier, A. Interest of corrective makeup in the management of patients in dermatology. Clin. Cosmet. Investig. Dermatol. 2012, 5, 123–128. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, T.; Mandal, A.K.; Chander, R. Quality of life in patients with acquired pigmentation: An observational study. J. Cosmet. Dermatol. 2018, 17, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Yadav, D.; Satapathy, S.; Upadhyay, A.; Mahajan, S.; Ramam, M.; Sharma, V.K. Psychosocial burden of lichen planus pigmentosus is similar to vitiligo, but greater than melasma: A cross-sectional study from a tertiary-care center in north India. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 341–347. [Google Scholar] [CrossRef]
- Kluger, N.; Misery, L.; Seité, S.; Taieb, C. Tattooing: A national survey in the general population of France. J. Am. Acad. Dermatol. 2019, 81, 607–610. [Google Scholar] [CrossRef]
- Shah, S.; Baskaran, N.; Vinay, K.; Bishnoi, A.; Parsad, D.; Kumaran, M.S. Acquired dermal macular hyperpigmentation: An overview of the recent updates. Int. J. Dermatol. 2023, 62, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Aurangabadkar, S.; Mysore, V. Standard guidelines of care: Lasers for tattoos and pigmented lesions. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 111. [Google Scholar]
- Fenton, A.; Elliott, E.; Shahbandi, A.; Ezenwa, E.; Morris, C.; McLawhorn, J.; Jackson, J.G.; Allen, P.; Murina, A. Medical students’ ability to diagnose common dermatologic conditions in skin of color. J. Am. Acad. Dermatol. 2020, 83, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Lallas, A.; Lazaridou, E.; Errichetti, E.; Apalla, Z. Race-Specific and Skin of Color Dermatoscopic Characteristics of Skin Cancer: A Literature Review. Dermatol. Pract. Concept. 2023, 13 (4 Suppl. S1), e2023311S. [Google Scholar] [CrossRef]
- Paasch, U.; Zidane, M.; Baron, J.M.; Bund, T.; Cappius, H.J.; Drosner, M.; Feise, K.; Fischer, T.; Gauglitz, G.; Gerber, P.A.; et al. S2k guideline: Laser therapy of the skin. J. Dtsch. Dermatol. Ges. 2022, 20, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hamblin, M.R.; Wen, X. An update on fractional picosecond laser treatment: Histology and clinical applications. Lasers Med. Sci. 2023, 38, 45. [Google Scholar] [CrossRef]
- Passeron, T.; Genedy, R.; Salah, L.; Fusade, T.; Kositratna, G.; Laubach, H.J.; Marini, L.; Badawi, A. Laser treatment of hyperpigmented lesions: Position statement of the European Society of Laser in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 987–1005. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, Z.; Xiang, L.F.; Zhang, C. Unveiling the mystery of Riehl’s melanosis: An update from pathogenesis, diagnosis to treatment. Pigment. Cell Melanoma Res. 2023, 36, 455–467. [Google Scholar] [CrossRef]
- Koh, Y.P.; Tan, A.W.M.; Chua, S.H. Treatment of Laser-Responsive Dermal Pigmentary Conditions in Type III-IV Asian Skin. With a 755-nm Picosecond Pulse Duration Laser: A Retrospective Review of Its Efficacy and Safety. Dermatol. Surg. 2020, 46, e82–e87. [Google Scholar] [CrossRef]
- Levin, M.K.; Ng, E.; Bae, Y.S.C.; Brauer, J.A.; Geronemus, R.G. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: A retrospective photographic review. Lasers Surg. Med. 2016, 48, 181–187. [Google Scholar] [CrossRef]
- Franceschini, D.; Dinulos, J.G. Dermal melanocytosis and associated disorders. Curr. Opin. Pediatr. 2015, 27, 480–485. [Google Scholar] [CrossRef]
- Belkin, D.A.; Jeon, H.; Weiss, E.; Brauer, J.A.; Geronemus, R.G. Successful and safe use of Q-switched lasers in the treatment of nevus of Ota in children with phototypes IV-VI. Lasers Surg. Med. 2018, 50, 56–60. [Google Scholar] [CrossRef]
- Imagawa, K.; Kono, T.; Hanai, U.; Groff, W.F.; Komaba, C.; Tsunoda, Y.; Nemoto, H.; Akamatsu, T. Prospective comparison study of a 550 picosecond 755 nm laser vs a 50 ns 755 nm laser in the treatment of nevus of Ota. Lasers Med. Sci. 2023, 38, 55. [Google Scholar] [CrossRef]
- Yang, C.Y.; Shih, I.H.; Huang, Y.L.; Hu, S. Efficacy and safety of picosecond 755-nm alexandrite laser for treatment of nevus of Ota in Taiwanese children: A retrospective study. Lasers Surg. Med. 2022, 54, 355–365. [Google Scholar] [CrossRef]
- Seo, H.M.; Choi, C.W.; Kim, W.S. Beneficial effects of early treatment of nevus of Ota with low-fluence 1,064-nm Q-switched Nd:YAG laser. Dermatol. Surg. 2015, 41, 142–148. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, Y.; Guo, L.; Zhang, M.; Wu, Q.; Zeng, R.; Rong, H.; Jia, G.; Shi, H.; Fang, J.; et al. Comparison of a picosecond alexandrite laser versus a Q-switched alexandrite laser for the treatment of nevus of Ota: A randomized, split-lesion, controlled trial. J. Am. Acad. Dermatol. 2020, 83, 397–403. [Google Scholar] [CrossRef]
- Hu, S.; Yang, C.S.; Chang, S.L.; Huang, Y.L.; Lin, Y.F.; Lee, M.C. Efficacy and safety of the picosecond 755-nm alexandrite laser for treatment of dermal pigmentation in Asians-a retrospective study. Lasers Med. Sci. 2020, 35, 1377–1383. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, J.; Yu, W.; Lyu, D.; Lin, X.; Zhang, Z. A split-face, single-blinded, randomized controlled comparison of alexandrite 755-nm picosecond laser versus alexandrite 755-nm nanosecond laser in the treatment of acquired bilateral nevus of Ota-like macules. J. Am. Acad. Dermatol. 2018, 79, 479–486. [Google Scholar] [CrossRef]
- Nam, J.H.; Kim, H.S.; Choi, Y.J.; Jung, H.J.; Kim, W.S. Treatment and Classification of Nevus of Ota: A Seven-Year Review of a Single Institution’s Experience. Ann. Dermatol. 2017, 29, 446–453. [Google Scholar] [CrossRef]
- Sarkar, R.; Vinay, K.; Bishnoi, A.; Poojary, S.; Gupta, M.; Kumaran, M.S.; Jain, A.; Gurumurthy, C.; Arora, P.; Kandhari, R.; et al. A Delphi consensus on the nomenclature and diagnosis of lichen planus pigmentosus and related entities. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 41–46. [Google Scholar] [CrossRef]
- Kumarasinghe, S.P.W.; Pandya, A.; Chandran, V.; Rodrigues, M.; Dlova, N.C.; Kang, H.Y.; Ramam, M.; Dayrit, J.F.; Goh, B.K.; Parsad, D. A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl’s melanosis. Int. J. Dermatol. 2019, 58, 263–272. [Google Scholar] [CrossRef]
- Kwon, H.H.; Ohn, J.; Suh, D.H.; Park, H.Y.; Choi, S.C.; Jung, J.Y.; Kwon, I.H.; Park, G.H. A pilot study for triple combination therapy with a low-fluence 1064 nm Q-switched Nd:YAG laser, hydroquinone cream and oral tranexamic acid for recalcitrant Riehl’s Melanosis. J. Dermatol. Treat. 2017, 28, 155–159. [Google Scholar] [CrossRef]
- Vinay, K.; Bishnoi, A.; Kamat, D.; Chatterjee, D.; Kumaran, M.S.; Parsad, D. Acquired Dermal Macular Hyperpigmentation: An Update. Indian Dermatol. Online J. 2021, 12, 663–673. [Google Scholar]
- Xu, L.; Huang, Q.; Wu, T.; Mu, Y. Research Advances in the Treatment of Riehl’s Melanosis. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1181–1189. [Google Scholar] [CrossRef]
- Xu, Z.; Xing, X.; Zhang, C.; Chen, L.; Flora Xiang, L. A pilot study of oral tranexamic acid and Glycyrrhizin compound in the treatment of recalcitrant Riehl’s melanosis. J. Cosmet. Dermatol. 2019, 18, 286–292. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, Y.J.; Baek, D.J.; Kwon, J.E.; Kang, H.Y. A novel treatment for Riehl’s melanosis targeting both dermal melanin and vessels. Photodermatol. Photoimmunol. Photomed. 2023, 39, 613–619. [Google Scholar] [CrossRef]
- Kim, S.M.; Hwang, S.; Almurayshid, A.; Park, M.Y.; Oh, S.H. Non-Ablative 1927 nm Fractional Thulium Fiber Laser: New, Promising Treatment Modality for Riehl’s Melanosis. Lasers Surg. Med. 2021, 53, 640–646. [Google Scholar] [CrossRef]
- Bhari, N.; Sharma, V.K.; Singh, S.; Parihar, A.; Arava, S. Effect of Q-switched Nd-YAG laser on the clinical, pigmentary, and immunological markers in patients with lichen planus pigmentosus: A pilot study. Dermatol. Ther. 2020, 33, e13208. [Google Scholar] [CrossRef]
- Cho, M.Y.; Roh, M.R. Successful Treatment of Riehl’s Melanosis With Mid-Fluence Q-Switched Nd:YAG 1064-nm Laser. Lasers Surg. Med. 2020, 52, 753–760. [Google Scholar] [CrossRef]
- Shah, D.S.D.; Aurangabadkar, D.S.; Nikam, D.B. An open-label non-randomized prospective pilot study of the efficacy of Q-switched Nd-YAG laser in management of facial lichen planus pigmentosus. J. Cosmet. Laser Ther. 2019, 21, 108–115. [Google Scholar] [CrossRef]
- Choi, C.W.; Jo, G.; Lee, D.H.; Jo, S.J.; Lee, C.; Mun, J.H. Analysis of Clinical Features and Treatment Outcomes Using 1,064-nm Nd-YAG Laser with Topical Hydroquinone in Patients with Riehl’s Melanosis: A Retrospective Study in 10 Patients. Ann. Dermatol. 2019, 31, 127–132. [Google Scholar] [CrossRef]
- Kossida, T.; Rigopoulos, D.; Katsambas, A.; Anderson, R.R. Optimal tattoo removal in a single laser session based on the method of repeated exposures. J. Am. Acad. Dermatol. 2012, 66, 271–277. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, M.H.; Noh, T.K.; Choi, K.H.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H. Successful Treatment of Tattoos with a Picosecond 755-nm Alexandrite Laser in Asian Skin. Ann. Dermatol. 2016, 28, 673–675. [Google Scholar] [CrossRef]
- Kasai, K. Picosecond Laser Treatment for Tattoos and Benign Cutaneous Pigmented Lesions (Secondary publication). Laser Ther. 2017, 26, 274–281. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Schomacker, K.T.; Basilavecchio, L.D.; Plugis, J.M.; Bhawalkar, J.D. A novel dual-wavelength, Nd:YAG, picosecond-domain laser safely and effectively removes multicolor tattoos. Lasers Surg. Med. 2015, 47, 542–548. [Google Scholar] [CrossRef]
- Sirithanabadeekul, P.; Vongchansathapat, P.; Sutthipisal, N.; Thanasarnaksorn, W.; Suwanchinda, A. Outcomes of 1064-nm picosecond laser alone and in combination with fractional 1064-nm picosecond laser in tattoo removal. J. Cosmet. Dermatol. 2022, 21, 2832–2839. [Google Scholar] [CrossRef]
- Au, S.; Liolios, A.M.; Goldman, M.P. Analysis of incidence of bulla formation after tattoo treatment using the combination of the picosecond Alexandrite laser and fractionated CO2 ablation. Dermatol. Surg. 2015, 41, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, A.N.B.; Keaney, T.C.; Alster, T. Laser Treatment of Professional Tattoos With a 1064/532-nm Dual-Wavelength Picosecond Laser. Dermatol. Surg. 2017, 43, 1434–1440. [Google Scholar] [CrossRef]
- Li, N.; Han, J.; Hu, D.; Cheng, J.; Wang, H.; Wang, Y.; Yang, X.; Liu, J.; Li, T.; Zhao, W. Intense pulsed light is effective in treating postburn hyperpigmentation and telangiectasia in Chinese patients. J. Cosmet. Laser Ther. 2018, 20, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Bao, S.; Qian, W.; Zhao, H. 755-nm Alexandrite Picosecond Laser with a Diffractive Lens Array or Zoom Handpiece for Post-Inflammatory Hyperpigmentation: Two Case Reports with a Three-Year Follow-Up. Cosmet. Investig. Dermatol. 2021, 14, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.C.; Rettig, S.; Weiss, E.; Bernstein, L.; Geronemus, R. Treatment of Post-Inflammatory Hyperpigmentation in Patients With Darker Skin Types Using a Low Energy 1927 nm Non-Ablative Fractional Laser: A Retrospective Photographic Review Analysis. Lasers Surg. Med. 2020, 52, 7–12. [Google Scholar] [CrossRef]
- Honigman, A.; Rodrigues, M. Differential diagnosis of melasma and hyperpigmentation. Dermatol. Rev. 2023, 4, 30–37. [Google Scholar] [CrossRef]
- Chen, J.; Yu, N.; Peng, L.; Li, H.; Tang, Y.; Ou, S.; Zhu, H. Efficacy of low-fluence 1064 nm Q-switched Nd: YAG laser for the treatment of melasma: A meta-analysis and systematic review. J. Cosmet. Dermatol. 2022, 21. [Google Scholar] [CrossRef]
- Chalermchai, T.; Rummaneethorn, P. Effects of a fractional picosecond 1064 nm laser for the treatment of dermal and mixed type melasma. J. Cosmet. Laser Ther. 2018, 20, 134–139. [Google Scholar] [CrossRef]
- Wong, C.S.M.; Chan, M.W.M.; Shek, S.Y.N.; Yeung, C.K.; Chan, H.H.L. Fractional 1064 nm Picosecond Laser in Treatment of Melasma and Skin Rejuvenation in Asians, A Prospective Study. Lasers Surg. Med. 2021, 53, 1032–1042. [Google Scholar] [CrossRef]
- Feng, J.; Shen, S.; Song, X.; Xiang, W. Efficacy and safety of picosecond laser for the treatment of melasma: A systematic review and meta-analysis. Lasers Med. Sci. 2023, 38, 84. [Google Scholar] [CrossRef]
- Manuskiatti, W.; Yan, C.; Tantrapornpong, P.; Cembrano, K.A.G.; Techapichetvanich, T.; Wanitphakdeedecha, R. A Prospective, Split-Face, Randomized Study Comparing a 755-nm Picosecond Laser With and Without Diffractive Lens Array in the Treatment of Melasma in Asians. Lasers Surg. Med. 2021, 53, 95–103. [Google Scholar] [CrossRef]
- Hong, J.K.; Shin, S.H.; Park, S.J.; Seo, S.J.; Park, K.Y. A prospective, split-face study comparing 1,064-nm picosecond Nd:YAG laser toning with 1,064-nm Q-switched Nd:YAG laser toning in the treatment of melasma. J. Dermatol. Treat. 2022, 33, 2547–2553. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lin, E.T.; Chen, Y.T.; Chiu, P.C.; Lin, B.S.; Chiang, H.M.; Huang, Y.H.; Wang, K.Y.; Lin, H.Y.; Chang, T.M.; et al. Prospective randomized controlled trial comparing treatment efficacy and tolerance of picosecond alexandrite laser with a diffractive lens array and triple combination cream in female asian patients with melasma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 624–632. [Google Scholar] [CrossRef]
- Chen, Y.T.; Lin, E.T.; Chang, C.C.; Lin, B.S.; Chiang, H.M.; Huang, Y.H.; Lin, H.Y.; Wang, K.Y.; Chang, T.M. Efficacy and Safety Evaluation of Picosecond Alexandrite Laser with a Diffractive Lens Array for Treatment of Melasma in Asian Patients by VISIA Imaging System. Photobiomodulation Photomed. Laser Surg. 2019, 37, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Nam, J.H.; Kim, J.Y.; Min, J.H.; Park, K.Y.; Ko, E.J.; Kim, B.J.; Kim, W.S. Efficacy and safety of a novel picosecond laser using combination of 1 064 and 595 nm on patients with melasma: A prospective, randomized, multicenter, split-face, 2% hydroquinone cream-controlled clinical trial. Lasers Surg. Med. 2017, 49, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sangwan, A. Dietary Protein Deficit and Deregulated Autophagy: A New Clinico-diagnostic Perspective in Pathogenesis of Early Aging, Skin, and Hair Disorders. Indian Dermatol. Online J. 2019, 10, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Tuknayat, A. Tips for Managing Post-Inflammatory Hyperpigmentation of Acne. CSDM [Internet]. 2021 July 23 [cited 2024 January 10]. Available online: https://cosmoderma.org/tips-for-managing-post-inflammatory-hyperpigmentation-of-acne/ (accessed on 8 January 2024).
- Cho, S.B.; Ahn, K.J.; Oh, D.; Kim, H.; Yoo, K.H. Sequential delivery of long-pulsed 755-nm alexandrite laser and long-pulsed 1,064-nm neodymium:yttrium-aluminum-garnet laser treatment for pigmented disorders. Skin Res. Technol. 2019, 25, 683–692. [Google Scholar] [CrossRef]





| Dermal Hyperpigmentation | Acquired Dermal Macular Hyperpigmentation
|
| Pigmented Contact Dermatitis, Riehl’s melanosis | |
| Dermal Melanocytosis | |
| Congenital Dermal Melanocytosis | |
| Nevus of Ota, Nevus of Ito, Hori’s Nevus, Sun’s Nevus | |
| Mixed Epidermal–Dermal Hyperpigmentation | Post-inflammatory Hyperpigmentation (PIH) |
| Melasma | |
| Drug-Induced Hyperpigmentation |
| Author | Study Type | Year | Laser | Therapy Parameters | Fitzpatrick Scale | No. of Cases | Clinical Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Imagawa et al. [24] | Prospective comparative study | 2023 | 550-ps 755 nm and 50-ns 755 nm lasers | Clinical endpoint for fluence choice was immediate whitening, with fluence ranging from 2.33 to 3.36 J/cm2 for ps laser and 5.5 to 7 J/cm2 for ns laser. PD: 550 ps and 50 ns for respective lasers; 2.5–3 mm spot | III–IV | 10 | Ps laser had superior efficacy, requiring fewer average sessions (4.2) than ns-laser (5.4) | Hyper- and hypopigmentation occurred in the ns group. The ps group had no side effects. |
| Yang et al. [25] | Retrospective study | 2022 | ps-755 nm Alexandrite laser | Fluence 1.96–2.08 J/cm2, 3.5–4.0-mm spot | III-IV | 86 | 96.5% of patients achieved >95% clearance in an average of 4.3 sessions. Early onset of lesions (<5 months of age) and darker skin types (type IV vs. III) significantly increased the number of sessions required for clearance. Age at first treatment, sex, and nevus color had no significant effect. | Transient hyper- and hypopigmentation |
| Koh et al. [20] | Retrospective review | 2020 | Picosecond 755-nm laser | For NO, the average fluence was 2.02 J/cm2, and for HN, it was 2.08 J/cm2 | III/IV | 29 | In the NO group, mean pre-and-post-treatment Physician global assessment scores were 3.1 and 1.3, respectively (1.8-point change, p = 0.0002). In HN group, mean pre-and-post-treatment PGA scores were 2.6 and 1.1, respectively (1.5-pt change, p = 0.004) | Eleven patients (37.9%) experienced post-laser erythema, and 1 (3.4%) developed transient post-laser hypopigmentation. |
| Ge et al. [27] | RCT (Split-lesion study in nevus of Ota) | 2020 | Ps-755-nm Alexandrite laser versus ns-QS 755-nm Alexandrite laser | Each lesion was treated with single-pass method in up to 6 sessions at 12-week intervals. ps-laser: 2–4 mm spot, 1.59–6.37 J/cm2, 5 Hz. Ns-laser: 3 mm spot, 5–7 J/cm2, 5 Hz. | III–IV | 56 | Higher efficacy, decreased pain scores, and post-inflammatory hyper/hypopigmentation in the ps laser-treated group. | There was an improved clearance, with fewer side effects and more patient satisfaction in the ps-laser arm. |
| Hu et al. [28] | Retrospective study | 2021 | Ps-755-nm Alexandrite laser | Fluence 2.73–3.98 J/cm2, spot size 2.9 to 2.4 mm, PD 650-ps, 1 to 4 sessions. | III–IV | 36 | 88.89% of patients had moderate to marked improvement. | Transient swelling and erythema. Transient PIH (in two patients) and hypopigmentation (in one patient) that resolved in 6 weeks. |
| Yu et al. [29] | RCT (Split-face study in Hori’s nevus) | 2018 | ps 755-nm Alexandrite laser vs. ns-QS 755-nm Alexandrite | 12-week interval. ps-laser: 2–2.5 mm spot, 4.07–6.37 J/cm2, 2.5 Hz. Ns-laser: 3 mm spot, 6–8 J/cm2, 2 Hz. | III–IV | 30 | The PSAL-treated area achieved significantly better clearance (3.73 vs. 2.4) with less severe pain (4.47 vs. 5.16) | the PSAL and QSAL treatments, lasting for nearly one and a half months |
| Belkin et al. [23] | Retrospective case series | 2018 | QS lasers (Ruby 694 nm in type IV and QS Nd:YAG 1064 nm in types V, VI) | Patients were treated without general anesthesia or sedation; corneal shields were used where appropriate. | IV–VI | 24 (children <18yrs) | Excellent response (76–100% improvement) in 70% of patients and good to excellent response (51–100% improvement) in 86%. Fewer sessions/lesser fluence required and fewer complications in younger patients. | Two patients (8%) had post-inflammatory hyperpigmentation, one of whom also had focal hypopigmentation. |
| Nam et al. [30] | Retrospective study | 2017 | Multiple QS laser modalities | Settings varied for different laser machines. | / | 67 | An average of 19 sessions (range 10–27, p = 0.001) is required for 95% clearance. | Two patients (3%) had persistent side effects, e.g., atrophic scarring, though not mentioned how many sessions these patients underwent. |
| Author | Study Type | Year | Laser | Therapy Parameters | Fitzpatrick Scale | No. of Cases | Clinical Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Kim et al. [37] | Open-label, Mixed-methods study | 2023 | Combination of fractional alexandrite picosecond laser and PDL | 3 to 7 sessions 4 weekly intervals. For ps-laser, single treatments, 2–3 passes, <10% overlap, ≥3000 pulses to full face, fluence 0.25 J/cm2, PD 750 ps, 10 mm spot, frequency 10 Hz. For PDL, fluence 7 J/cm2, PD of 6ms, 7 mm spot | III–IV | 11 | Significant reduction in assessment scores in all patients—10 out of 11 reported being very satisfied or satisfied. Effective for both pigmentation and dilated vessels | Transient erythema and oedema |
| Kim et al. [38] | Retrospective Study | 2021 | Non-ablative 1927 nm Fractional Thulium Fiber Laser | 5 W for the output power and 10–20 mJ pulse energy at monthly intervals | III–IV | 9 | 6/9 had “marked”, 1/9 had “significant” and 2/9 had “moderate” improvement | Mild erythema, which faded away within 3 days |
| Bhari et al. [39] | Prospective, pilot study | 2020 | QS Nd:YAG 1064 nm laser | QS-NdYAG laser, toning protocol (3 J/cm2/6 mm/10 Hz), 6 sessions, 2 weekly intervals | IV–V | 9 | 25.7% improvement as per physician assessment. Moreover, 8/9 did not perceive any improvement. No change in melanin index or erythema index | Post-inflammatory hypopigmentation in one patient |
| Cho et al. [40] | Retrospective Study | 2020 | Mid-fluence QS Nd:YAG 1064-nm laser | 3.5–5 J/cm2, 5-mm spot, 10 Hz, 6 sessions, at 5-week interval | III–IV | 21 | 6/21 had “moderate”, 8/21 had “much” and 2/21 had “very much” improvement” | No severe side effect |
| Shah et al. [41] | Open-label, non-randomized prospective pilot study | 2019 | QS Nd-YAG 1064 nm laser | 5 sessions, 4–8 weekly, 5-mm spot, fluence 3–4.6 J/cm2, 5 Hz, 5-mm, single pass with minimal overlap | IV–V | 13 | 38.4% had >90%, 38.4% had >75%, and 23% had >50% improvement | Confetti-like depigmentation in one patient, mild scarring in the supraorbital area in one patient |
| Choi et al. [42] | Retrospective Study | 2019 | 1064-nm Nd:YAG laser | Fluence 0.9 J/cm2 to 2.0 J/cm2, 7 mm spot, 10 Hz, 3-week intervals with 4% HQ cream | III–IV | 10 | 7/10 “near total improvement” 2/10 “marked improvement” 1/10 “minimal improvement | Three patients complained of guttate hypopigmentation |
| Kwon et al. [33] | Pilot study | 2017 | Low-fluence Q-switched 1064-nm Nd:YAG, combined with other lightening agents. | Fluence 1–1.3 J/cm2, 10 mm spot, 2–3 passes, at 3-week intervals, 4% HQ cream and oral tranexamic acid 250mg/day | III–V | 8 | 3/8 “almost clear” grade 5/8“marked improvement” grading | None |
| Author | Study Type | Year | Laser | Therapy Parameters | Fitzpatrick Scale | No. of Cases | Clinical Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Sirithanabadeekul et al. [47] | RCT | 2022 | Fractionated 1064-nm picosecond lasers and unfractional 1064-nm ps-laser | 1064-nm ps-laser: fluence 1.5–7.24 J/cm2; 3–4.5 mm spot, 2–5 Hz; fractionated 1064-nm ps-lasers: fluence 0.8 J/cm2; 8 mm spot, 2–5 Hz | III–V | 11 | The combination side showed greater clearance scores and fewer adverse events than the side of unfractional 1064-nm picosecond laser alone. | Temporary crusting, purpura, edema, erythema, burning sensation, and petechiae |
| Kasai et al. [45] | Retrospective case series | 2017 | Multi-colored tattoos with a ps 755 nm Alexandrite laser and a ps Nd:YAG laser | / | / | 4 | Ps-lasers require fewer sessions than ns-QS lasers for treating black-colored tattoos and are more efficient than ns-QS lasers for multi-colored tattoos. | Transient PIH in 1 patient |
| Kauvar et al. [49] | Prospective clinical study | 2017 | Dual-wavelength, 1064/532-nm, ps-laser | Both wavelengths are used on the same tattoo. Spot sizes 2 to 6 mm, maximum fluence 1.9 J/cm2 for 532-nm and 8.5 J/cm2 for 1064-nm handpieces | Only 26% of subjects were non-Caucasian | 34 (34 subjects with 39 tattoos) | 86% (31 of 36 tattoos) showed at least a 50% clearance after three treatments. Patient satisfaction and treatment tolerability were high. | Adverse events were few and transient in nature. |
| Sirithanabadeekul et al. [47] | RCT | 2022 | Fractionated 1064-nm picosecond lasers and unfractional 1064-nm ps-laser | 1064-nm ps-laser: fluence 1.5–7.24 J/cm2; 3–4.5 mm spot, 2–5 Hz; fractionated 1064-nm ps-lasers: fluence 0.8 J/cm2; 8 mm spot, 2–5 Hz | III–V | 11 | The combination side showed greater clearance scores and fewer adverse events than the side of unfractional 1064-nm picosecond laser alone. | Temporary crusting, purpura, edema, erythema, burning sensation, and petechiae |
| Author | Study Type | Year | Laser | Therapy Parameters | Fitzpatrick Scale | No. of Cases | Clinical Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Bae et al. [52] | Retrospective photographic analysis | 2020 | Non-ablative fractional 1927 nm laser | Low energy, low-density parameters (fluence 5 mJ, spot size 140 μm, depth 170 μm, 5% coverage) | IV–VI | 61 | A mean improvement of 43.24% was assessed by two independent dermatologists, and there was a statistically significant correlation between the raters. | No side effects reported. |
| Li et al. [50] | Prospective interventional study | 2018 | Intense pulsed light | Wavelength 560–615 nm, 2–6 treatments over 3–5 weeks | III–IV | 35 | IPL significantly lessened PIH as per physician assessment with an 82.9% satisfaction rate | No post-treatment complications |
| Kasai et al. [45] | Retrospective case series | 2017 | Fractionated 755 nm Alexandrite ps-laser | 8 mm spot, fluence 0.4 J/cm2, 750-ps, 10 Hz, 3 sessions at 1- or 2-month intervals | III | 1 | At the 3-year follow-up, the PIH lesions had a 50–75% improvement | Deepening of local skin lesions |
| Author | Study Type | Year | Laser | Therapy Parameters | Fitzpatrick Scale | No. of Cases | Clinical Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Manuskiatti et al. [58] | RCT | 2022 | 755 nm fractionated and un-fractionated ps-laser | Fractionated on one side of the face and flat optics (unfractionated)on the other side: 8-mm spot, fluence 0.40 J/cm2, 2.5 Hz, 5 sessions at 5-month intervals | IV–V | 18 (14 patients completed) | All patients showed significant improvement in pigment clearance without notable differences between treatment areas. | Mild PIH and erythema |
| Hong et al. [59] | RCT | 2022 | Ps Nd:YAG vs. conventional 1064 nm Nd:YAG | 10-mm spot, fluence 1.5–2.5 J/cm2 | III–IV | 19 | Both arms had comparable outcomes in terms of modified MASI and melanin index | Mild pain |
| Wong et al. [56] | Prospective study | 2021 | Fractional 1064-nm ps-laser | 450-ps pulses with maximum microbeam energy per pulse 3 mJ | III–IV | 10 | Compared to baseline, seven patients had moderate to marked improvement 6 weeks post-therapy, and five had sustained improvement. | Erythema, edema, and hyper/hypopigmentation |
| Wang et al. [60] | RCT | 2020 | Fractionated ps-Alexandrite 755 nm laser versus TCC | 8 mm spot, fluence 0.4 J/cm2 | / (Asian ethnicity) | 29 | Fractionated ps-Alexandrite laser proved as effective as TCC for treating melasma. | Dryness, PIH Itching focal desquamation |
| Chen et al. [61] | Prospective study | 2019 | Fractionated 755 nm ps-Alexandrite laser | 8-mm spot, fluence 0.4 J/cm2, 750 ps for 3 treatment sessions at 4-to-6-week intervals | IV | 20 | The mean MASI score improved to 6.9 ± 3.7 after 3 sessions of treatment, with the baseline being 9.4 ± 4.7 | Erythema, pruritus, scaling, one developed PIH. |
| Garg et al. [6] | RCT | 2019 | SSR 540 nm, low fluence pixel-QS Nd:YAG, and pixel-Er: YAG | SSR: 8–9 J/cm2, one pass of double shots; pixel-QS Nd:YAG 1064-nm: 4 Hz, low fluence 1.2 J/cm2, 4 passes; pixel Er:YAG 2940 nm: 1100–1200 mJ/pass, long pulse, stack mode, 4–5 passes | III–IV | 60 | All groups had a significant reduction in mMASI (p < 0.001). SSR and pixel-QS Nd:YAG worked best on epidermal melasma (p < 0.001), while pixel-Er: YAG laser was most effective for dermal and mixed melasma (p < 0.001). | Transient and treatable side effects (PIH, acneiform eruptions. One patient in pixel-Er: YAG developed herpes labialis, treated with Valaciclovir |
| Chalermchai et al. [55] | RCT | 2018 | Fractional picosecond 1064-nm laser and 4% HQ | Fractional ps-1064-nm laser: fluence 1.3–1.5 mJ/microbeam, PD 450 ps, 4 Hz versus daily 4% HQ cream | III–IV | 30 | The intervention sides had considerably lower melasma area severity index scores than 4% HQ cream alone. | Mild erythema, desquamation, burning sensation |
| Choi et al. [62] | RCT | 2017 | Dual wavelength ps-laser 1064 and 595 nm with 2% HQ, vs. 2% HQ alone | 7–10 mm spot, fluence 0.2–1.5 J/cm2/5 mm spot, and fluence 0.1–0.55 J/cm2 | III–IV | 39 | Ps-laser with 2% HQ outperformed 2% HQ alone; satisfaction scores supported the results. | Mild dermatitis |
| Diagnosis | No. of Patients | Age in Years (Mean ± SD) | Laser Protocols (Alma Harmony XL Platform) | Rationale behind Laser Sequence | No. of Sessions (Mean ± SD) | Follow-Up in Months (Mean ± SD) |
|---|---|---|---|---|---|---|
| Mixed/dermal Melasma | 51 | 44.5 ± 8.2 | Sessions 1, 2: Non-ablative laser (SSR 540), 7–8 J/cm2, 30 Hz Sessions 3, 4: Pixel QS 1064 nm, 1200 mJ/cm2, 4–8 passes 5th session: Ablative (pixel Er: YAG 2940 nm) laser, 1100–1200 mJ/pass, stack mode, long pulse, 4–5 passes Residual areas: “Hybrid” laser (Alma) with CO2 and 1570 nm Erb glass fiber wavelengths | Targets various mechanisms of melasma, especially in resistant cases | 8.3 ± 2.2 | 10.4 ± 3.5 |
| Dermal Nevi | 21 | 19.7 ± 4.5 | Nevus of Ota, Hori’s nevus: Pixel QS 1064 nm laser for 4–6 sessions starting with 1200 mj, 20 passes, with gradual increase to 40–50 passes. After ≥3 sessions for resistant areas, conventional 3- or 5-mm tips were used at 1000–1200 mJ/pass; single/double passes. Becker’s nevus *: Hair removal: Diode laser (10 J/cm2 × 4 passes) → Alexandrite laser (13 J/cm2 × 10 passes) for finer hair in subsequent sessions. Pigment reduction: QS-Nd:YAG laser (5–6 sessions) using a 5- or 3-mm tip with 1000 mJ/pass X 1–2 passes. | Lasers customized to address different depths and components of Nevi. For instance, treating Becker’s nevus involved sequential use of hair removal and pigment lasers. | 6.4 ± 1.9 | 8.6 ± 2.1 |
| ADMH | 11 | 21.4 ± 5.2 | Sessions 1–3: Pixel QS lasers, 1200 mJ, 4–6 passes Sessions 4, 5: QS 1064 nm laser, 1000 mJ, spot size 3–5 mm Sessions 6, 7: Ablative (pixel Er:YAG) laser, 1200 mJ/pass, 5 passes, long pulse Resistant areas: “Hybrid” laser comprising of CO2 and 1570 nm Erbium glass fiber lasers | Serial use of different wavelengths targets chromophores at varying depths. | 5.9 ± 1.4 | 9.1 ± 2.1 |
| Tattoo | 39 | 28.5 ± 4.2 | Total of 6 to 7 sessions of the “ABLIQ” technique. This is a single-session cocktail laser therapy that stands for Ablative laser, IPL, and QS laser. But the sequence to be used is as follows: IPL → QS Nd:YAG laser → Ablative lasers viz. either pixel Er:YAG or fractional CO2 lasers. (Figure 1) | Facilitates pigment extrusion, reduction in the number of sessions, and “ghost effect” (residual faint outline) | 5.9 ± 1.4 | 9.4 ± 3.1 |
| Type of Dermal Pigmentation (Number of Patients) | Improvement in Pigmentation at 6 Months Post-Therapy, Compared to Baseline | |||||
|---|---|---|---|---|---|---|
| Patient Feedback | Physician Assessment | |||||
| Likert Scale (0 to 10) | Excellent (>90% Improvement; Score: 5) | Good (75–90% Improvement; Score: 4) | Moderate (50–74% Improvement; Score: 3) | Mild (25–49% Improvement; Score: 2) | None (<25% Improvement; Score: 1) | |
| Mixed/dermal Melasma (51) | 6.9 ± 2.5 | 18 (35.3%) | 13 (25.4%) | 15 (29.4%) | 4 (7.8%) | 1 (1.9%) |
| Dermal Nevus (21) | 6.2 ± 1.4 | 8 (38%) | 5 (23.8%) | 5 (23.8%) | 2 (9.5%) | 1 (4.7%) |
| ADMH (11) | 5.9 ± 1.4 | 4 (36.3%) | 4 (36.3%) | 1 (9%) | 1 (9%) | 1 (9%) |
| Tattoo (39) | 7.9 ± 1.1 | 23 (58.9%) | 11 (28.3%) | 5 (12.8%) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, S.; Vashisht, K.R.; Garg, D.; Oberoi, B.; Sharma, G. Advancements in Laser Therapies for Dermal Hyperpigmentation in Skin of Color: A Comprehensive Literature Review and Experience of Sequential Laser Treatments in a Cohort of 122 Indian Patients. J. Clin. Med. 2024, 13, 2116. https://doi.org/10.3390/jcm13072116
Garg S, Vashisht KR, Garg D, Oberoi B, Sharma G. Advancements in Laser Therapies for Dermal Hyperpigmentation in Skin of Color: A Comprehensive Literature Review and Experience of Sequential Laser Treatments in a Cohort of 122 Indian Patients. Journal of Clinical Medicine. 2024; 13(7):2116. https://doi.org/10.3390/jcm13072116
Chicago/Turabian StyleGarg, Suruchi, Kanya Rani Vashisht, Diksha Garg, Bhavni Oberoi, and Geeta Sharma. 2024. "Advancements in Laser Therapies for Dermal Hyperpigmentation in Skin of Color: A Comprehensive Literature Review and Experience of Sequential Laser Treatments in a Cohort of 122 Indian Patients" Journal of Clinical Medicine 13, no. 7: 2116. https://doi.org/10.3390/jcm13072116
APA StyleGarg, S., Vashisht, K. R., Garg, D., Oberoi, B., & Sharma, G. (2024). Advancements in Laser Therapies for Dermal Hyperpigmentation in Skin of Color: A Comprehensive Literature Review and Experience of Sequential Laser Treatments in a Cohort of 122 Indian Patients. Journal of Clinical Medicine, 13(7), 2116. https://doi.org/10.3390/jcm13072116