Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Screening and Selection
- -
- Health status ASA I;
- -
- Patients with periodontal disease: periodontitis stage I and II;
- -
- Topical agents with an action of biofilm disaggregation;
- -
- Primary outcomes: clinical outcomes of PPD (pocket probing depth), CAL (clinical attachment level), BOP (bleeding on probing);
- -
- Secondary outcomes: clinical outcomes of PI (plaque index) and REC (recession) and microbiological outcomes.
- -
- Patients treated with surgical therapy for periodontitis;
- -
- Dental implants;
- -
- Systemic diseases (ASA II, III);
- -
- No previous antibiotic prophylaxis;
- -
- Topical agents with a chemical action on biofilm (systemic administration of drugs, antibiotics, probiotics);
2.4. Risk of Bias Assessment
2.5. Data Extraction
2.6. Data Analysis
2.7. Grading the Body of Evidence
3. Results
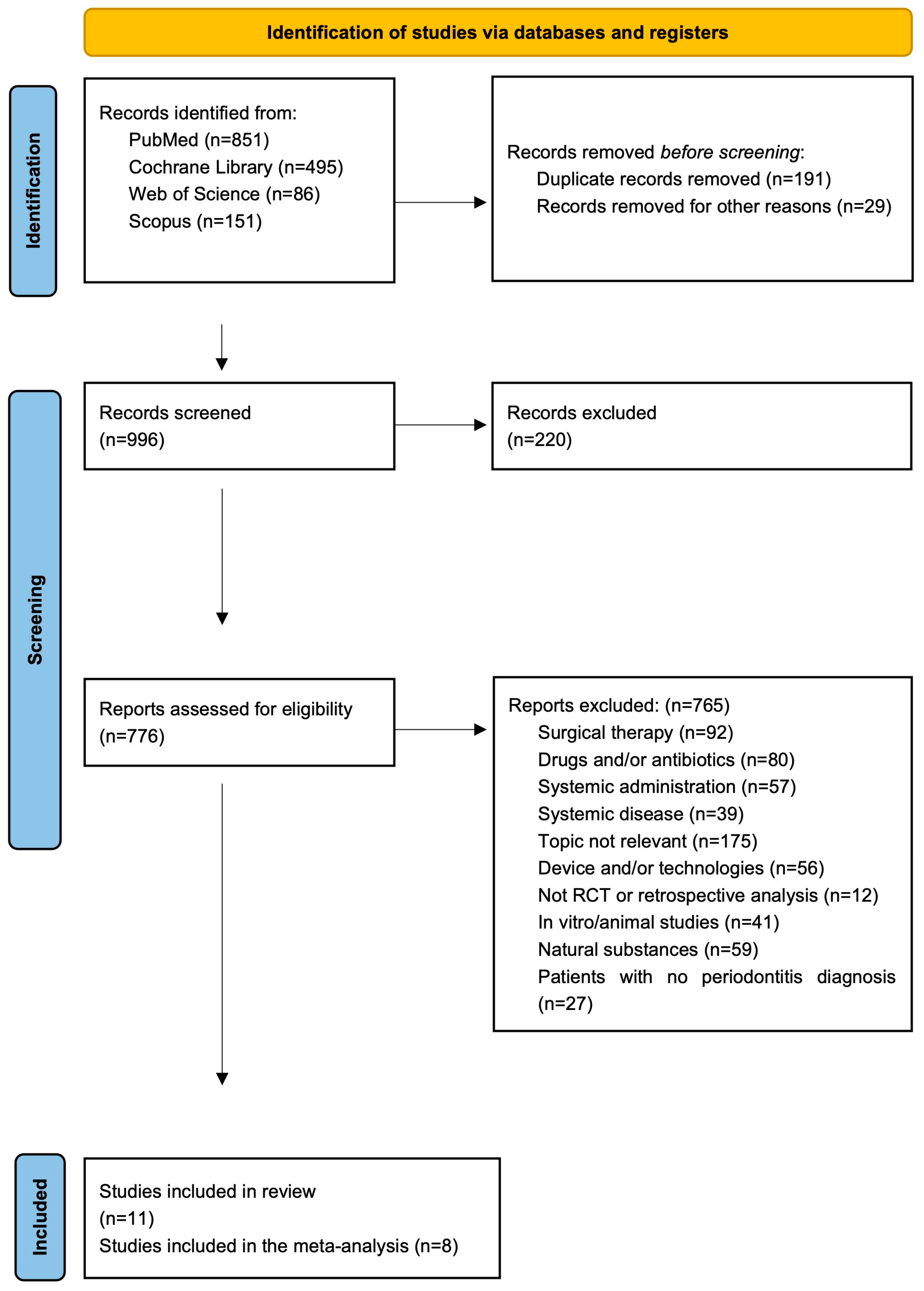
3.1. Search and Selection Results
3.2. Characteristics of Included Studies
3.3. Sodium Hypochlorite
3.4. Desiccant Agent
3.5. Risk of Bias Assessment
3.6. Study Outcomes Results: Qualitative Analysis
3.7. Meta-Analysis
3.8. Grading the Body of Evidence
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Signoriello, A.; Signoretto, C.; Messina, E.; Carelli, M.; Tessari, M.; De Manna, N.D.; Rossetti, C.; Albanese, M.; Lombardo, G.; et al. Detection of Periodontal Pathogens in Oral Samples and Cardiac Specimens in Patients Undergoing Aortic Valve Replacement: A Pilot Study. J. Clin. Med. 2021, 10, 3874. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Sculean, A. Nonsurgical therapy for teeth and implants-When and why? Periodontology 2000 2019, 79, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.M.; Ash, M.M., Jr.; Caffesse, R.G. The effectiveness of subgingival scaling and root planning in calculus removal. J. Periodontol. 1981, 52, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, E.; Tetè, G. Manual vs Mechanical Oral Hygiene Procedures: Has the Role of the Dental Hygienist in Phase 2 Post-lockdown Really Changed? Oral Health Prev. Dent. 2020, 18, 1031–1037. [Google Scholar] [PubMed]
- Devi, S.S.; Divyapriya, G.K.; Viswanathan, K.; Murugappan, S.; Balu, B.P.; Sweta, V.S.A. Oral Hygiene Practices and Knowledge on Periodontal Diseases and Therapy: A Cross-Sectional Questionnaire Study on Gypsy Narikuravars in Puducherry. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), S739–S743. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Mischler, D.C.; Schmidlin, K.; Matuliene, G.; Pjetursson, B.E.; Brägger, U.; Lang, N.P. Risk factors associated with the longevity of multi-rooted teeth. Long-term outcomes after active and supportive periodontal therapy. J. Clin. Periodontol. 2014, 41, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Sweeney, P.L.; Smith, B.A. Scaling and root planing with and without periodontal flap surgery. J. Clin. Periodontol. 1986, 13, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Lindhe, J. Parodontologia Clinica e Implantologia Orale, 6th ed.; Edi.Ermes: Milan, Italy, 2020; Volume 2. [Google Scholar]
- Žiemytė, M.; Lopez-Roldan, A.; Carda-Diéguez, M.; Reglero-Santaolaya, M.; Rodriguez, A.; Ferrer, M.D.; Mira, A. Personalized antibiotic selection in periodontal treatment improves clinical and microbiological outputs. Front. Cell. Infect. Microbiol. 2023, 13, 1307380. [Google Scholar] [CrossRef]
- Greenstein, G.; Polson, A. The role of local drug delivery in the management of periodontal diseases: A comprehensive review. J. Periodontol. 1998, 69, 507–520. [Google Scholar] [CrossRef]
- Gegout, P.Y.; Stutz, C.; Huck, O. Gels as adjuvant to non-surgical periodontal therapy: A systematic review and meta-analysis. Heliyon 2023, 9, 17789. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, E.; Machiulskiene, V. Antiseptics as adjuncts to scaling and root planing in the treatment of periodontitis: A systematic literature review. BMC Oral Health 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Butera, A.; Giordano, A.; Albanese, M. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 1086. [Google Scholar] [CrossRef]
- Gandhi, K.K.; Cappetta, E.G.; Pavaskar, R. Effectiveness of the adjunctive use of ozone and chlorhexidine in patients with chronic periodontitis. BDJ Open. 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Samaranayake, L.P. Topical and systemic antibiotics in the management of periodontal diseases. Int. Dent. J. 2004, 54, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- RevMan Web. The Cochrane Collaboration. 2023. Available online: https://revman.cochrane.org (accessed on 5 November 2023).
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- McMaster University and Evidence Prime, GRADEpro GDT: GRADEpro Guideline Development Tool. 2023. Available online: https://gradepro.org (accessed on 5 November 2023).
- Megally, A.; Zekeridou, A.; Cancela, J.; Giannopoulou, C.; Mombelli, A. Short ultrasonic debridement with adjunctive low-concentrated hypochlorite/amino acid gel during periodontal maintenance: Randomized clinical trial of 12 months. Clin. Oral Investig. 2020, 24, 201–209. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef]
- Diehl, D.; Friedmann, A.; Liedloff, P.; Jung, R.M.; Sculean, A.; Bilhan, H. Adjunctive Application of Hyaluronic Acid in Combination with a Sodium Hypochlorite Gel for Non-Surgical Treatment of Residual Pockets Reduces the Need for Periodontal Surgery-Retrospective Analysis of a Clinical Case Series. Materials 2022, 15, 6508. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, S.; Van der Velden, U.; Loos, B.G. Local disinfection with sodium hypochlorite as adjunct to basic periodontal therapy: A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Sethiya, K.R.; Dhadse, P.; Bajaj, P.; Chimote, M.; Subhadarsanee, C.; Hassan, C. Comparative evaluation of the effectiveness of sodium hypochlorite and chlorhexidine in one-stage full-mouth disinfection treatment of patients with generalized aggressive periodontitis: A randomized controlled clinical trial. J. Datta Meghe Inst. Med. Sci. Univ. 2021, 16, 4. [Google Scholar] [CrossRef]
- Radulescu, V.; Boariu, M.I.; Rusu, D.; Roman, A.; Surlin, P.; Voicu, A.; Didilescu, A.C.; Jentsch, H.; Siciliano, V.I.; Ramaglia, L.; et al. Clinical and microbiological effects of a single application of sodium hypochlorite gel during subgingival re-instrumentation: A triple-blind randomized placebo-controlled clinical trial. Clin. Oral Investig. 2022, 26, 6639–6652. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, E.; Machiulskiene, V.; Shirakata, Y.; Dvyliene, U.M.; Nedzelskiene, I.; Sculean, A. Clinical evaluation of sodium hypochlorite/amino acids and cross-linked hyaluronic acid adjunctive to non-surgical periodontal treatment: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 6645–6656. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Williams, R.C.; Iorio-Siciliano, V.; Alibrandi, A. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Signoretto, C.; Corrocher, G.; Pardo, A.; Pighi, J.; Rovera, A.; Caccuri, F.; Nocini, P.F. A topical desiccant agent in association with ultrasonic debridement in the initial treatment of chronic periodontitis: A clinical and microbiological study. New Microbiol. 2015, 38, 393–407. [Google Scholar] [PubMed]
- Khalil, B.; Abou Sulaiman, A.; Al Hajjar, B. The effects of adjunctive use of a desiccant agent in the treatment of stage III periodontitis (Randomized controlled clinical trial). Saudi Dent. J. 2023, 35, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Soancă, A.; Leucuța, D.C.; Roman, A.; Ciurea, A.; Negucioiu, M.; Pascu, L.C.; Picoș, A.; Delean, A.G.; Micu, I.C.; Popa Wagner, A.; et al. The Treatment of Severe Periodontitis Using a Local Antiseptic Desiccant and Subgingival Mechanical Instrumentation: A Pilot Study. J. Clin. Med. 2023, 12, 4286. [Google Scholar] [CrossRef]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants; Merete Aass, A.; et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Englund, K.; Nikrad, J.; Jones, R. Assessing the dynamic biofilm removal of sulfonated phenolics using CP-OCT P. Laser Dent. 2017, 9, 10044. [Google Scholar]
- El Mobadder, M.; Nammour, S.; Grzech-Leśniak, Z.; Grzech-Leśniak, K. Efficacy of the Adjunct Use of Povidone-Iodine or Sodium Hypochlorite with Non-Surgical Management of Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6593. [Google Scholar] [CrossRef] [PubMed]
- Guarnelli, M.E.; Vecchiatini, R.; Farina, R. Somministrazione professionale locale di un trattamento a base di cloramina in associazione a strumentazione meccanica ad ultrasuoni: Esiti clinici in pazienti con tasche parodontali profonde persistenti dopo terapia attiva non chirurgica. Minerva Stomatol. 2015, 64, 158–159. [Google Scholar]
- Eliezer, M.; Imber, J.C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.M.; Sabatini, S.; Lauritano, D.; Denisi, C.; Grassi, F.R. Management of biofilm control in an elderly patient suffering from rheumatoid arthritis: A case report. Int. J. Immunopathol. Pharmacol. 2013, 26, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Baccini, F.; De Manzoni, R.; Viviani, M.; Brentaro, S.; Zangani, A.; Faccioni, P.; Luciano, U.; Zuffellato, N.; Signoriello, A.; et al. Air polishing therapy in supportive periodontal treatment: A systematic review. J. Appl. Cosmetol. 2023, 41, 13–24. [Google Scholar] [CrossRef]
- Zafar, F.; Romano, F.; Citterio, F.; Ferrarotti, F.; Dellavia, C.; Chang, M.; Aimetti, M. Chemical cleansing as an adjunct to subgingival instrumentation with ultrasonic and hand devices in deep periodontal pockets: A randomized controlled study. J. Periodontal Implant. Sci. 2021, 51, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Bracke, J.; Basara, M.; Savord, E.; Dunaway, A.; Watkins, M. Pilot evaluation of a simple adjunctive method for improved removal of oral biofilm during conventional scaling and root planing therapy. J. Biol. Regul. Homeost. Agents 2015, 29, 6–9. [Google Scholar]
- Lauritano, D. The efficacy of hybenx® oral tissue decontaminant for periodontal disease treatment: A case series study. Int. J. Adv. Case Rep. 2015, 2, 405–408. [Google Scholar]
| Authors | Year | Study Design | Country | Sample Number | N Test/Age N Control/Age | Gender | Test and Control Interventions | Follow-Up | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Bizzarro et al. [26] | 2016 | RCT, full-mouth | Amsterdam, the Netherlands | 56 | n 27/47.7 n 29/46.9 | F: 20, M: 36 | 1. BPT + NaOCl 2. BPT + saline solution | 12 months | PPD, CAL, BOP, PS |
| Daniel Diehl et al. [25] | 2022 | Retrospective analysis | Witten, Germany | 29 | n 29/54.6 | F: 20, M: 9 | 1. gel NaOCl/aa + UD e/o SRP + HA | 6 months | PPD, CAL, BOP, GR |
| Iorio-Siciliano et al. [24] | 2021 | RCT, full-mouth | Messina, Italy | 37 | n 18/53.3 n 19/48.5 | F: 21, M: 16 | 1. MINST + gel NaOCl/aa 2. MINST | 6 months | PD, CAL, BOP, FMPS, GR |
| Isola et al. [30] | 2018 | RCT, split-mouth | Messina, Italy | 36 | n 36/46.7 | F: 17, M: 19 | 1. SRP + Hybenx 2. SRP | 12 months | PD, CAL, BOP, PS, GR |
| Khalil et al. [32] | 2023 | RCT, split-mouth | Damascus, Syria | 25 | n 25/45.2 | F: 15, M: 10 | 1. SRP + Hybenx 2. SRP | 6 months | PPD, RAL, GI, BOP, PLI, GH |
| Lombardo et al. [31] | 2015 | RCT, split-mouth | Verona, Italy | 16 | n 16/55 | F: 9, M: 7 | 1. UD + Hybenx 2. UD | 3 months | PPD, CAL, GI, BOP, VPI, GM, microbiology |
| Megally et al. [23] | 2020 | RCT, full-mouth | Geneva, Switzerland | 32 | n 16/61.7 n 16/62.1 | F: 11, M 21 | 1. US + gel NaOCl/aa 2. US | 12 months | PD, BOP, GR, microbiology |
| Radulescu et al. [28] | 2022 | RCT, full-mouth | Timisoara, Romania | 42 | n 20/44.60 n 18/50.61 | F: 22, M 16 | 1. UMI + air polish + gel NaOCl/aa 2. UMI + air polish + placebo gel | 12 months | PPD, CAL, FMBS, FMPS, GR, microbiology |
| Ramanauskaite et al. [29] | 2023 | RCT, full-mouth | Kaunas, Lithuania | 48 | n 24/47.3 n 24/49.3 | F: 35, M 13 | 1. SRP + NaOCl/aa + HA 2. SRP | 6 months | PPD, CAL, BOP, PI |
| Sethiya et al. [27] | 2021 | RCT, full-mouth | Maharashtra, India | 29 | n 11/28.27 n 11/29.09 | F: 11, M 11 | 1. OSFMD + NaOCl gel + mouthwash 2. OSFMD + CHX gel + mouthwash | 6 months | PPD, CAL, mSBI, PI |
| Soancă et al. [33] | 2023 | RCT, split-mouth | Cluj-Napoca, Romania | 36 | n 30/44.8 | F: 13, M 17 | 1. SRP + Hybenx 2. SRP | 3 months | PD, CAL, GBI, OHI, GR |
| Author | Periodontitis | Study Design | SRP (Test and Control) | Topical Agent (Test) | Topical Agent (Control) |
|---|---|---|---|---|---|
| Bizzarro et al., 2016 [26] | CAL ≥ 3 mm PPD ≥ 5 mm | Full-mouth | Ultrasound (Hu-Friedy EMS piezon; Hu-Friedy, Chicago, IL, USA); manual instrumentation | After 3 days: NaOCl solution | After 3 days: saline solution |
| Diehl et al., 2022 [25] | PPD ≥ 5 mm | Full-mouth | Ultrasound supra-gingivally; manual instrumentation with Gracey curettes (Deppeler, American Dental Systems, Monaco, Germany) | After SRP: 30–45 s gel NaOCl/aa (Perisolv; Regedent AG, Zurig, Switzerland); further application of the gel as needed After SRP: gel IA (xHyA; hyaDENT BG, Regedent AG, Zurig, Switzerland) Further application within 7 days | No placebo |
| Iorio-Siciliano et al., 2022 [24] | Stage III/IV, grade A/B PPD ≥ 5 mm | Full-mouth | Ultrasound (Instrument PS®EMS Electro Medical System S.A., Nyon, Switzerland); Gracey micro-curette (Hu-Friedy®, Chicago, IL, USA); polishing | Before SRP: 30 s gel NaOCl/aa (Perisolv®, Regedent AG, Zurich, Switzerland) No rinse After SRP: further application of the hyaluronic gel | No placebo |
| Isola et al., 2018 [30] | Cronic periodontitis, PPD ≥ 5 mm | Split-mouth | Ultrasound with insert number 5/6/7 (Satelec Ultrasonics, Acteon, VA, Italy); manual instrumentation (Gracey curettes, ASA Dental, Bozzano, Italy) | Before SRP: 60 s sulfonated gel (HYBENX, oral tissue decontaminant, EPIEN Medical, St Paul, MN, USA); Saline solution removal | Before SRP: 60 s saline solution |
| Khalil et al., 2023 [32] | Stage III, PPD ≥ 6 mm | Split-mouth | Manual instrumentation (CK6 e U-15, ZaffiroTM, Beckum, Germany; Gracey curettes: ZaffiroTM, Germany) | Before SRP: 30 s sulfonated gel (HYBENX, oral tissue decontaminant, EPIEN Medical, St Paul, MN, USA); Saline solution removal | No placebo |
| Lombardo et al., 2015 [31] | Moderate or severe PPD ≥ 5 mm | Split-mouth | Ultrasound (Piezon Master 400, EMS, Nyon, Switzerland) with standards inserts | Before SRP: 45–60 s sulfonated gel (HYBENX, oral tissue decontaminant, EPIEN Medical, St Paul, MN, USA); Saline solution removal | No placebo |
| Megally et al., 2020 [23] | PPD ≥ 5 mm | Full-mouth | Ultrasound (Piezon® LED, EMS Electro Medical System S.A., Nyon, Switzerland) | Before SRP: 30 s gel NaOCl/aa (Perisolv®, Regedent AG, Zürich, Switzerland) After SRP: further application of hyaluronic gel | No placebo |
| Radulescu et al., 2022 [28] | Stage III/IV, PPD ≥ 4 mm | Full-mouth | Supragingival ultrasound (EMS Piezon® Master, EMS, Nyon, Switzerland); air polishing (standard air-flow nozzle, AIRFLOW® PLUS powder EMS, Nyon, Switzerland); sites with PPD > 4 mm: ultrasound with subgingival insert (PS, EMS, Nyon, Switzerland) | Before SRP: 30 s gel NaOCl/aa (Perisolv®, Regedent AG, Zürich, Switzerland) After 15 min, further application of hyaluronic gel and SRP | Before SRP: placebo |
| Ramanauskaite et al., 2023 [29] | Stage II-III, grade A/B | Full-mouth | Ultrasound (Satelec/Acteon Suprasson Newtron ultrasonic scaler); manual instrumentation (LM SharpDiamond 1/2, 7/8, 11/12, 13/14 SD Gracey curettes and mini-curettes, LM Dental™, Pargas, Finland); polish (Lunos Super Soft, RDA < 5, Dürr Dental, Germany) | Before SRP: 60 s gel NaOCl/aa (Perisolv®, Regedent AG, Zurig, Switzerland) Further application of gel as needed (max 2–3 months)After SRP: gel IA (Hyadent® BG, Regedent AG, Zurigo, Switzerland) | No placebo |
| Sethiya et al., 2021 [27] | PPD ≥ 4 mm and BOP or PPD ≥ 5 mm | Full-mouth | SRP in 24 h supra- and subgingivally | After SRP: gel NaOCl 0.05% (5 mL 10% NaOCl and 995 mL sterile water) Gel application for 2 months | After SRP: gel CHX |
| Soancă et al., 2023 [33] | Stage III/IV, PPD ≥ 4 mm | Split-mouth | Ultrasound (Unit-P5 Booster Suprasson-Satelec, Acteon, Mount Laurel, NJ, USA); manual instrumentation: Gracey curettes (Hu-Friedy, Chicago, IL, USA) | Before SRP: 20 s sulfonated gel | No placebo |
| ΔPPD (mm) | |||
|---|---|---|---|
| Control (Mean ± SD) | Test (Mean ± SD) | Follow-Up | |
| Bizzarro et al., 2016 [26] | 1 ± 0.5 | 0.9 ± 0.4 | Baseline—6 months |
| Iorio-Siciliano et al., 2021 [24] | 1.98 ± 0.8 | 2.49 ± 0.76 | Baseline—6 months |
| Isola et al., 2018 [30] | 2.3 ± 0.43 | 2.85 ± 0.48 | Baseline—6 months |
| Khalil et al., 2023 [32] | 0.83 ± 0.52 | 1.57 ± 0.42 | Baseline—6 months |
| Lombardo et al., 2015 [31] | 0.6 ± 1.4 | 0.85 ± 1.5 | Baseline—3 months |
| Radulescu et al., 2022 [28] | 0.68 ± 0.73 | 0.98 ± 0.31 | Baseline—3 months |
| Ramanauskaite et al., 2023 [29] | 1.9 ± 0.57 | 2.5 ± 0.29 | Baseline—3 months |
| Soancă et al., 2023 [33] | 0.85 ± 0.91 | 0.82 ± 0.72 | Baseline—3 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, A.; Fiorini, V.; Zangani, A.; Faccioni, P.; Signoriello, A.; Albanese, M.; Lombardo, G. Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 2179. https://doi.org/10.3390/jcm13082179
Pardo A, Fiorini V, Zangani A, Faccioni P, Signoriello A, Albanese M, Lombardo G. Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(8):2179. https://doi.org/10.3390/jcm13082179
Chicago/Turabian StylePardo, Alessia, Vera Fiorini, Alessandro Zangani, Paolo Faccioni, Annarita Signoriello, Massimo Albanese, and Giorgio Lombardo. 2024. "Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 8: 2179. https://doi.org/10.3390/jcm13082179
APA StylePardo, A., Fiorini, V., Zangani, A., Faccioni, P., Signoriello, A., Albanese, M., & Lombardo, G. (2024). Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(8), 2179. https://doi.org/10.3390/jcm13082179









