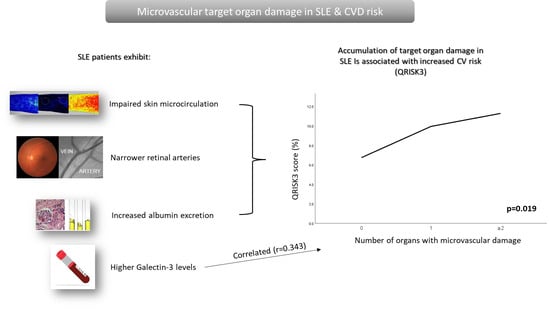
Accumulation of Microvascular Target Organ Damage in Systemic Lupus Erythematosus Patients Is Associated with Increased Cardiovascular Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Laboratory Measurements
2.4. Cardiovascular Risk Assessment
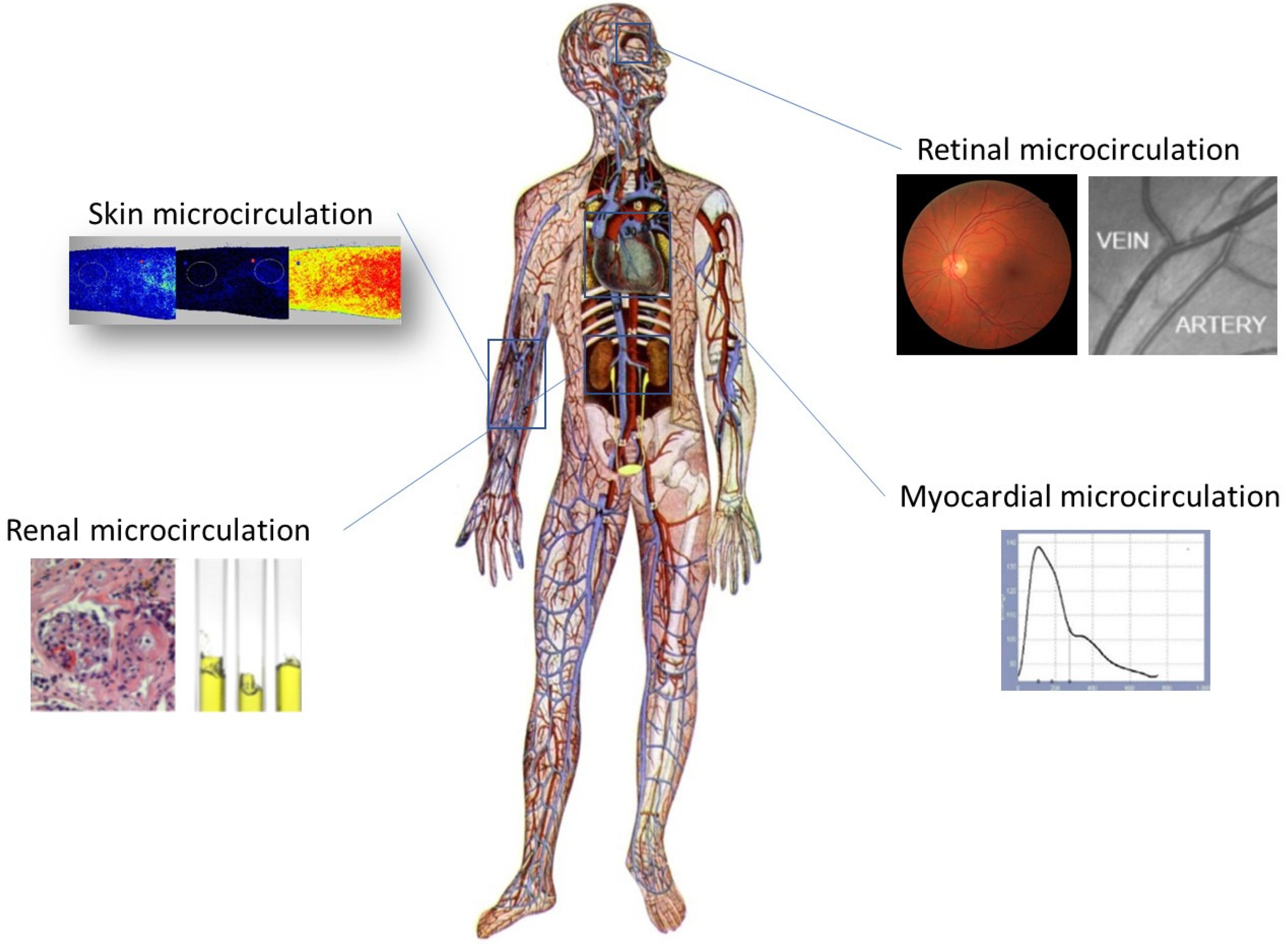
2.5. Microcirculation Assessment
2.5.1. Assessment of Skin Microvascular Function
2.5.2. Retinal Vessel Analysis
2.5.3. Assessment of Microvascular Myocardial Perfusion
2.5.4. Assessment of Urinary Albumin Excretion
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Vascular Measurements
3.3. Prevalence of Microvascular Target Organ Damage
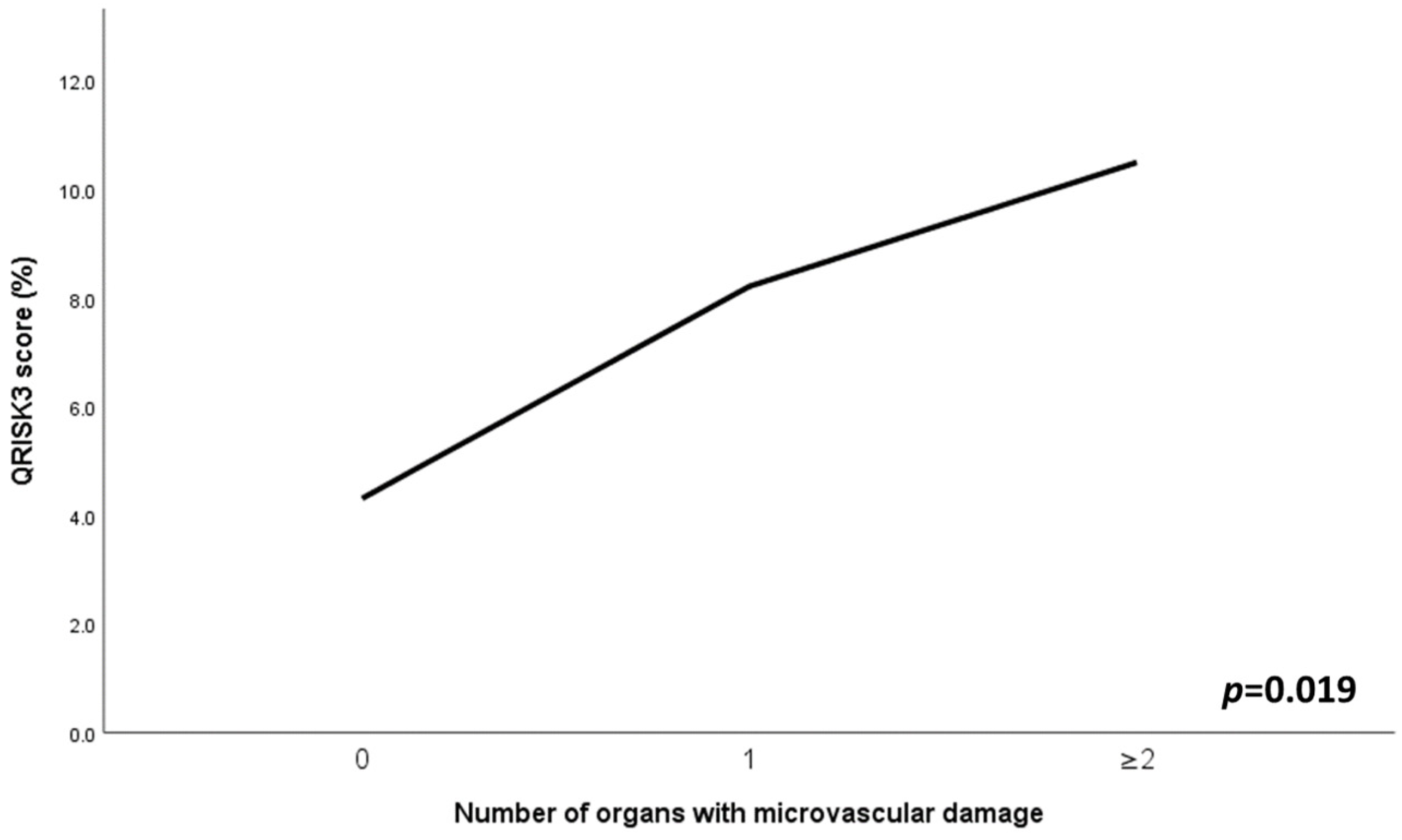
3.4. Associations of Combined Microvascular Target Organ Damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; Van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016, 2, 16039. [Google Scholar] [CrossRef]
- Dörner, T.; Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 2019, 393, 2344–2358. [Google Scholar] [CrossRef]
- Ocampo-Piraquive, V.; Nieto-Aristizábal, I.; Cañas, C.A.; Tobón, G.J. Mortality in systemic lupus erythematosus: Causes, predictors and interventions. Expert Rev. Clin. Immunol. 2018, 14, 1043–1053. [Google Scholar] [CrossRef]
- Zen, M.; Salmaso, L.; Barbiellini Amidei, C.; Fedeli, U.; Bellio, S.; Iaccarino, L.; Doria, A.; Saia, M. Mortality and causes of death in systemic lupus erythematosus over the last decade: Data from a large population-based study. Eur. J. Intern. Med. 2023, 112, 45–51. [Google Scholar] [CrossRef]
- Bello, N.; Meyers, K.J.; Workman, J.; Hartley, L.; McMahon, M. Cardiovascular events and risk in patients with systemic lupus erythematosus: Systematic literature review and meta-analysis. Lupus 2023, 32, 325–341. [Google Scholar] [CrossRef]
- Tselios, K.; Gladman, D.D.; Sheane, B.J.; Su, J.; Urowitz, M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971–2013). Ann. Rheum. Dis. 2019, 78, 802–806. [Google Scholar] [CrossRef]
- Esdaile, J.M.; Abrahamowicz, M.; Grodzicky, T.; Li, Y.; Panaritis, C.; du Berger, R..; Côte, R.; Grover, S.A.; Fortin, P.R.; Clarke, A.E.; et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 2331–2337. [Google Scholar] [CrossRef]
- Ballocca, F.; D’Ascenzo, F.; Moretti, C.; Omedè, P.; Cerrato, E.; Barbero, U.; Abbate, A.; Bertero, M.T.; Zoccai, G.B.; Gaita, F. Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 22, 1435–1441. [Google Scholar] [CrossRef]
- Conrad, N.; Verbeke, G.; Molenberghs, G.; Goetschalckx, L.; Callender, T.; Cambridge, G.; Mason, J.C.; Rahimi, K.; McMurray, J.J.V.; Verbakel, J. Autoimmune diseases and cardiovascular risk: A population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022, 400, 733–743. [Google Scholar] [CrossRef]
- Gustafsson, J.T.; Svenungsson, E. Definitions of and contributions to cardiovascular disease in systemic lupus erythematosus. Autoimmunity 2014, 47, 67–76. [Google Scholar] [CrossRef]
- Panopoulos, S.; Drosos, G.C.; Konstantonis, G.; Sfikakis, P.P.; Tektonidou, M.G. Generic and disease-adapted cardiovascular risk scores as predictors of atherosclerosis progression in SLE. Lupus Sci. Med. 2023, 10, e000864. [Google Scholar] [CrossRef]
- Boland, J.; Long, C. Update on the Inflammatory Hypothesis of Coronary Artery Disease. Curr. Cardiol. Rep. 2021, 23, 6. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Wang, P.; Mao, Y.M.; Zhao, C.N.; Liu, L.N.; Li, X.M.; Li, X.P.; Pan, H.F. Increased Pulse Wave Velocity in Systemic Lupus Erythematosus: A Meta-Analysis. Angiology 2018, 69, 228–235. [Google Scholar] [CrossRef]
- Wu, G.C.; Liu, H.R.; Leng, R.X.; Li, X.P.; Li, X.M.; Pan, H.F.; Ye, D.Q. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun. Rev. 2016, 15, 22–37. [Google Scholar] [CrossRef]
- Henrot, P.; Foret, J.; Barnetche, T.; Lazaro, E.; Duffau, P.; Seneschal, J.; Schaeverbeke, T.; Truchetet, M.E.; Richez, C. Assessment of subclinical atherosclerosis in systemic lupus erythematosus: A systematic review and meta-analysis. Jt. Bone Spine 2018, 85, 155–163. [Google Scholar] [CrossRef]
- Kravvariti, E.; Konstantonis, G.; Sfikakis, P.P.; Tektonidou, M.G. Progression of subclinical atherosclerosis in systemic lupus erythematosus versus rheumatoid arthritis: The impact of low disease activity. Rheumatology 2018, 57, 2158–2166. [Google Scholar] [CrossRef]
- Agabiti-Rosei, E.; Rizzoni, D. Microvascular structure as a prognostically relevant endpoint. J. Hypertens. 2017, 35, 914–921. [Google Scholar] [CrossRef]
- Dipla, K.; Triantafyllou, A.; Koletsos, N.; Papadopoulos, S.; Sachpekidis, V.; Vrabas, I.S.; Gkaliagkousi, E.; Zafeiridis, A.; Douma, S. Impaired Muscle Oxygenation and Elevated Exercise Blood Pressure in Hypertensive Patients: Links with Vascular Stiffness. Hypertension 2017, 70, 444–451. [Google Scholar] [CrossRef]
- Lazaridis, A.; Triantafyllou, A.; Dipla, K.; Dolgyras, P.; Koletsos, N.; Anyfanti, P.; Aslanidis, S.; Douma, S.; Gkaliagkousi, E. Skin microvascular function, as assessed with laser speckle contrast imaging, is impaired in untreated essential and masked hypertension. Hypertens. Res. 2022, 45, 445–454. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gavriilaki, E.; Dolgyras, P.; Nikolaidou, B.; Dimitriadou, A.; Lazaridis, A.; Mastrogiannis, K.; Koletsos, N.; Triantafyllou, A.; Dimitroulas, T.; et al. Skin microcirculation dynamics are impaired in patients with rheumatoid arthritis and no cardiovascular comorbidities. Clin. Exp. Rheumatol. 2023, 41, 1507–1515. [Google Scholar] [CrossRef]
- Koletsos, N.; Gkaliagkousi, E.; Lazaridis, A.; Triantafyllou, A.; Anyfanti, P.; Dolgyras, P.; Dipla, K.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Skin microvascular dysfunction in systemic lupus erythematosus patients with and without cardiovascular risk factors. Rheumatology 2021, 60, 2834–2841. [Google Scholar] [CrossRef]
- Koletsos, N.; Dipla, K.; Triantafyllou, A.; Lazaridis, A.; Papadopoulos, N.G.; Dolgyras, P.; Gavriilaki, E.; Anyfanti, P.; Zafeiridis, A.; Koravou, E.E.; et al. Blunted cerebral oxygenation during exercise in systemic lupus erythematosus patients. Clin. Exp. Rheumatol. 2022, 41, 6–14. [Google Scholar] [CrossRef]
- Hirata, K.; Kadirvelu, A.; Kinjo, M.; Sciacca, R.; Sugioka, K.; Otsuka, R.; Choy, A.M.; Chow, S.K.; Yoshiyama, M.; Yoshikawa, J.; et al. Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 1904–1909. [Google Scholar] [CrossRef]
- Shi, Y.; Tang, D.; Li, X.; Xie, X.; Ye, Y.; Wang, L. Galectin Family Members: Emerging Novel Targets for Lymphoma Therapy? Front. Oncol. 2022, 12, 2092. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef]
- Hogas, S.; Bilha, S.C.; Branisteanu, D.; Hogas, M.; Gaipov, A.; Kanbay, M.; Covic, A. Potential novel biomarkers of cardiovascular dysfunction and disease: Cardiotrophin-1, adipokines and galectin-3. Arch. Med. Sci. 2017, 13, 897–913. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef]
- Koopmans, S.M.; Bot, F.J.; Schouten, H.C.; Janssen, J.; van Marion, A.M. The involvement of Galectins in the modulation of the JAK/STAT pathway in myeloproliferative neoplasia. Am. J. Blood Res. 2012, 2, 119. [Google Scholar]
- Song, X.; Qian, X.; Shen, M.; Jiang, R.; Wagner, M.B.; Ding, G.; Chen, G.; Shen, B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim. Biophys. Acta 2015, 1853, 513–521. [Google Scholar] [CrossRef]
- Jagodzinski, A.; Havulinna, A.S.; Appelbaum, S.; Zeller, T.; Jousilahti, P.; Skytte-Johanssen, S.; Hughes, M.F.; Blankeberg, S.; Salomaa, V. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int. J. Cardiol. 2015, 192, 33–39. [Google Scholar] [CrossRef]
- de Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and autoimmune diseases. Exp. Biol. Med. 2015, 240, 1019. [Google Scholar] [CrossRef]
- Shi, Z.; Meng, Z.; Han, Y.; Cao, C.; Tan, G.; Wang, L. The involvement of galectin-3 in skin injury in systemic lupus erythematosus patients. Lupus 2018, 27, 621–627. [Google Scholar] [CrossRef]
- Kang, E.H.; Moon, K.C.; Lee, E.Y.; Lee, Y.J.; Lee, E.B.; Ahn, C.; Song, Y.W. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus 2009, 18, 22–28. [Google Scholar] [CrossRef]
- Anyfanti, P.; Gkaliagkousi, E.; Gavriilaki, E.; Triantafyllou, A.; Dolgyras, P.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Association of galectin-3 with markers of myocardial function, atherosclerosis, and vascular fibrosis in patients with rheumatoid arthritis. Clin. Cardiol. 2019, 42, 62–68. [Google Scholar] [CrossRef]
- Anyfanti, P.; Dimitriadou, A.; Dara, A.; Angeloudi, E.; Gavriilaki, E.; Nikolaidou, B.; Triantafyllou, A.; Dimitroulas, T.; Gkaliagkousi, E. Circulating levels of galectin-3 and coronary microvascular perfusion in rheumatoid arthritis patients with suppressed inflammation. Clin. Rheumatol. 2023, 42, 2881–2887. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef]
- Cook, R.J.; Dickens, B.M.; Fathalla, M.F. World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191. [Google Scholar]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Gladman, D.; Ginzler, E.; Goldsmith, C.; Fortin, P.; Liang, M.; Urowitz, M.; Bacon, P.; Bombardieri, S.; Hanly, J.; Hay, E.; et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996, 39, 363–369. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Anyfanti, P.; Koletsos, N.; Malliora, A.; Lamprou, S.; Dipla, K.; Gkaliagkousi, E. Clinical Significance of Altered Vascular Morphology and Function in Normotension. Curr. Hypertens. Rep. 2023, 25, 287–297. [Google Scholar] [CrossRef]
- Mahé, G.; Humeau-Heurtier, A.; Durand, S.; Leftheriotis, G.; Abraham, P. Assessment of Skin Microvascular Function and Dysfunction with Laser Speckle Contrast Imaging. Circ. Cardiovasc. Imaging 2012, 5, 155–163. [Google Scholar] [CrossRef]
- Roustit, M.; Cracowski, J.L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol. Sci. 2013, 34, 373–384. [Google Scholar] [CrossRef]
- Roustit, M.; Millet, C.; Blaise, S.; Dufournet, B.; Cracowski, J.L. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc. Res. 2010, 80, 505–511. [Google Scholar] [CrossRef]
- Humeau-Heurtier, A.; Abraham, P.; Durand, S.; Mahé, G. Excellent inter- and intra-observer reproducibility of microvascular tests using laser speckle contrast imaging. Clin. Hemorheol. Microcirc. 2014, 58, 439–446. [Google Scholar] [CrossRef]
- Wellek, S.; Lackner, K.J.; Jennen-Steinmetz, C.; Reinhard, I.; Hoffmann, I.; Blettner, M. Determination of reference limits: Statistical concepts and tools for sample size calculation. Clin. Chem. Lab. Med. 2014, 52, 1685–1694. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Doumas, M.; Anyfanti, P.; Gkaliagkousi, E.; Zabulis, X.; Petidis, K.; Gavriilaki, E.; Karamaounas, P.; Gkolias, V.; Pyrpasopoulou, A.; et al. Divergent retinal vascular abnormalities in normotensive persons and patients with never-treated, masked, white coat hypertension. Am. J. Hypertens. 2013, 26, 318–325. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Anyfanti, P.; Gavriilaki, E.; Zabulis, X.; Gkaliagkousi, E.; Petidis, K.; Triantafyllou, G.; Gkolias, V.; Pyrpasopoulou, A.; Douma, S. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am. J. Hypertens. 2014, 27, 1472–1478. [Google Scholar] [CrossRef]
- Hubbard, L.D.; Brothers, R.J.; King, W.N.; Clegg, L.X.; Klein, R.; Cooper, L.S.; Sharrett, A.R.; Davis, M.D.; Cai, J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study1. Ophthalmology 1999, 106, 2269–2280. [Google Scholar] [CrossRef]
- Tsiachris, D.; Tsioufis, C.; Syrseloudis, D.; Roussos, D.; Tatsis, I.; Dimitriadis, K.; Toutouzas, K.; Tsiamis, E.; Stefanadis, C. Subendocardial viability ratio as an index of impaired coronary flow reserve in hypertensives without significant coronary artery stenoses. J. Hum. Hypertens. 2012, 26, 64–70. [Google Scholar] [CrossRef]
- Salvi, P.; Grillo, A.; Gautier, S.; Labat, C.; Salvi, L.; Valbusa, F.; Baldi, C.; Rovina, M.; Simon, G.; Gao, L.; et al. Myocardial oxygen supply and demand imbalance predicts mortality in older nursing home residents: The PARTAGE study. J. Am. Geriatr. Soc. 2024, 1–12. [Google Scholar] [CrossRef]
- Salvi, P.; Baldi, C.; Scalise, F.; Grillo, A.; Salvi, L.; Tan, I.; De Censi, L.; Sorropago, A.; Moretti, F.; Sorropago, G.; et al. Comparison Between Invasive and Noninvasive Methods to Estimate Subendocardial Oxygen Supply and Demand Imbalance. J. Am. Heart Assoc. 2021, 10, e021207. [Google Scholar] [CrossRef]
- Jekell, A.; Kalani, M.; Kahan, T. The interrelation of endothelial function and microvascular reactivity in different vascular beds, and risk assessment in hypertension: Results from the Doxazosin-ramipril study. Heart Vessel. 2019, 34, 484–495. [Google Scholar] [CrossRef]
- Gómez-Sánchez, M.; Gómez-Sánchez, L.; Patino-Alonso, C.; Recio-Rodríguez, J.I.; Alonso-Domínguez, R.; Sánchez-Aguadero, N.; Lugones-Sánchez, C.; Rodríguez- Sánchez, E.; García-Ortiz, L.; Gomez-Marcos, M.A. Reference values of central blood pressure and central haemodynamic parameters and their relationship with cardiovascular risk factors in a Spanish population: Early vascular ageing study. J. Hypertens. 2021, 39, 2147–2156. [Google Scholar] [CrossRef]
- Ott, C.; Schneider, M.P.; Delles, C.; Schlaich, M.P.; Schmieder, R.E. Reduction in basal nitric oxide activity causes albuminuria. Diabetes 2011, 60, 572–576. [Google Scholar] [CrossRef]
- Deckert, T.; Feldt-Rasmussen, B.; Borch-Johnsen, K.; Jensen, T.; Kofoed-Enevoldsen, A. Albuminuria reflects widespread vascular damage. Steno Hypothesis. Diabetol. 1989, 32, 219–226. [Google Scholar] [CrossRef]
- Paterson, E.N.; Cardwell, C.; MacGillivray, T.J.; Trucco, E.; Doney, A.S.; Foster, P.; Maxwell, A.P.; McKay, G.K. Investigation of associations between retinal microvascular parameters and albuminuria in UK Biobank: A cross-sectional case-control study. BMC Nephrol. 2021, 22, 72. [Google Scholar] [CrossRef]
- Kakutani, Y.; Morioka, T.; Mori, K.; Yamazaki, Y.; Ochi, A.; Kurajoh, M.; Fukumoto, S.; Shioi, A.; Shoji, T.; Inaba, M.; et al. Albuminuria rather than glomerular filtration rate is associated with vascular endothelial function in patients with type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107702. [Google Scholar] [CrossRef]
- Khosla, N.; Sarafidis, P.A.; Bakris, G.L. Microalbuminuria. Clin. Lab. Med. 2006, 26, 635–653. [Google Scholar] [CrossRef]
- ADA. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S151–S167. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.S.; Kim, G.T. Microvascular findings in patients with systemic lupus erythematosus assessed by fundus photography with fluorescein angiography. Clin. Exp. Rheumatol. 2013, 31, 871–876. [Google Scholar]
- Aissopou, E.; Protogerou, A.; Papaioannou, T.; Tektonidou, M.; Tentolouris, N.; Theodossiadis, P.; Stehouwer, C.D.A.; Kitas, G.D.; Sfikakis, P.P. Retinal vascular calibers in contemporary patients with chronic systemic inflammatory diseases: The Greek REtinal Microcirculation (GREM) study. Artery Res. 2017, 18, 1–6. [Google Scholar] [CrossRef]
- Anyfanti, P.; Triantafyllou, A.; Gkaliagkousi, E.; Koletsos, N.; Athanasopoulos, G.; Zabulis, X.; Galanopoulou, V.; Aslanidis, S.; Douma, S. Retinal vessel morphology in rheumatoid arthritis: Association with systemic inflammation, subclinical atherosclerosis, and cardiovascular risk. Microcirculation 2017, 24, e12417. [Google Scholar] [CrossRef]
- Zhu, L.; Singh, M.; Lele, S.; Sahakian, L.; Grossman, J.; Hahn, B.; McMahon, M. Assessing the validity of QRISK3 in predicting cardiovascular events in systemic lupus erythematosus. Lupus Sci. Med. 2022, 9, e000564. [Google Scholar] [CrossRef]
- Vázquez-Del Mercado, M.; de, J. Perez-Vazquez, F.; Gomez-Bañuelos, E.; Chavarria-Avila, E.; Llamas-García, A.; Arrona-Rios, K.I.; Diaz-Rubio, G.I.; Durán-Barragán, S.; Navarro-Hernández, R.E.; Jordán-Estrada, B.P.; et al. Subclinical parameters of arterial stiffness and arteriosclerosis correlate with QRISK3 in systemic lupus erythematosus. PLoS ONE 2018, 13, e0207520. [Google Scholar]
- Quevedo-Abeledo, J.C.; Caceres, L.; Palazuelos, C.; Llorca, J.; González-Gay, M.A.; Ferraz-Amaro, I. QRISK3 relation to carotid plaque is higer than that of score in patients with systemic lupus erythematosus. Rheumatology 2022, 61, 1408–1416. [Google Scholar] [CrossRef]
- Edwards, N.; Langford-Smith, A.W.W.; Parker, B.J.; Bruce, I.N.; Reynolds, J.A.; Alexander, M.Y.; McCarthy, E.M.; Wilkinson, F.L. QRISK3 improves detection of cardiovascular disease risk in patients with systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000272. [Google Scholar] [CrossRef]
- Atehortúa, L.; Rojas, M.; Vásquez, G.M.; Castaño, D. Endothelial Alterations in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Potential Effect of Monocyte Interaction. Mediat. Inflamm. 2017, 2017, 9680729. [Google Scholar] [CrossRef]
- Papaspyridonos, M.; McNeill, E.; De Bono, J.P.; Smith, A.; Burnand, K.G.; Channon, K.M.; Greaves, D.R. Galectin-3 Is an Amplifier of Inflammation in Atherosclerotic Plaque Progression Through Macrophage Activation and Monocyte Chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 433–440. [Google Scholar] [CrossRef]
- Seropian, I.M.; Cassaglia, P.; Miksztowicz, V.; González, G.E. Unraveling the role of galectin-3 in cardiac pathology and physiology. Front. Physiol. 2023, 14, 1304735. [Google Scholar] [CrossRef]
- Mehina, E.M.F.; Taylor, S.; Boghozian, R.; White, E.; Choi, S.E.; Cheema, M.S.; Korbelin, J.; Brown, C.E. Invasion of phagocytic Galectin 3 expressing macrophages in the diabetic brain disrupts vascular repair. Sci. Adv. 2021, 7, eabg2712. [Google Scholar] [CrossRef]
- Radulova, G.; Kapogianni, A.; Cholakova, G.; Iliev, S.; Ivanova, A.; Bogoeva, V.; Tsacheva, I. Galectin-3—A novel ligand of complement protein C1q. Int. J. Biol. Macromol. 2024, 262 Pt 2, 129930. [Google Scholar] [CrossRef]
- Piani, F.; Tossetta, G.; Cara-Fuentes, G.; Agnoletti, D.; Marzioni, D.; Borghi, C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules 2023, 13, 910. [Google Scholar] [CrossRef]
| SLE (n = 47) | Control (n = 50) | p Value | |
|---|---|---|---|
| Age (years), mean ± S.D. | 48.5 ± 9.2 | 45.0 ± 10.7 | 0.092 |
| BMI (Kg/m2), mean ± S.D. | 25.5 ± 4.4 | 26.4 ± 4.8 | 0.337 |
| Female sex, n (%) | 41 (87.2) | 38 (76.0) | 0.155 |
| Smoking, yes, n (%) | 23 (48.9) | 17 (34.0) | 0.135 |
| Office SBP (mmHg), mean ± S.D. | 118.9 ± 14.1 | 118.8 ± 13.2 | 0.991 |
| Office DBP (mmHg), mean ± S.D. | 77.3 ± 11.6 | 75.6 ± 8.5 | 0.406 |
| Office HR (pulses/min), median (IQR) | 73.0 (16.0) | 75.0 (15.0) | 0.411 |
| Glucose (mg/dL), mean ± S.D. | 85.9 ± 8.9 | 88.7 ± 8.8 | 0.152 |
| Uric acid (mg/dL), mean ± S.D. | 4.6 ± 1.0 | 4.8 ± 1.1 | 0.501 |
| eGFR (mL/min/1.73 m2), median (IQR) | 93.0 (24.0) | 96.0 (17.0) | 0.200 |
| Total Cholesterol (mg/dL), mean ± S.D. | 177.4 ± 28.9 | 191.4 ± 35.5 | 0.041 |
| Triglycerides (mg/dL), median (IQR) | 84.0 (60.0) | 87.0 (53.0) | 0.622 |
| HDL Cholesterol (mg/dL), mean ± S.D. | 50.5 ± 15.5 | 50.6 ± 9.1 | 0.981 |
| LDL Cholesterol (mg/dL), mean ± S.D. | 107.0 ± 24.4 | 117.8 ± 30.7 | 0.066 |
| Galectin-3 (ng/dL), median (IQR) | 21.5 (6.1) | 6.6 (6.6) | <0.001 |
| QRISK3 score, median (IQR) | 7.0 (8.6) | 1.3 (3.6) | <0.001 |
| Clinical Characteristics | |
|---|---|
| Age (years), mean ± S.D. | 48.5 ± 9.2 |
| Disease duration (years), median (IQR) | 12.0 (13.0) |
| Female sex, n (%) | 41 (87.2) |
| Raynaud’s phenomenon, n (%) | 24 (54.5) |
| Lupus nephritis history, n (%) | 8 (18.6) |
| SLEDAI-2K, median (IQR) | 2.0 (2.0) |
| SDI, median (IQR) | 0.6 (1.0) |
| Serology | |
| ANA, positive, (%) | 91.5 |
| Anti-dsDNA, (%) | 45.7 |
| aPL positivity (%) | 32.6 |
| ESR (mm), median (IQR) | 13.0 (19.0) |
| CRP (mg/dL), median (IQR) | 0.26 (0.47) |
| C3, mean ± S.D. | 75.2 ± 20.2 |
| C4, mean ± S.D. | 14.0 ± 5.6 |
| Treatment | |
| Hydroxychloroquine, yes (%) | 66.0 |
| Corticosteroid use, yes (%) | 46.8 |
| Immunosuppressants, yes (%) | 44.7 |
| Azathioprine (%) | 29.8 |
| Mycophenolate mofetil (%) | 6.4 |
| Cyclophosphamide (%) | 4.3 |
| Methotrexate (%) | 4.3 |
| Belimumab (%) | 2.1 |
| SLE (n = 47) | Control (n = 50) | p Value | |
|---|---|---|---|
| Baseline flux (PU), mean ± S.D. | 43.4 ± 7.8 | 38.4 ± 9.9 | 0.012 |
| Baseline-to-occlusion change (%), median (IQR) | −79.0 (11.5) | −79.0 (12.4) | 0.580 |
| Peak flux (PU), mean ± S.D. | 112.0 ± 23.2 | 114.7 ± 26.6 | 0.627 |
| Peak magnitude (%), mean ± S.D. | 160.2 ± 41.0 | 203.6 ± 40.1 | <0.001 |
| CRAE (μm), mean ± S.D. | 88.1 ± 11.1 | 94.6 ± 13.5 | 0.022 |
| CRVE (μm), mean ± S.D. | 116.1 ± 14.0 | 117.5 ± 15.4 | 0.664 |
| AVR, median (IQR) | 0.78 (0.18) | 0.78 (0.15) | 0.346 |
| SEVR (%), mean ± S.D. | 150.2 ± 20.7 | 154.1 ± 28.8 | 0.493 |
| UACR (mg/g), median (IQR) | 8.9 (16.2) | 5.7 (2.6) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koletsos, N.; Lazaridis, A.; Triantafyllou, A.; Anyfanti, P.; Lamprou, S.; Stoimeni, A.; Papadopoulos, N.G.; Koravou, E.-E.; Gkaliagkousi, E. Accumulation of Microvascular Target Organ Damage in Systemic Lupus Erythematosus Patients Is Associated with Increased Cardiovascular Risk. J. Clin. Med. 2024, 13, 2140. https://doi.org/10.3390/jcm13072140
Koletsos N, Lazaridis A, Triantafyllou A, Anyfanti P, Lamprou S, Stoimeni A, Papadopoulos NG, Koravou E-E, Gkaliagkousi E. Accumulation of Microvascular Target Organ Damage in Systemic Lupus Erythematosus Patients Is Associated with Increased Cardiovascular Risk. Journal of Clinical Medicine. 2024; 13(7):2140. https://doi.org/10.3390/jcm13072140
Chicago/Turabian StyleKoletsos, Nikolaos, Antonios Lazaridis, Areti Triantafyllou, Panagiota Anyfanti, Stamatina Lamprou, Anastasia Stoimeni, Nikolaos G. Papadopoulos, Evaggelia-Evdoxia Koravou, and Eugenia Gkaliagkousi. 2024. "Accumulation of Microvascular Target Organ Damage in Systemic Lupus Erythematosus Patients Is Associated with Increased Cardiovascular Risk" Journal of Clinical Medicine 13, no. 7: 2140. https://doi.org/10.3390/jcm13072140