Abstract
Multi-system inflammatory syndrome in children (MIS-C) in the setting of COVID-19 can be associated with severe cardiopulmonary dysfunction. This clinical deterioration may sometimes necessitate veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support. We describe an algorithmic approach including the role of balloon atrial septostomy in this cohort. This is the first reported series of percutaneous VA-ECMO in pediatric patients with MIS-C for better outcomes. The lessons from this approach can be replicated in other pediatric clinical conditions and adds to the armament of multiple pediatric specialties.
1. Introduction
Coronavirus Disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Initial reports suggested that this disease was mild in children and the majority were asymptomatic [1]. However, in April 2020, the first reports in children of a hyperinflammatory syndrome hemodynamically characterized by shock associated with being positive for COVID-19 came to light [2]. The clinical features appeared to be similar to atypical or incomplete Kawasaki disease, toxic shock syndrome, or bacterial sepsis. In addition to the hyperinflammatory state, this syndrome was characterized by injury to multiple organ systems including the heart [3]. The United States Centers for Disease Control and Prevention named this multisystem inflammatory syndrome in children (MIS-C) [4]. MIS-C secondary to COVID-19 has been shown to be associated with significant morbidity and prolonged intensive care admission [5]. From the time of its initial description until March 2022, more than 7880 reported cases of MIS-C have been described throughout US, resulting at least 66 known deaths [6]. Hemodynamic and cardiopulmonary decompensation can occur rapidly in these children if not identified and managed in a timely fashion. Extra-corporeal membrane oxygenation (ECMO) is employed to support patients with severe cardiopulmonary failure to avoid irreversible organ injury. A few cases of veno-arterial (VA) ECMO using open surgical cannulation for severe MIS-C patients presenting in cardiogenic shock have been performed, [7,8,9] including at our center, with variable outcomes.
At our institution, we adopted a protocol for COVID-19 [10] and MIS-C patients [11]. We also instituted a protocol for the cohort presenting in cardiogenic shock. Time-saving measures including percutaneous VA-ECMO cannulation, followed by immediate balloon atrial septostomy (BAS), were instituted in children with MIS-C based on clinical presentation, a left ventricular ejection fraction (LVEF) <25% by echocardiography, and a vasoactive inotropic score (VIS) >25. We describe the outcomes of five pediatric patients with MIS-C complicated by severe cardiopulmonary dysfunction who required VA-ECMO support from a cohort of 162 patients admitted to the hospital between April 2020 and October 2021.
2. Case Presentation
All five patients presented with symptoms and physical exam findings consistent with heart failure or respiratory failure secondary to MIS-C in the setting of recent COVID-19 exposure and positive COVID-19 antibodies. The demographics, presenting symptoms, echocardiographic findings, and outcomes of these patients are presented in the Table 1. All patients had elevated inflammatory markers, liver enzymes, troponin, BNP, and D-dimer. They were treated with corticosteroids and IVIG for MIS-C. Echocardiograms prior to ECMO cannulation demonstrated severely decreased LVEF except for patients 1 and 2 who had diastolic dysfunction and respiratory failure. These five patients required mechanical ventilation secondary to LV dysfunction and pulmonary edema, refractory hypotension, and persistent metabolic acidosis requiring inotropic support.

Table 1.
Management and outcomes.
VA-ECMO cannulation was initiated in all five cases due to severe, persistent hypotension requiring multiple inotropic support with VIS >25 and LVEF <25%, or persistent metabolic acidosis leading to other end organ dysfunction. In the first patient, VA-ECMO cannulation was attempted using standard surgical cut-down with direct cannulation of the internal jugular and carotid arteries. However, the carotid artery developed severe vasospasm during the surgical dissection. Therefore, a cut-down was performed to cannulate the femoral artery instead. The patient developed acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT). The attempted carotid cannulation led to cerebral hemorrhage and neurologic complications leading to prolonged hospitalization for 123 days with the patient eventually being transferred to a rehabilitation facility. This patient did not undergo BAS, which prolonged the days on the ventilator; ultimately, they required tracheostomy for prolonged mechanical ventilation.
The second patient also underwent open surgical VA-ECMO cannulation of the common carotid artery and the internal jugular vein. This patient underwent immediate BAS, which improved the pulmonary edema by decompressing the left atrium. However, the cannulation time was prolonged (58 min), with the patient ultimately developing a stroke involving the middle cerebral artery territory as well as thrombosis of the right common carotid artery following ECMO decannulation 5 days later. This patient also developed neurologic sequela requiring transfer to a rehabilitation facility after 28 days of hospitalization. Based on the outcomes of these two patients, an institutional protocol was established to ensure time saving measures for better patient outcomes. This led to percutaneous VA-ECMO cannulation and immediate BAS in the next three patients.
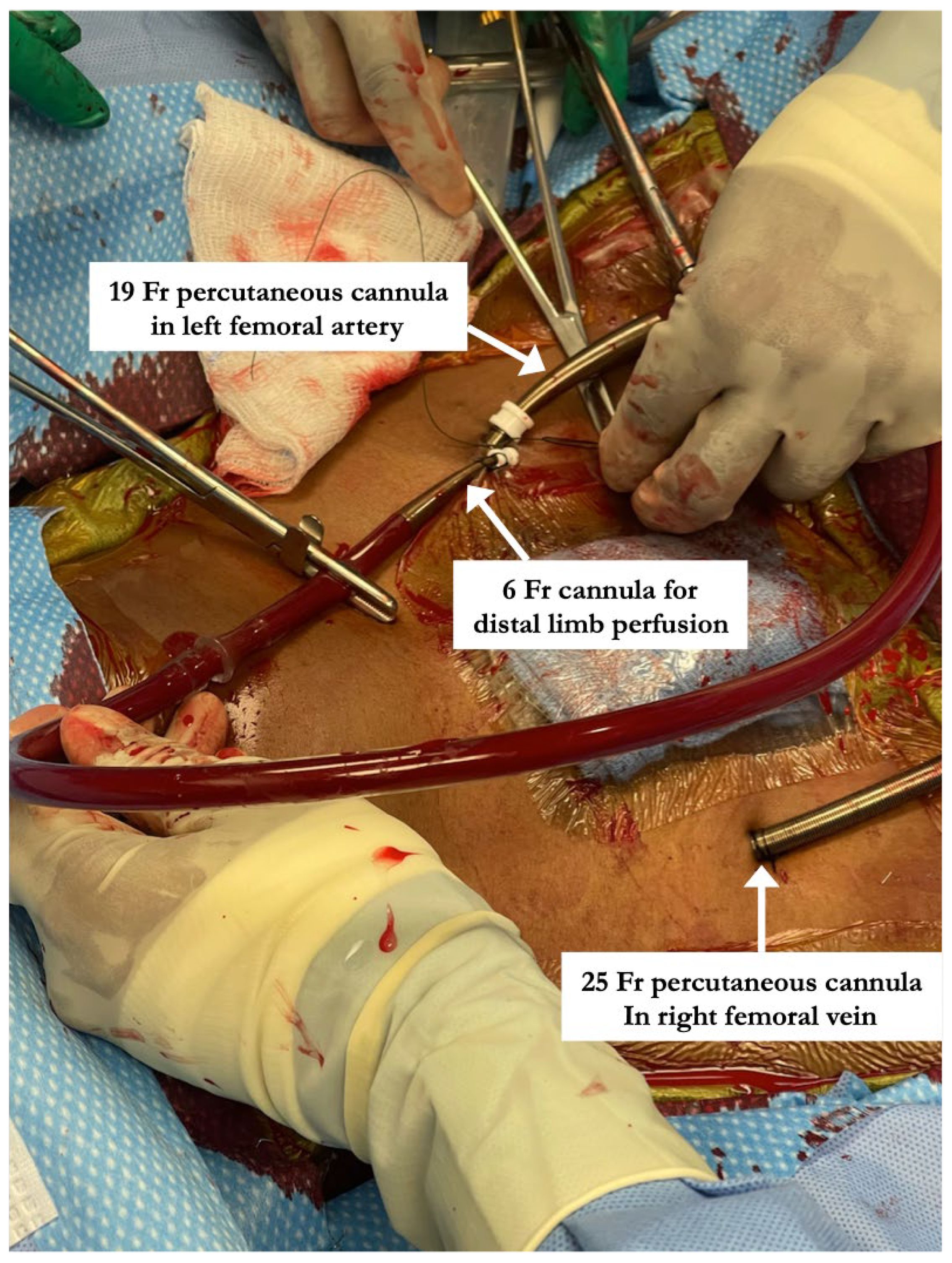
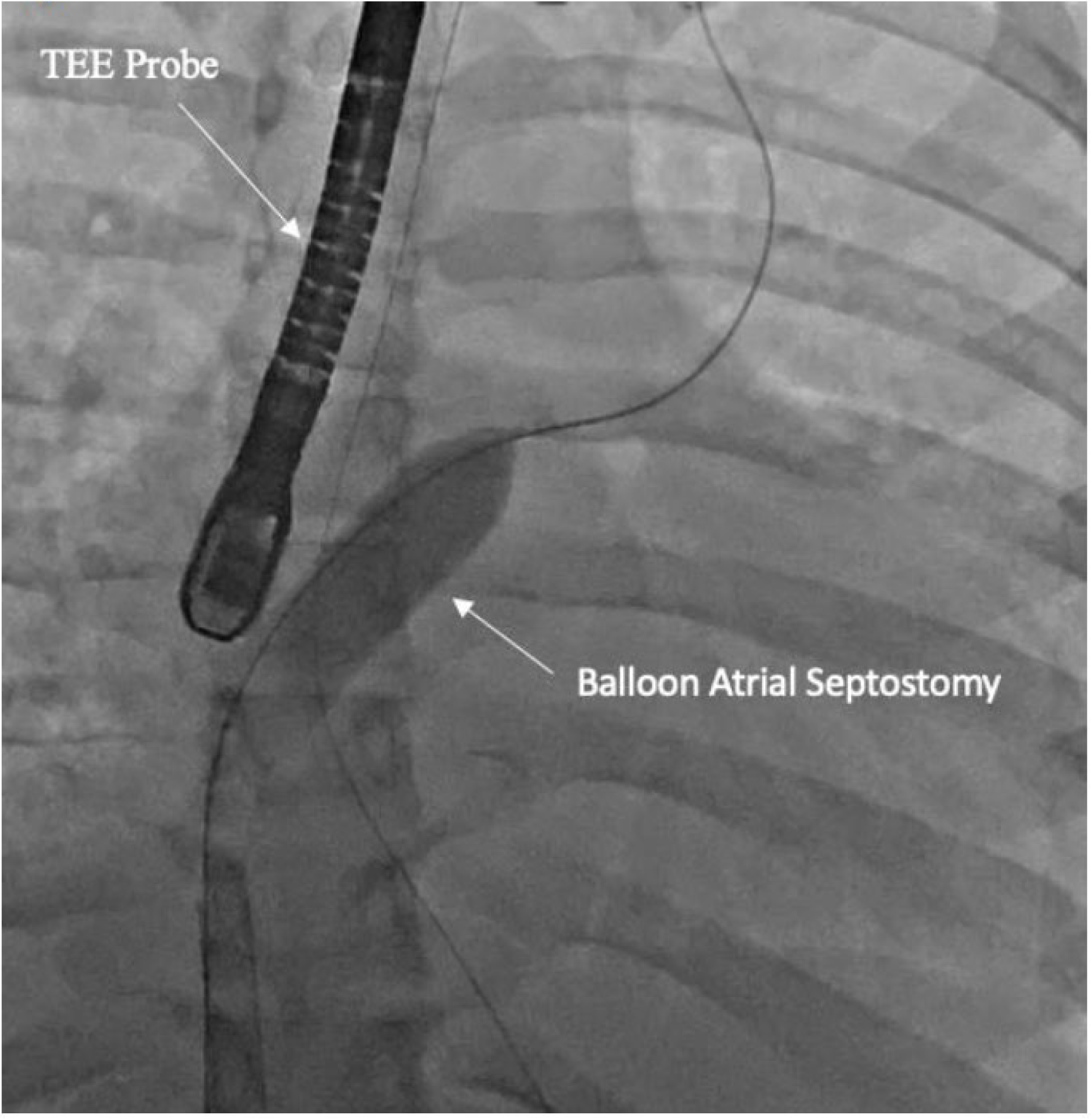
All percutaneous VA-ECMO cannulations were performed in the lower extremity vessels under ultrasound guidance with an additional limb reperfusion cannula attached to the arterial cannula (Figure 1). Figure 1 shows Patient 4 with a 25 Fr venous cannula in the right femoral vein. Insertion of a 19 Fr arterial cannula in the left femoral artery and a 6 Fr cannula for distal limb perfusion simultaneously. The time from ECMO activation to ECMO support was 13, 17 and 34 min, respectively, suggesting that percutaneous cannulation was achieved rapidly. All three patients who were cannulated percutaneously underwent BAS (Figure 2) within 2 h of ECMO support for immediate left atrial decompression. This was achieved through the percutaneous approach via transseptal puncture using real-time trans-esophageal echocardiography guidance. An atrial level communication was made using radio-frequency perforation of the atrial septum. The atrial level communication was slowly dilated using serial dilations with sequentially increasing balloon sizes. The adequacy of the atrial septum was then assessed by angiography and trans-esophageal echocardiography. There were no procedural complications, including no evidence for limb hypoperfusion. Patient 3 required CRRT for AKI, which developed even prior to cannulation. The number of days on ECMO was 3–8 days for these patients, with all of them transferred out of the ICU within 4 days after decannulation. All three patients were eventually discharged home with recovered cardiac function.
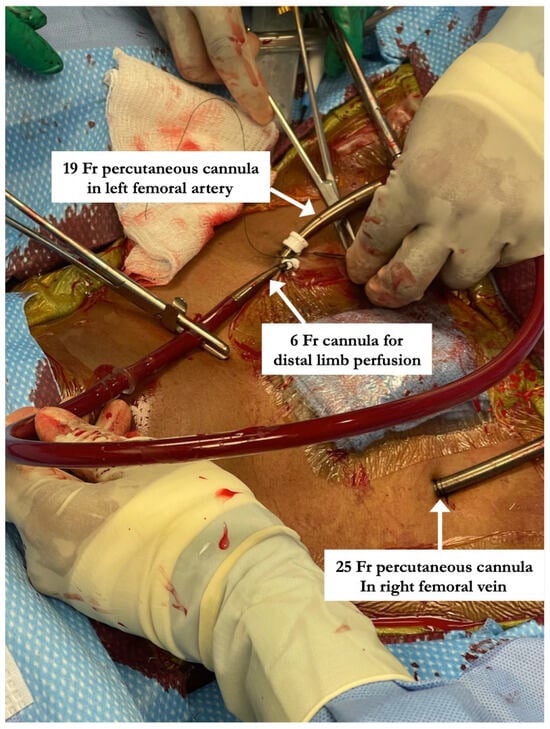
Figure 1.
Steps involved in percutaneous arterio-venous ECMO cannulation including placement of limb perfusion cannula. “Patient 4” with a 25 Fr venous cannula in the right femoral vein. Insertion of a 19 Fr arterial cannula in the left femoral artery and a 6 Fr cannula for distal limb perfusion simultaneously.
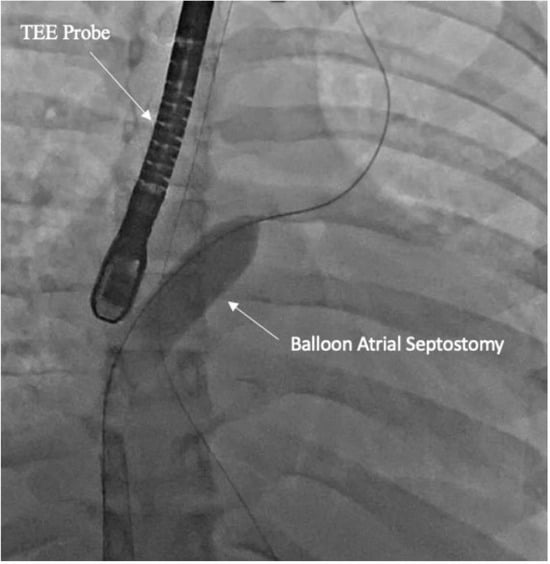
Figure 2.
Fluoroscopy showing balloon atrial septostomy for left atrial decompression. Transesophageal (TEE) probe is seen for echo-guidance.
3. Discussion
This series is the first report of the successful use of percutaneous VA-ECMO to support children developing cardiorespiratory collapse from MIS-C in the setting of COVID-19. This report emphasizes the rare but serious consequences of severe rapid clinical deterioration associated with COVID-19 in children. In the cohort of 162 patients, 5 needed ECMO support. Of the 157 that did not need ECMO, there were patients with depressed cardiac function that improved with medical therapy such as IVIG and steroids. What distinguished these five patients from the rest of the cohort was the rapid clinical and cardiopulmonary deterioration that needed emergency support. This report highlights the life-saving role of VA-ECMO in these patients while anticipating reversal of the inflammatory response at a time when there was a shortage of resources in terms of medical personnel. Time saving measures including percutaneous VA-ECMO cannulation along with emergency BAS were the key features that improved outcomes for these children at least in our hospital.
Open surgical VA-ECMO cannulation is the current standard in children who have very limited experience with percutaneous cannulation [12]. However, with availability of smaller percutaneous cannulas that can be advanced over a guidewire, the percutaneous approach to VA-ECMO cannulation is appealing. Percutaneous VA-ECMO could potentially offer faster cannulation and uninterrupted CPR during cannulation in addition to increasing accessibility to more providers (interventional cardiologists, radiologists, and intensivists), especially when a surgeon is not available for cannulation. Obese children are more commonly affected by MIS-C, which makes the percutaneous route with ultrasound guidance a good alternative to open cannulation from a technique perspective for these patients.
With severe shock and cardiovascular collapse, identification of blood vessels for cannulation is challenging. The use of ultrasound for access of vessels in the cardiac catheterization laboratory has significantly improved the time taken for and the safety profile of obtaining arterial and venous access [13]. Early recognition of the potential need for ECMO helps with a more efficient cannulation process. There is a lower incidence of cannula site bleeding with the percutaneous route compared to open cannulation, possibly due to the larger incision required for open cannulation [14]. For similar reasons, there is a higher rate of vascular site repair with open surgical cannulation [15]. Complications from blood clots are also more common when ECMO is achieved through the open surgical route in comparison to the percutaneous route [16]. Patients with MIS-C are already at a higher risk of thrombotic complications especially with left ventricular dysfunction and coronary artery aneurysms [17,18,19]. Tailored anti-coagulation strategies have been suggested by groups when there is significant left ventricular dysfunction and coronary artery aneurysms. The degree of D-dimer elevation has been used by groups to determine the need for anti-coagulation [20]. This can further complicate anti-thrombotic strategies whilst on ECMO. Although cannula site bleeding and thrombotic complications are likely to be less common with percutaneous cannulation in comparison to open cannulation, concerns about limb ischemia remain [21]. Limb ischemia could be avoided by introducing a distal limb perfusion cannula at the time of the arterial cannulation. It is almost impossible to insert the perfusion cannula after femoral arterial cannulation; this must be accomplished simultaneously. We achieved this by introducing two guide wires concurrently into the femoral artery, one passing towards the heart and another towards the feet, to synchronously introduce the femoral arterial and the distal limb perfusion cannulas at the same time. With continued technical evolution and advances in technology, adoption of percutaneous VA-ECMO in pediatrics should be feasible and complications can be minimized.
Immediate BAS after VA-ECMO cannulation must be considered to decompress the left atrium to prevent or treat the flash pulmonary edema that develops with rising left ventricular end diastolic pressure [22]. This may preserve lung compliance and can potentially decrease the number of days on ECMO, thereby improving survival. One might argue that we could have attempted BAS prior to consideration of ECMO. This is also a good option when left ventricular end diastolic pressures are high. However, due to the rapid clinical deterioration in these patients, ECMO was carried out prior to BAS.
This report reiterates the fact that ECMO should be strongly considered in a small cohort of MIS-C patients since the inflammatory process is transient, as evidenced by the low average number of days on ECMO in this series. With newer COVID-19 variants on the horizon and vaccine adoption rates in the pediatric population being low, children continue to be the most vulnerable group in society. This is also of grave importance during a pandemic that has stretched medical resources in terms of the number of available ICU beds and staffing shortages [23]. These lessons from the pandemic can be extended to sepsis and other inflammatory conditions featuring cardiopulmonary compromise. This necessitates collaboration between ECMO providers, pediatric intensivists, interventional cardiologists, and pediatric and cardio-thoracic surgeons to provide accessibility and implement an organized, tailored, institutional approach that is essential for improving outcomes.
4. Conclusions
The use of percutaneous VA-ECMO in pediatric patients with MIS-C is feasible and a good alternative to open surgical ECMO. Immediate atrial septostomy for left atrial decompression should be considered for these patients. A programmatic approach to specific disease processes like MIS-C and possibly extension to sepsis and other inflammatory conditions is essential for good outcomes.
Author Contributions
Conceptualization: S.S., R.R.P., U.B. and K.R.; Methodology: J.N.J., A.M., C.S. and K.A.R.; Formal analysis, Investigation: R.R.P. and S.S.; Writing—original draft preparation: R.R.P.; writing—review and editing: A.M., J.N.J. and S.S.; supervision; S.S. and R.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of The University of Tennessee Health Science Center (protocol code 20-07741-XP, date of approval 25 October 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study. These were 5 patients from a larger retrospective cohort.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ECMO: extracorporeal membrane oxygenation; MIS-C: multisystem inflammatory syndrome in children.
References
- Centers for Disease Control and Prevention. United States COVID-19 Cases and Deaths by State. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html (accessed on 24 August 2020).
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; Center for Preparedness and Response. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19), Clinician Outreach and Communication (COCA) Webinar. 2020. Available online: https://emergency.cdc.gov/coca/calls/2020/callinfo_051920asp (accessed on 13 June 2020).
- Sperotto, F.; Friedman, K.G.; Son, M.B.F.; VanderPluym, C.J.; Newburger, J.W.; Dionne, A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur. J. Pediatr. 2021, 180, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC. Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 18 April 2022).
- Watanabe, A.; Yasuhara, J.; Karube, T.; Watanabe, K.; Shirasu, T.; Takagi, H.; Sumitomo, N.; Lee, S.; Kuno, T. Extracorporeal Membrane Oxygenation in Children With COVID-19: A Systematic Review and Meta-Analysis. Pediatr. Crit. Care Med. 2023, 24, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Aydin, S.I.; Derespina, K.R.; Bansal, P.B.; Kowalsky, S.; Trachtman, R.; Gillen, J.K.; Perez, M.M.; Soshnick, S.H.; Conway, E.E., Jr.; et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J. Pediatr. 2020, 224, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.P.; Walker, T.C.; Kihlstrom, M.; Isani, M.; Smith, M.M.; Smith, R.L.; McLean, S.E.; Clement, K.C.; Phillips, M.R. Extracorporeal Membrane Oxygenation for COVID-19-Associated Multisystem Inflammatory Syndrome in a 5-year-old. Am. Surg. 2022, 88, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.S.; Stephens, R.E.; Chang, J.V.; Amburn, J.M.; Pierotti, L.L.; Johnson, J.L.; Hyden, J.C.; Johnson, J.N.; Philip, R.R. Cardiovascular Evaluation After COVID-19 in 137 Collegiate Athletes: Results of an Algorithm-Guided Screening. Circulation 2021, 143, 1926–1928. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Johnson, J.N.; Spagnoli, J.; Amin, N.; Mccoy, M.; Swaminathan, N.; Yohannan, T.; Philip, R. Long-Term Cardiovascular Outcomes of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 Using an Institution Based Algorithm. Pediatr. Cardiol. 2023, 44, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Cairo, S.B.; Arbuthnot, M.; Boomer, L.; Dingeldein, M.W.; Feliz, A.; Gadepalli, S.; Newton, C.R.; Puligandla, P.; Ricca, R.; Rycus, P.; et al. Comparing Percutaneous to Open Access for Extracorporeal Membrane Oxygenation in Pediatric Respiratory Failure. Pediatr. Crit. Care Med. 2018, 19, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Yohannan, T.; Abutineh, I.; Agrawal, V.; Lloyd, H.; Zurakowski, D.; Waller, B.R.; Sathanandam, S. Ultrasound-guided femoral arterial access in pediatric cardiac catheterizations: A prospective evaluation of the prevalence, risk factors, and mechanism for acute loss of arterial pulse. Catheter. Cardiovasc. Interv. 2016, 88, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.M.; Hirose, H.; Cavarocchi, N.C. Preparation and Technical Considerations for Percutaneous Cannulation for Veno-Arterial Extracorporeal Membrane Oxygenation. J. Card. Surg. 2013, 28, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, A.; McGahren, E.D.; Rodgers, B.M. Effects of carotid artery repair following neonatal extracorporeal membrane oxygenation. Pediatr. Surg. Int. 2000, 16, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Schad, C.A.; Fallon, B.P.; Monteagudo, J.; Okochi, S.; Cheung, E.W.; Morrissey, N.J.; Kadenhe-Chiweshe, A.V.; Aspelund, G.; Stylianos, S.; Middlesworth, W. Routine Use of Distal Arterial Perfusion in Pediatric Femoral Venoarterial Extracorporeal Membrane Oxygenation. Artif. Organs 2017, 41, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, H.B.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Bokman, C.L.; Tashiro, J.; Perez, E.A.; Lasko, D.S.; Sola, J.E. Determinants of survival and resource utilization for pediatric extracorporeal membrane oxygenation in the United States 1997–2009. J. Pediatr. Surg. 2015, 50, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, M.; Meani, P.; Cotza, M.; Kowalewski, M.; Raffa, G.M.; Kuriscak, E.; Popkova, M.; Pilato, M.; Arcadipane, A.; Ranucci, M.; et al. Atrial Septostomy for Left Ventricular Unloading During Extracorporeal Membrane Oxygenation for Cardiogenic Shock: Animal Model. J. Am. Coll. Cardiol. Intv. 2021, 14, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Morray, B.H.; Gordon, B.M.; Crystal, M.A.; Goldstein, B.H.; Qureshi, A.M.; Torres, A.J.; Epstein, S.M.; Crittendon, I.; Ing, F.F.; Sathanandam, S.K. Resource Allocation and Decision Making for Pediatric and Congenital Cardiac Catheterization during the Novel Coronavirus SARS-CoV-2 (COVID-19) Pandemic: A U.S. Multi-Institutional Perspective. J. Invas. Cardiol. 2020, 32, E103–E109. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).