Impaired Carbohydrate Metabolism among Women with Chronic Low Back Pain and the Role of Dietary Carbohydrates: A Randomized Controlled Cross-Over Experiment
Abstract
:1. Introduction
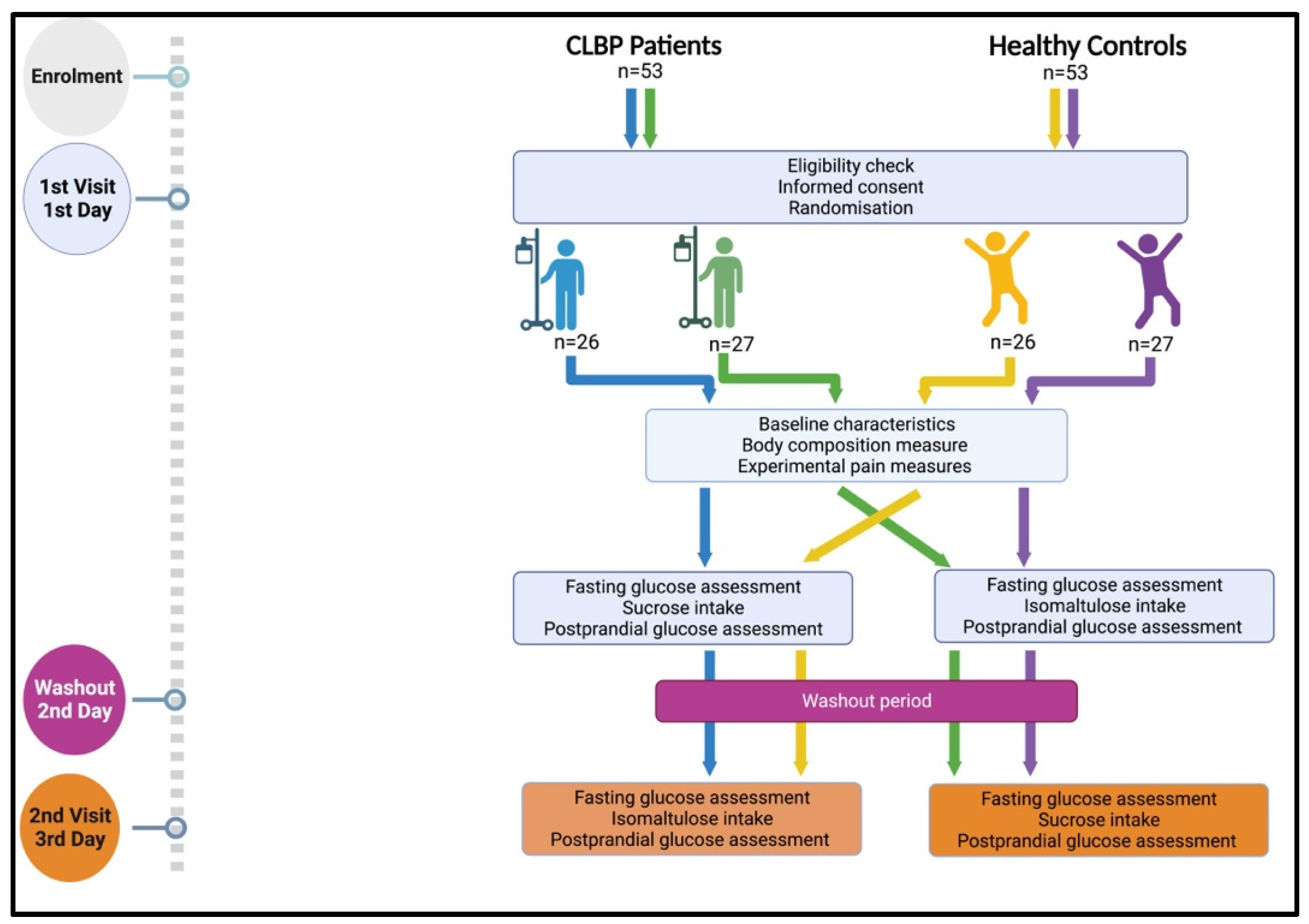
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sample Size Calculation
2.3. Participants
2.4. Procedure
2.5. Randomisation and Blinding
2.6. Outcome Measures
2.6.1. Baseline Characteristics
2.6.2. Blood Glycemic Response Measurements
2.6.3. Experimental Pain Measures
Electrical Detection and Electrical Pain Thresholds
Temporal Summation
Electrical Offset Analgesia
Pressure Pain Thresholds
2.6.4. Anthropometry
2.6.5. Self-Reported Questionnaires
Brief Pain Inventory (BPI)
Central Sensitization Inventory
Physical Activity
Quality of Life
2.7. Data Analysis
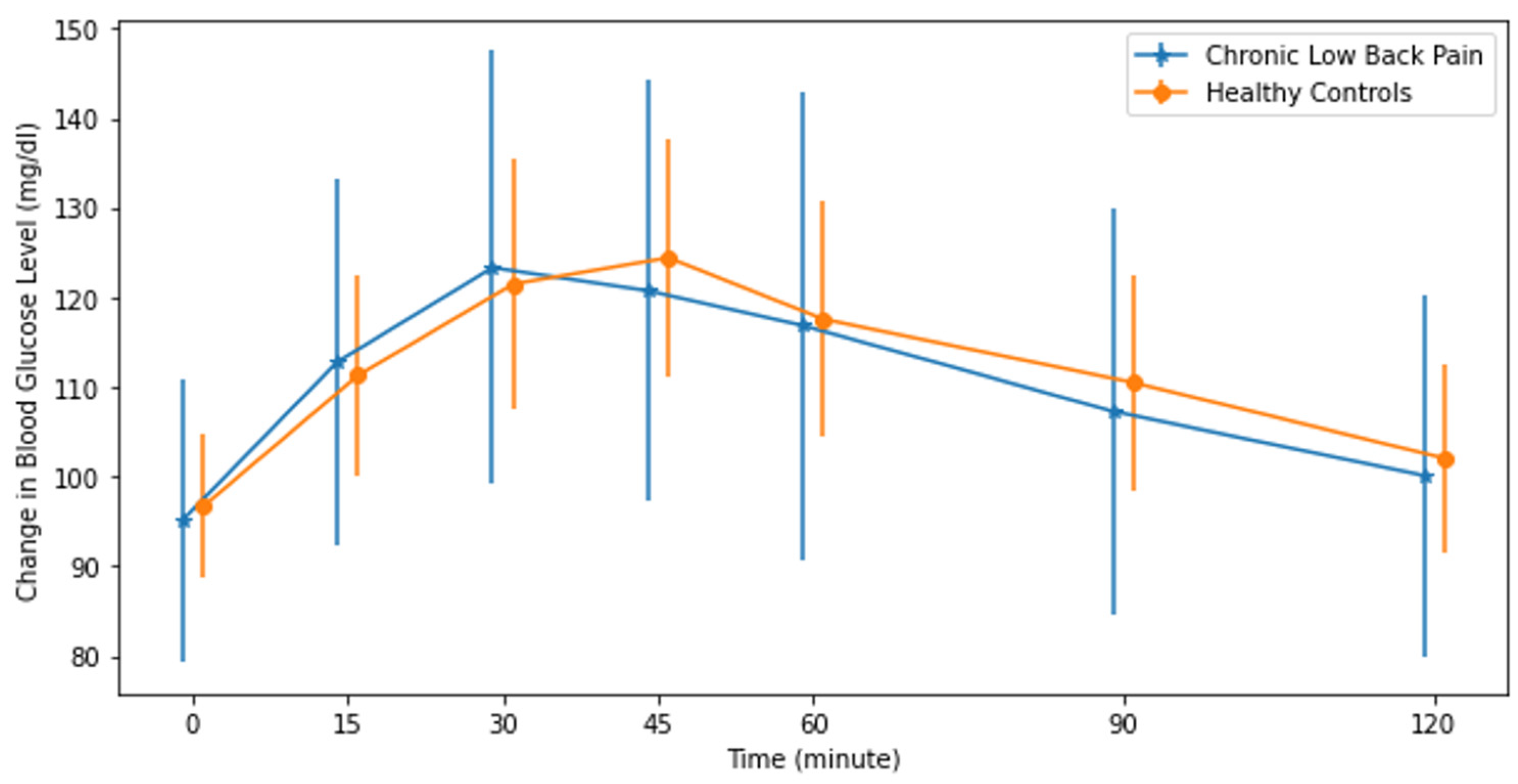
3. Results
4. Discussion
4.1. Limitations and Strengths
4.2. Practical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Python Code for the Calculation of the Area Under the Curve (AUC)
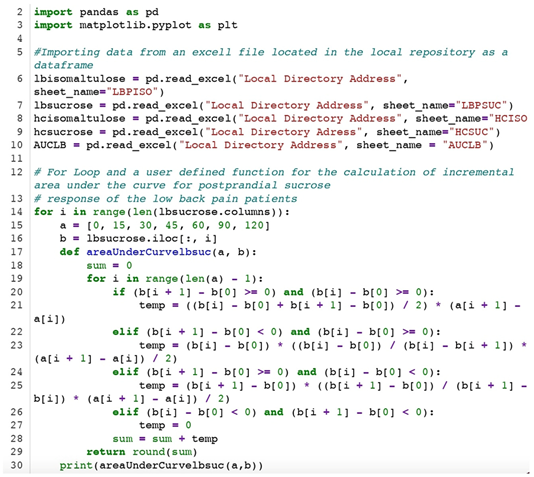
Appendix B. Description of the Elements of the Python Code
| Line Number | Code Element | Description |
| 2 | Import module | “Import module” imports pandas and matplotlib libraries for required functions |
| 14 | for loop | Iterates through the list containing all participants’ data |
| 17 | def areaUnderCurvelbpsu | Creates a function that calculates the area under curve using the trapezoidal rule, ignoring the values below the fasting blood glucose level |
| 19 | for loop | Iterates through the list containing individual participant data |
| 20 | If statement | If all the values are higher than the fasting blood glucose level, use this function to calculate the area of the trapezoid |
| 22 | elif statement | If the first value is lower than the fasting blood glucose level and the second value is higher and equal to fasting blood glucose level, use this function to calculate the area of the trapezoid |
| 24 | elif statement | If the first value is higher than or equal to the fasting blood glucose level and the second value is lower than the fasting blood glucose level, use this function to calculate the area of the trapezoid |
| 26 | elif statement | If the fasting blood glucose level is higher than all the value, use this function to calculate the area of the trapezoid |
| 28 | sum variable | Total sum of the area under the curve value for each individual |
References
- Freburger, J.K.; Holmets, G.M.; Agans, R.P.; Jackman, A.M.; Darter, J.D.; Wallace, A.S.; Castel, L.D.; Kalsbeek, W.D.; Carey, T.S. The rising prevalence of chronic low back pain. Intern. Med. 2009, 169, 251–258. [Google Scholar] [CrossRef]
- Rubin, D.I. Epidemiology and risk factors for spine pain. Neurol. Clin. 2007, 25, 353–371. [Google Scholar] [CrossRef]
- Meucci, R.D.; Fassa, A.G.; Faria, N.M.X. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 73. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.L.G.; Bromet, E.J.; de Girolamo, G.; de Graaf, R.; et al. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Di Cesare, M.; Danaei, G.; Gaciong, Z. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4· 4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Berenbaum, F. Diabetes-induced osteoarthritis: From a new paradigm to a new phenotype. Postgrad. Med. J. 2012, 88, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, L. Atherosclerosis and disc degeneration/low-back pain—A systematic review. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Bestall, S.M.; Hulse, R.P.; Blackley, Z.; Swift, M.; Ved, N.; Paton, K.; Beazley-Long, N.; Bates, D.O.; Donaldson, L.F. Sensory neuronal sensitisation occurs through HMGB-1–RAGE and TRPV1 in high-glucose conditions. J. Cell Sci. 2018, 131, jcs215939. [Google Scholar] [CrossRef]
- Afshari, K.; Dehdashtian, A.; Haddadi, N.-S.; Haj-Mirzaian, A.; Iranmehr, A.; Ebrahimi, M.A.; Tavangar, S.M.; Faghir-Ghanesefat, H.; Mohammadi, F.; Rahimi, N.; et al. Anti-inflammatory effects of metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: Introduction of an alternative therapy. Spinal Cord 2018, 56, 1032–1041. [Google Scholar] [CrossRef]
- Mäntyselkä, P.; Miettola, J.; Niskanen, L.; Kumpusalo, E. Chronic pain, impaired glucose tolerance and diabetes: A community-based study. Pain® 2008, 137, 34–40. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, M.; Pazos-Couselo, M.; García-López, J.M.; Rodríguez-Segade, S.; Rodríguez-García, J.; Túñez-Bastida, C.; Gude, F. Postprandial glycemic response in a non-diabetic adult population: The effect of nutrients is different between men and women. Nutr. Metab. 2019, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Corrales, P.P.; Castañeda, V.B.; Ampudia-Blasco, F. Update on postprandial hyperglycemia: The pathophysiology, prevalence, consequences and implications of treating diabetes. Rev. Clín. Española 2020, 220, 57–68. [Google Scholar] [CrossRef]
- Wolever, T.M.; Yang, M.; Zeng, X.Y.; Atkinson, F.; Brand-Miller, J.C. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am. J. Clin. Nutr. 2006, 83, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.K.; Tan, S.-Y.; Henry, C.J. Ethnic variability in glycemic response to sucrose and isomaltulose. Nutrients 2017, 9, 347. [Google Scholar] [CrossRef]
- Amano, T.; Watanabe, D.; Otsuka, J.; Okamoto, Y.; Takada, S.; Fujii, N.; Kenny, G.P.; Enoki, Y.; Maejima, D. Comparison of hydration efficacy of carbohydrate-electrolytes beverages consisting of isomaltulose and sucrose in healthy young adults: A randomized crossover trial. Physiol. Behav. 2022, 249, 113770. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed]
- May, S.; Serpell, M. Diagnosis and assessment of neuropathic pain. F1000 Med. Rep. 2009, 1, 76. [Google Scholar] [CrossRef]
- Bailey, T.; Chang, A.; Rosenblit, P.D.; Jones, L.; Teft, G.; Setford, S.; Mahoney, J. A comprehensive evaluation of the performance of the test strip technology for OneTouch Verio glucose meter systems. Diabetes Technol. Ther. 2012, 14, 701–709. [Google Scholar] [CrossRef]
- France, C.R.; France, J.L.; Al’Absi, M.; Ring, C.; McIntyre, D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain 2002, 99, 459–463. [Google Scholar] [CrossRef]
- Vuilleumier, P.H.; Manresa, J.A.B.; Ghamri, Y.; Mlekusch, S.; Siegenthaler, A.; Arendt-Nielsen, L.; Curatolo, M. Reliability of quantitative sensory tests in a low back pain population. Reg. Anesth. Pain Med. 2015, 40, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, S.; Winefield, A.H.; Rolan, P.; Lushington, K. Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res. Manag. 2009, 14, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Uddin, Z.; MacDermid, J.C. Quantitative sensory testing in chronic musculoskeletal pain. Pain Med. 2016, 17, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.; Laeubli, T.; Klipstein, A. Validity of pressure pain thresholds in female workers with and without recurrent low back pain. Eur. Spine J. 2007, 16, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Verney, J.; Miguet, M.; Masurier, J.; Cardenoux, C.; Lambert, C.; Courteix, D.; Metz, L.; Pereira, B. The accuracy of bioelectrical impedance to track body composition changes depends on the degree of obesity in adolescents with obesity. Nutr. Res. 2018, 54, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, J. Brief Pain Inventory review. Occup. Med. 2016, 66, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Scerbo, T.; Colasurdo, J.; Dunn, S.; Unger, J.; Nijs, J.; Cook, C. Measurement properties of the central sensitization inventory: A systematic review. Pain Pract. 2018, 18, 544–554. [Google Scholar] [CrossRef]
- Vandelanotte, C.; De Bourdeaudhuij, I.; Philippaerts, R.; Sjöström, M.; Sallis, J. Reliability and validity of a computerized and Dutch version of the International Physical Activity Questionnaire (IPAQ). J. Phys. Acta Health 2005, 2, 63–75. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Muller, M.; Cohen, P.D.; Essink-Bot, M.-L.; Fekkes, M.; Sanderman, R.; Sprangers, M.A.; Velde, A.t.; Verrips, E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J. Clin. Epidemiol. 1998, 51, 1055–1068. [Google Scholar] [CrossRef]
- Heuch, I.; Heuch, I.; Hagen, K.; Sørgjerd, E.P.; Åsvold, B.O.; Zwart, J.A. Is chronic low back pain a risk factor for diabetes? The Nord-Trøndelag Health Study. BMJ Open Diabetes Res. Care 2018, 6, e000569. [Google Scholar] [CrossRef] [PubMed]
- Mäntyselkä, P.; Miettola, J.; Niskanen, L.; Kumpusalo, E. Glucose regulation and chronic pain at multiple sites. Rheumatology 2008, 47, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Sun, C.; Rong, P.; Li, S.; McCabe, M.F.; Wang, X.; Mao, J.; Wang, S. A correlative relationship between chronic pain and insulin resistance in Zucker fatty rats: Role of downregulation of insulin receptors. J. Pain 2016, 17, 404–413. [Google Scholar] [CrossRef]
- Pain, M.; Wasilla, A.; Pappolla, M.A. Insulin resistance is associated with central pain in patients with fibromyalgia. Pain Physician 2021, 24, 175–184. [Google Scholar]
- O’rahilly, S.; Gray, H.; Hattersley, A.; Vaag, A. Insulin resistance as the major cause of impaired glucose tolerance: A self-fulfilling prophesy? Lancet 1994, 344, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Ferrannini, E.; Miyazaki, Y.; Matsuda, M.; DeFronzo, R.A. Beta-cell dysfunction and glucose intolerance: Results from the San Antonio metabolism (SAM) study. Diabetologia 2004, 47, 31–39. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Williams, K.; DeFronzo, R.; Stern, M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006, 29, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group; European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-h diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: An integrative approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef]
- Ye, D.; Fairchild, T.J.; Vo, L.; Drummond, P.D. High Blood Glucose and Excess Body fat Enhance Pain Sensitivity and Weaken Pain Inhibition in Healthy Adults: A Single-blind Cross-over Randomized Controlled Trial. J. Pain 2023, 24, 128–144. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Gavin, J.R., III. Pathophysiologic mechanisms of postprandial hyperglycemia. Am. J. Cardiol. 2001, 88, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Elma, Ö.; Lebuf, E.; Marnef, A.Q.; Yilmaz, S.T.; Coppieters, I.; Clarys, P.; Nijs, J.; Malfliet, A.; Deliens, T. Diet can exert both analgesic and pronociceptive effects in acute and chronic pain models: A systematic review of preclinical studies. Nutr. Neurosci. 2021, 25, 2195–2217. [Google Scholar] [CrossRef] [PubMed]
- Arkkila, P.E.; Gautier, J.-F. Musculoskeletal disorders in diabetes mellitus: An update. Best Pract. Res. Clin. Rheumatol. 2003, 17, 945–970. [Google Scholar] [CrossRef]
- Fields, A.J.; Berg-Johansen, B.; Metz, L.N.; Miller, S.; La, B.; Liebenberg, E.C.; Coughlin, D.G.; Graham, J.L.; Stanhope, K.L.; Havel, P.J.; et al. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes. J. Orthop. Res. 2015, 33, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Bjorck-Van Dijken, C.; Fjellman-Wiklund, A.; Hildingsson, C. Low back pain, lifestyle factors and physical activity: A population based-study. J. Rehabil. Med. 2008, 40, 864. [Google Scholar] [CrossRef]
- Venn, B.; Williams, S.; Mann, J. Comparison of postprandial glycaemia in Asians and Caucasians. Diabet. Med. 2010, 27, 1205–1208. [Google Scholar] [CrossRef]

| Pain Group (n = 53) Mean (SD) | Healthy Group (n = 53) Mean (SD) | Effect Size (Cohen’s d) | p-Value | |
|---|---|---|---|---|
| Age (years) | 37.4 (12.8) | 34.1 (9.8) | 0.292 | 0.136 |
| Weight (kg) | 72.3 (15.8) | 69.7 (12.1) | 0.187 | 0.338 |
| Height (cm) | 163.5 (6.0) | 164.6 (7.1) | −0.153 | 0.433 |
| BMI (kg/m2) | 27.1 (6.1) | 25.8 (4.9) | 0.225 | 0.249 |
| Body fat mass% | 32.7 (7.1) | 31.8 (6.8) | 0.140 | 0.472 |
| Body muscle mass % | 64.0 (6.6) | 64.6 (6.3) | −0.094 | 0.630 |
| Body water mass % | 48.2 (5.2) | 48.8 (4.9) | −0.120 | 0.537 |
| SF-36—PF | 70.8 (20.7) | 89.4 (13.0) | −1.076 | <0.001 * |
| SF-36—RF | 56.6 (40.5) | 86.3 (26.2) | −0.872 | <0.001 * |
| SF-36—SF | 70.0 (25.9) | 77.2 (21.9) | −0.303 | 0.122 |
| SF-36—EH | 57.2 (43.1) | 66.6 (39.3) | −0.228 | 0.243 |
| SF-36—BP | 51.0 (23.9) | 78.4 (20.8) | −1.224 | <0.001 * |
| SF-36—MH | 61.6 (20.4) | 69.7 (15.6) | −0.445 | 0.024 * |
| SF-36—V | 48.8 (20.4) | 59.1 (17.7) | −0.538 | 0.007 * |
| SF-36—GH | 52.7 (21.4) | 65.8 (15.6) | −0.700 | <0.001 * |
| IPAQ Low (min/week) | 2747.4 (6000.1) | 1620.7 (2754.3) | 0.241 | 0.217 |
| IPAQ Moderate (min/week) | 2416.8 (2674.9) | 1315.0 (1060.3) | 0.542 | 0.006 * |
| IPAQ Vigorous (min/week) | 1071.7 (1769.8) | 588.7 (949.9) | 0.340 | 0.083 |
| IPAQ Total (min/week) | 4643.8 (3894.4) | 3589.6 (3659.3) | 0.279 | 0.154 |
| Mean Fasting Blood Glucose Level (mg/dL) | 95.9 (8.4) | 96.8 (7.8) | −0.104 | 0.594 |
| Mean 2 h Blood Glucose Level (mg/dL) | 96.7 (11.0) | 98.3 (9.7) | −0.160 | 0.411 |
| EDT | 3.3 (0.6) | 3.2 (0.5) | 0.200 | 0.305 |
| EPT | 9.9 (3.9) | 8.9 (3.2) | 0.281 | 0.151 |
| TS | 22.5 (17.5) | 23.0 (17.7) | −0.029 | 0.882 |
| OA | 6.0 (15.0) | 11.4 (15.4) | −0.354 | 0.072 |
| PPT—LBP | 6.6 (2.1) | 7.8 (3.1) | −0.470 | 0.017 * |
| PPT—TA | 7.0 (2.0) | 7.5 (2.7) | −0.223 | 0.254 |
| CSI | 42.0 (14.2) | 27.2 (12.4) | 1.110 | <0.001 * |
| BPI—Severity | 3.3 (1.9) | 0 (0) | 2.422 | <0.001 * |
| -Worst pain during the last 24 h | 4.7 (2.6) | 0 (0) | 2.510 | <0.001 * |
| -Least pain during the last 24 h | 2.0 (1.8) | 0 (0) | 1.536 | <0.001 * |
| -Pain now | 2.8 (2.4) | 0 (0) | 1.608 | <0.001 * |
| -Average pain during the last 24 h | 3.5 (2.0) | 0 (0) | 2.537 | <0.001 * |
| BPI—Interference | 3.1 (2.4) | 0 (0) | 1.839 | <0.001 * |
| -General activity | 3.8 (3.6) | 0 (0) | 1.692 | <0.001 * |
| -Mood | 3.9 (3.2) | 0 (0) | 1.723 | <0.001 * |
| -Walking ability | 2.8 (2.8) | 0 (0) | 1.409 | <0.001 * |
| -Normal work | 2.3 (2.6) | 0 (0) | 1.255 | <0.001 * |
| -Relationships with others | 2.3 (2.6) | 0 (0) | 1.253 | <0.001 * |
| -Sleep | 3.3 (3.1) | 0 (0) | 1.518 | <0.001 * |
| -Enjoyment of life | 3.4 (3.4) | 0 (0) | 1.427 | <0.001 * |
| Sucrose Mean (SD) | Isomaltulose Mean (SD) | Effect Size (Cohen’s d) | p-Value | |
|---|---|---|---|---|
| Chronic Low Back Pain (min × mg/dL) (n = 53) | 3470.4 (1524.7) | 2049.3 (942.0) | 0.959 | <0.001 * |
| Healthy Controls (min × mg/dL) (n = 53) | 2854.9 (1147.9) | 2011.1 (864.3) | 0.628 | <0.001 * |
| Pain Group (n = 53) Mean (SD) | Healthy Group (n = 53) Mean (SD) | Effect Size (Cohen’s d) | p-Value | |
|---|---|---|---|---|
| Sucrose (min × mg/dL) | 3470.4 (1524.7) | 2854.9 (1147.9) | 0.456 | 0.021 * |
| Isomaltulose (min × mg/dL) | 2049.3 (942.0) | 2011.1 (864.3) | 0.420 | 0.828 |
| Difference (min × mg/dL) | 1379.7(1374.7) | 844.3 (1344.3) | 0.394 | 0.045 * |
| Sucrose (n = 53) | Isomaltulose (n = 53) | Difference (n = 53) | |
|---|---|---|---|
| EDT | −0.016 | 0.042 | −0.022 |
| EPT | 0.070 | −0.021 | 0.063 |
| TS | 0.151 | −0.088 | 0.194 |
| OA | −0.188 | −0.013 | −0.208 |
| PPT—LB | −0.034 | −0.075 | −0.007 |
| PPT—TA | −0.134 | −0.241 | −0.006 |
| CSI | −0.171 | −0.142 | 0.124 |
| BPI—Severity | 0.023 | 0.003 | 0.033 |
| -Worst pain during the last 24 h | 0.188 | −0.99 | 0.238 |
| -Least pain during the last 24 h | 0.300 | −0.114 | 0.378 |
| -Pain now | 0.234 | −0.108 | 0.281 |
| -Average Pain during the last 24 h | 0.394 | −0.043 | 0.413 |
| BPI—Interference | 0.147 | −0.169 | 0.059 |
| -General activity | 0.296 | 0.110 | 0.260 |
| -Mood | 0.403 | 0.118 | 0.347 |
| -Walking ability | 0.134 | −0.039 | 0.185 |
| -Normal work | 0.117 | 0.188 | 0.015 |
| -Relationships with others | 0.257 | 0.065 | 0.244 |
| -Sleep | 0.320 | 0.089 | 0.296 |
| -Enjoyment of Life | 0.269 | 0.048 | 0.269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elma, Ö.; Tümkaya Yılmaz, S.; Nijs, J.; Clarys, P.; Coppieters, I.; Mertens, E.; Malfliet, A.; Deliens, T. Impaired Carbohydrate Metabolism among Women with Chronic Low Back Pain and the Role of Dietary Carbohydrates: A Randomized Controlled Cross-Over Experiment. J. Clin. Med. 2024, 13, 2155. https://doi.org/10.3390/jcm13072155
Elma Ö, Tümkaya Yılmaz S, Nijs J, Clarys P, Coppieters I, Mertens E, Malfliet A, Deliens T. Impaired Carbohydrate Metabolism among Women with Chronic Low Back Pain and the Role of Dietary Carbohydrates: A Randomized Controlled Cross-Over Experiment. Journal of Clinical Medicine. 2024; 13(7):2155. https://doi.org/10.3390/jcm13072155
Chicago/Turabian StyleElma, Ömer, Sevilay Tümkaya Yılmaz, Jo Nijs, Peter Clarys, Iris Coppieters, Evelien Mertens, Anneleen Malfliet, and Tom Deliens. 2024. "Impaired Carbohydrate Metabolism among Women with Chronic Low Back Pain and the Role of Dietary Carbohydrates: A Randomized Controlled Cross-Over Experiment" Journal of Clinical Medicine 13, no. 7: 2155. https://doi.org/10.3390/jcm13072155






