Nationwide Screening Practices for Tamoxifen Retinal Toxicity in South Korea: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
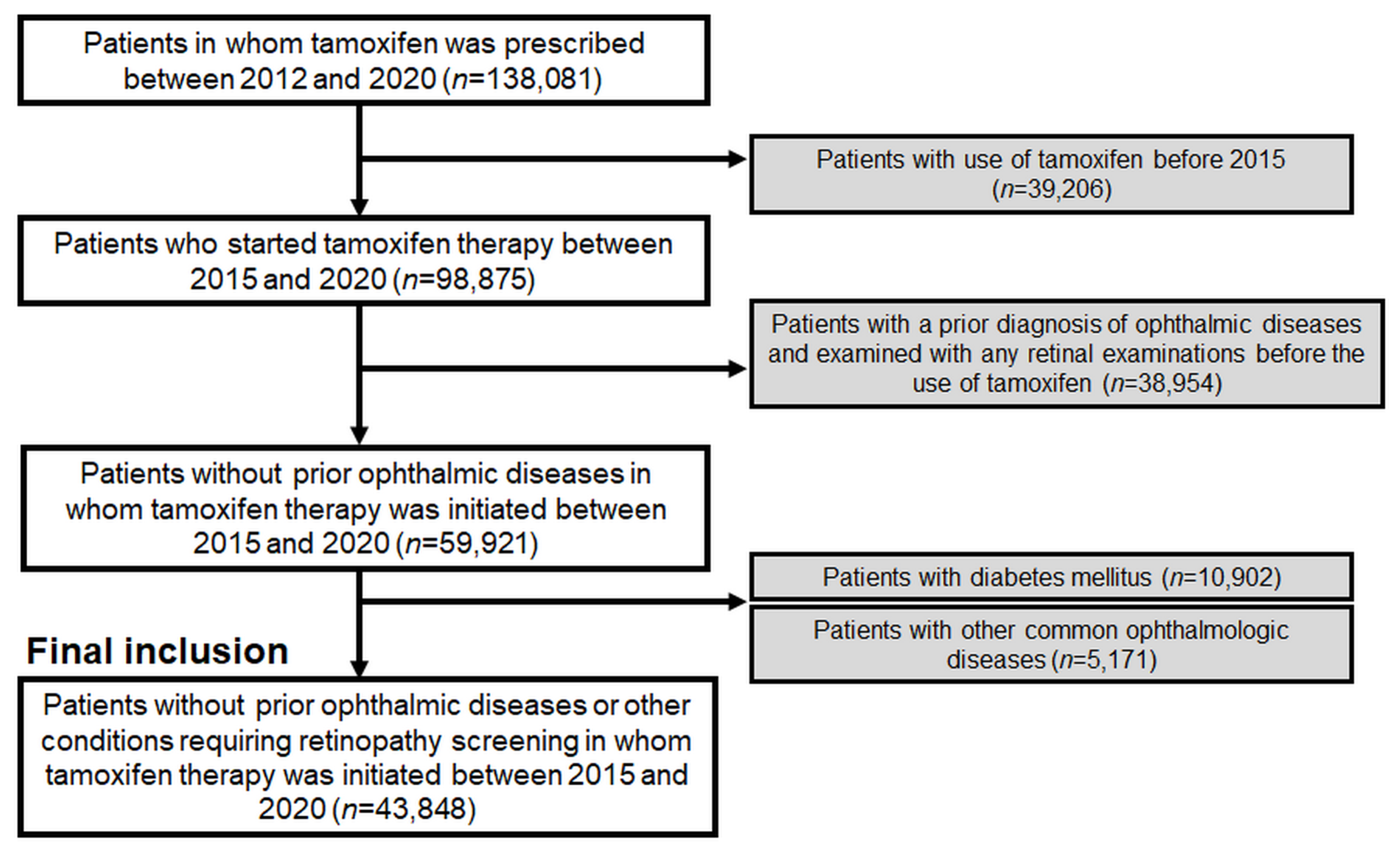
2.1. Study Population
2.2. Definitions and Evaluations
2.3. Data Analysis
3. Results
3.1. Population of Tamoxifen Users and Trends over Time
3.2. Performance, Timing, and Modalities of Baseline and Subsequent Monitoring Examinations
3.3. Trends of Retinopathy Screening among Tamoxifen Users over the Study Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazvand, F.; Mahdizad, Z.; Mohammadi, N.; Shahi, F.; Mirghorbani, M.; Riazi-Esfahani, H.; Modjtahedi, B.S. Tamoxifen retinopathy. Surv. Ophthalmol. 2023, 68, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Lee, S.; Eah, K.S.; Yoon, Y.H. Prevalence and Risk Factors of Tamoxifen Retinopathy. Ophthalmology 2020, 127, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer. Br. J. Pharmacol. 1993, 110, 507–517. [Google Scholar] [CrossRef]
- Vogel, V.G.; Costantino, J.P.; Wickerham, D.L.; Cronin, W.M.; Cecchini, R.S.; Atkins, J.N.; Bevers, T.B.; Fehrenbacher, L.; Pajon, E.R., Jr.; Wade, J.L., 3rd; et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006, 295, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Kaiser-Kupfer, M.I.; Kupfer, C.; Rodrigues, M.M. Tamoxifen retinopathy. A clinicopathologic report. Ophthalmology 1981, 88, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Kaiser-Kupfer, M.I.; Lippman, M.E. Tamoxifen retinopathy. Cancer Treat. Rep. 1978, 62, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Mauget-Faysse, M.; Gambrelle, J.; Quaranta-El Maftouhi, M. Optical coherence tomography in tamoxifen retinopathy. Breast Cancer Res. Treat. 2006, 99, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.E.; Kim, J.H.; Ahn, S.J. Practice Patterns of Screening for Hydroxychloroquine Retinopathy in South Korea. JAMA Netw. Open 2023, 6, e2314816. [Google Scholar] [CrossRef]
- Moir, J.; Amin, S.V.; Khanna, S.; Komati, R.; Shaw, L.T.; Dao, D.; Hariprasad, S.M.; Skondra, D. Use of OCT Angiography to Diagnose and Manage Atypical Presentations of Macular Telangiectasia Type 2. Int. J. Mol. Sci. 2022, 23, 7849. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, S.; Yoon, Y.H. One-year follow-up of optical coherence tomography angiography microvascular findings: Macular telangiectasia type 2 versus tamoxifen retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3479–3488. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.A.; Yoon, Y.H. OCT Angiography Findings of Tamoxifen Retinopathy: Similarity with Macular Telangiectasia Type 2. Ophthalmol. Retin. 2019, 3, 681–689. [Google Scholar] [CrossRef]
- Kovach, J.L.; Isildak, H.; Sarraf, D. Crystalline retinopathy: Unifying pathogenic pathways of disease. Surv. Ophthalmol. 2019, 64, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Charbel Issa, P.; Gillies, M.C.; Chew, E.Y.; Bird, A.C.; Heeren, T.F.; Peto, T.; Holz, F.G.; Scholl, H.P. Macular telangiectasia type 2. Prog. Retin. Eye Res. 2013, 34, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L.; Zhang, Y.; Ma, W.; Gonzalez, S.R.; Fan, J.; Kretschmer, F.; Badea, T.C.; Qian, H.H.; Wong, W.T. Tamoxifen Provides Structural and Functional Rescue in Murine Models of Photoreceptor Degeneration. J. Neurosci. 2017, 37, 3294–3310. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi, S.; Kandemir Gursel, O.; Cakir, A.; Erden, B.; Karatas, G. Retinal structural changes in patients receiving tamoxifen therapy by spectral-domain optical coherence tomography. Cutan. Ocul. Toxicol. 2020, 39, 115–121. [Google Scholar] [CrossRef]
- Crisostomo, S.; Vieira, L.; Cardigos, J.; Fernandes, D.H.; Luis, M.E.; Nunes, S.; Morujao, I.; Anjos, R.; Flores, R. TAMOXIFEN-INDUCED CHORIORETINAL CHANGES: An Optical Coherence Tomography and Optical Coherence Tomography Angiography Study. Retina 2020, 40, 1185–1190. [Google Scholar] [CrossRef]
- Bicer, T.; Imamoglu, G.I.; Caliskan, S.; Bicer, B.K.; Gurdal, C. The Effects of Adjuvant Tamoxifen Use on Macula Pigment Epithelium Optical Density, Visual Acuity and Retinal Thickness in Patients with Breast Cancer. Curr. Eye Res. 2020, 45, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Gorin, M.B.; Day, R.; Costantino, J.P.; Fisher, B.; Redmond, C.K.; Wickerham, L.; Gomolin, J.E.; Margolese, R.G.; Mathen, M.K.; Bowman, D.M.; et al. Long-term tamoxifen citrate use and potential ocular toxicity. Am. J. Ophthalmol. 1998, 125, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.R.; Fortun, J.A.; Kim, B.T.; Dubovy, S.R.; Rosenfeld, P.J. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am. J. Ophthalmol. 2014, 157, 1291–1298.e3. [Google Scholar] [CrossRef]
- Gualino, V.; Cohen, S.Y.; Delyfer, M.N.; Sahel, J.A.; Gaudric, A. Optical coherence tomography findings in tamoxifen retinopathy. Am. J. Ophthalmol. 2005, 140, 757–758. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.H.; Foot, B.; Lotery, A.J. The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): Executive summary. Eye 2021, 35, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.W.; Wheatley, H.M. Intravitreal triamcinolone acetonide treatment of tamoxifen maculopathy with associated cystoid macular edema. Retin. Cases Brief. Rep. 2015, 9, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiao, J.; Zou, H.; Yang, B.; Luo, L. The response of anti-VEGF therapy and tamoxifen withdrawal of tamoxifen-induced cystoid macular edema in the same patient. BMC Ophthalmol. 2021, 21, 201. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.Y.; Kim, J.H.; Ahn, S.J. Nationwide Usage of Pentosan Polysulfate and Practice Patterns of Pentosan Polysulfate Maculopathy Screening in South Korea. Ophthalmol. Retin. 2024, 8, 246–253. [Google Scholar] [CrossRef]
| Characteristics | Overall Users (n = 43,848) |
|---|---|
| Sex | |
| Male | 5003 (11.4%) |
| Female | 38,845 (88.6%) |
| Mean age (±SD), years | 45.0 ± 9.7 |
| <20 | 688 (1.6%) |
| 20–29 | 1860 (4.2%) |
| 30–39 | 6968 (15.9%) |
| 40–49 | 23,667 (54.0%) |
| 50–59 | 7903 (18.0%) |
| 60–69 | 1945 (4.4%) |
| ≥70 | 817 (1.9%) |
| Indication for tamoxifen use | |
| Breast cancer | 30,629 (69.9%) |
| Ductal carcinoma in situ | 7744 (17.7%) |
| Gynecomastia | 4606 (10.5%) |
| Others | 869 (2.0%) |
| Mean duration of tamoxifen use (±SD), months | 36.0 ± 21.8 |
| Less than 1 year | 7858 (17.9%) |
| 1–2 years | 6287 (14.3%) |
| 2–3 years | 7857 (17.9%) |
| 3–4 years | 6849 (15.6%) |
| 4–5 years | 8715 (19.9%) |
| 5 years or longer | 6282 (14.3%) |
| Mean daily dose of tamoxifen (±SD), mg/day | 20.0 ± 3.0 |
| Less than 15 mg | 1064 (2.4%) |
| 15–20 mg | 808 (1.8%) |
| 20–25 mg | 41,261 (94.1%) |
| 25 mg or greater | 715 (1.6%) |
| Year | Total Number of Patients Using Tamoxifen (% among Entire Korean Population in Each Year †) | Annual Number of Patients Who Initiated Tamoxifen Therapy (% among Korean Population †) |
|---|---|---|
| 2015 | 54,056 (0.106%) | 14,065 (0.028%) |
| 2016 | 59,426 (0.116%) | 15,690 (0.031%) |
| 2017 | 64,760 (0.126%) | 16,689 (0.032%) |
| 2018 | 69,957 (0.136%) | 17,272 (0.033%) |
| 2019 | 74,860 (0.145%) | 17,990 (0.035%) |
| 2020 | 77,943 (0.150%) | 17,169 (0.033%) |
| 2021 | 81,720 (0.158%) | 18,012 (0.035%) |
| Characteristics | Value |
|---|---|
| Timing | |
| No. of patients receiving any ophthalmic examination after tamoxifen use/No. of users (%) | 8961/43,848 (20.4%) |
| No. of patients receiving ophthalmic examination within 1 year of tamoxifen use/No. of users (%) | 2836/43,848 (6.5%) |
| No. of patients receiving any subsequent monitoring examination/No. of patients receiving baseline screening (%) | 2492/8961 (27.8%) |
| No. of monitoring examinations per year after baseline ones, numbers/year | 0.68 ± 0.45 |
| Timing of the baseline examination since tamoxifen use, median (Q1–Q3), days | 645 (280–1161) |
| Mean/median (Q1–Q3) interval between baseline examination and 1st monitoring exam, months | 12.5 ± 14.2/7.1 (1.2–19.3) |
| Mean/median (Q1–Q3) interval of monitoring between 1st and 2nd monitoring exam, months | 6.9 ± 8.7/3.4 (0.7–10.1) |
| Mean/median (Q1–Q3) interval of monitoring between 2nd and 3rd monitoring exam, months | 5.8 ± 8.4/2.8 (0.6–7.1) |
| Modalities used Funduscopy/fundus photography Optical coherence tomography Automated visual fields Fundus autofluorescence Fluorescein angiography Others | Baseline/Monitoring (%) 8873 (99.0%)/2458 (98.6%) 1960 (21.9%)/738 (29.6%) 602 (6.7%)/205 (8.2%) 253 (2.8%)/138 (5.5%) 70 (0.8%)/52 (2.1%) 270 (3.0%)/162 (6.5%) |
| Year | Baseline Examination within 1 Year | Monitoring | |
|---|---|---|---|
| Examined within 6 Months from Baseline Exam (% among Those with Baseline) | Examined within 1 Year from Baseline Exam (% among Those with Baseline) | ||
| 2015 | 290 (4.6%) | 210 (11.4%) | 286 (16.3%) |
| 2016 | 411 (5.7%) | 252 (13.4%) | 358 (19.0%) |
| 2017 | 453 (6.0%) | 225 (12.9%) | 302 (17.4%) |
| 2018 | 524 (6.9%) | 196 (13.2%) | 268 (18.0%) |
| 2019 | 585 (7.4%) | 150 (11.7%) | 221 (17.3%) |
| 2020 | 573 (7.7%) | 102 (12.5%) | 131 (16.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.J.; Kim, J.; Kwon, H.Y. Nationwide Screening Practices for Tamoxifen Retinal Toxicity in South Korea: A Population-Based Cohort Study. J. Clin. Med. 2024, 13, 2167. https://doi.org/10.3390/jcm13082167
Ahn SJ, Kim J, Kwon HY. Nationwide Screening Practices for Tamoxifen Retinal Toxicity in South Korea: A Population-Based Cohort Study. Journal of Clinical Medicine. 2024; 13(8):2167. https://doi.org/10.3390/jcm13082167
Chicago/Turabian StyleAhn, Seong Joon, Jiyeong Kim, and Hyeon Yoon Kwon. 2024. "Nationwide Screening Practices for Tamoxifen Retinal Toxicity in South Korea: A Population-Based Cohort Study" Journal of Clinical Medicine 13, no. 8: 2167. https://doi.org/10.3390/jcm13082167
APA StyleAhn, S. J., Kim, J., & Kwon, H. Y. (2024). Nationwide Screening Practices for Tamoxifen Retinal Toxicity in South Korea: A Population-Based Cohort Study. Journal of Clinical Medicine, 13(8), 2167. https://doi.org/10.3390/jcm13082167







