Clinical Application of Exercise Stress Echocardiography in an Outpatient Pediatric Population
Abstract
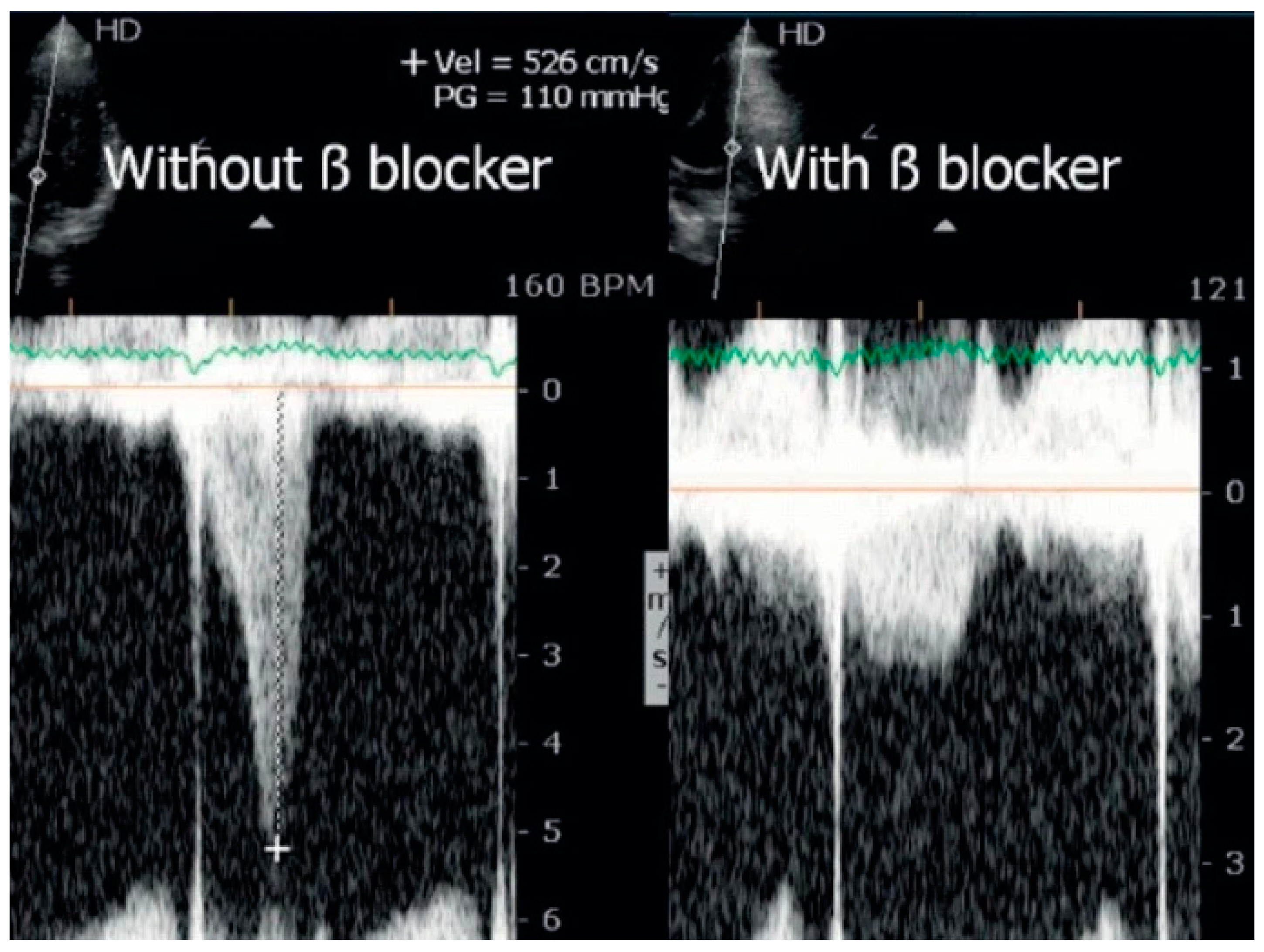
:1. Introduction and Aims
2. Materials and Methods
3. Results and Statistical Analysis
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotrim, C.A.; Café, H.; João, I.; Cotrim, N.; Guardado, J.; Cotrim, H.; Cordeiro, P.; Baquero, L. Exercise stress echocardiography: Where are we now? World J. Cardiol. 2022, 14, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Friedman, H.; Allen, N.; Stark, M.; Ferguson, E.; Sachdeva, R.; Border, W.L. Exercise stress echocardiography: Impact on clinical decision-making in pediatric patients. Echocardiography 2019, 36, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Kimball, T.R. Pediatric stress echocardiography. Pediatr. Cardiol. 2002, 23, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R. Stress echocardiography in paediatrics: Implications for the evaluation of anomalous aortic origin of the coronary arteries. Cardiol. Young 2015, 25, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, P.; McLeod, I.; Cairello, F.; Kaski, J.P.; Fenton, M.; Giardini, A.; Marek, J. Semi-supine exercise stress echocardiography in children and adolescents: Feasibility and safety. Pediatr. Cardiol. 2015, 36, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Cifra, B.; Dragulescu, A.; Border, W.L.; Mertens, L. Stress echocardiography in pediatric cardiology. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Ermis, P. Stress echocardiography: An overview for use in pediatric and congenital cardiology. Congenit. Heart Dis. 2017, 12, 624–626. [Google Scholar] [CrossRef] [PubMed]
- El Assaad, I.; Gauvreau, K.; Rizwan, R.; Margossian, R.; Colan, S.; Chen, M.H. Value of Exercise Stress Echocardiography in Children with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2020, 33, 888–894.e2. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.T.; Rizwan, R.; Gauvreau, K.; Daly, K.P.; Deng, E.S.; Blume, E.D.; Singh, T.P.; Chen, M.H. Prognostic Value of Exercise Stress Echocardiography in Pediatric Cardiac Transplant Recipients. J. Am. Soc. Echocardiogr. 2022, 35, 1133–1138.e2. [Google Scholar] [CrossRef]
- Forton, K.; Motoji, Y.; Caravita, S.; Faoro, V.; Naeije, R. Exercise stress echocardiography of the pulmonary circulation and right ventricular-arterial coupling in healthy adolescents. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 688–694. [Google Scholar] [CrossRef]
- Araujo, J.J. Stress Echocardiography in Pediatric and Adult Congenital Heart Disease: A Complement in Anatomical and Functional Assessment. Curr. Probl. Cardiol. 2021, 46, 100762. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Abernathey, E.; Lunze, F.; Colan, S.D.; O’Neill, S.; Bergersen, L.; Geva, T.; Blume, E.D. Utility of exercise stress echocardiography in pediatric cardiac transplant recipients: A single-center experience. J. Heart Lung Transplant. 2012, 31, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Tedla, B.A.; Burns, J.C.; Tremoulet, A.H.; Shimizu, C.; Gordon, J.B.; El-Said, H.; Golding, F.; Davis, C.K.; Dummer, K.B. Exercise Stress Echocardiography in Kawasaki Disease Patients with Coronary Aneurysms. Pediatr. Cardiol. 2023, 44, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pahl, E.; Duffy, C.E.; Chaudhry, F.A. The role of stress echocardiography in children. Echocardiography 2000, 17, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Rigsby, C.K.; McKenney, S.E.; Hill, K.D.; Chelliah, A.; Einstein, A.J.; Han, B.K.; Robinson, J.D.; Sammet, C.L.; Slesnick, T.C.; Frush, D.P. Radiation dose management for pediatric cardiac computed tomography: A report from the Image Gently ‘Have-A-Heart’ campaign. Pediatr. Radiol. 2018, 48, 5–20. [Google Scholar] [CrossRef]
- Picano, E.; Henein, M. Stress Echocardiography in Children. In Stress Echocardiography; Picano, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Hill, K.; Frush, D.; Han, K.; Abbott, B.; Armstrong, A.; DeKemp, R.A.; Glatz, A.C.; Greenberg, S.B.; Herbert, A.S.; Justino, H.; et al. Radiation Safety in Children with Congenital and Acquired Heart Disease: A Scientific Position Statement on Multimodal Dose Optimization from the Image Gently Alliance. JACC Cardiovasc. Imaging 2017, 10, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Khong, P.L.; Ringertz, H.; Donoghue, V.; Frush, D.; Rehani, M.; Appelgate, K.; Sanchez, R. ICRP Publication 121: Radiological Protection in Paediatric Diagnostic and Interventional Radiology. Ann. ICRP 2013, 42, 1–63. [Google Scholar] [CrossRef] [PubMed]
- EANM: European Association of Nuclear Medicine. Paediatric Nuclear Imaging: Minimizing the Dose—Maintaining the Diagnostic Value. EANM PRESS RELEASE. 2015. Available online: https://www.eanm.org/content-eanm/uploads/2016/12/EANM_PR_2015-07-13_Paediatric-nuclear-imaging-en.pdf (accessed on 4 January 2019).
- Lancellotti, P.; Pellikka, P.; Werner Budts, W.; Chaudhry, F.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.—Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Cotrim, H.; Granja, C.; Carvalho, A.S.; Cotrim, C.; Martins, R. Children’s Understanding of Informed Assents in Research Studies. Healthcare 2021, 9, 871. [Google Scholar] [CrossRef]
- Vilela, E.M.; Oliveira, C.; Oliveira, C.; Torres, S.; Sampaio, F.; Primo, J.; Ribeiro, J.; Teixeira, M.; Oliveira, M.; Bettencourt, N.; et al. Sixty years of the Bruce protocol: Reappraising the contemporary role of exercise stress testing with electrocardiographic monitoring. Porto Biomed. J. 2023, 8, e235. [Google Scholar] [CrossRef]
- Cotrim, C.; Palinkas, E.D.; Cotrim, N. The Importance of Left Ventricular Outflow Tract and Mid-Ventricular Gradients in Stress Echocardiography: A Narrative Review. J. Clin. Med. 2023, 12, 5292. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Cotrim, C.; Cruz, I.; Picano, E.; Pinto, F.; Pereira, H. Left ventricular outflow tract obstruction as a primary phenotypic expression of hypertrophic cardiomyopathy in mutation carriers without hypertrophy. Int. J. Cardiol. 2014, 176, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Bursi, F.; Bellino, M.; Picano, E. The Role of Stress Echocardiography in Valvular Heart Disease. Curr. Cardiol. Rep. 2022, 24, 1477–1485. [Google Scholar] [CrossRef]
- Picano, E.; Pierard, L.; Peteiro, J.; Djordjevic-Dikic, A.; Sade, L.E.; Cortigiani, L.; Van De Heyning, C.M.; Celutkiene, J.; Gaibazzi, N.; Ciampi, Q.; et al. The clinical use of stress echocardiography in chronic coronary syndromes and beyond coronary artery disease: A clinical consensus statement from the European Association of Cardiovascular Imaging of the ESC. Eur. Heart J. Cardiovasc. Imaging 2024, 25, e65–e90. [Google Scholar] [CrossRef]
- Moscatelli, S.; Bianco, F.; Cimini, A.; Panebianco, M.; Leo, I.; Bucciarelli-Ducci, C.; Perrone, M.A. The Use of Stress Cardiovascular Imaging in Pediatric Population. Children 2023, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.F.; Ma, K.L.; Shan, H.; Liu, T.F.; Zhao, S.Q.; Wan, Y.; Zhang, J.; Wang, H.Q. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer 2022, 22, 1238. [Google Scholar] [CrossRef]
- Nievelstein, R.A.; Frush, D.P. Should we obtain informed consent for examinations that expose patients to radiation? AJR Am. J. Roentgenol. 2012, 199, 664–669. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Kyle, W.B.; Macicek, S.L.; Lindle, K.A.; Kim, J.J.; Cannon, B.C. Limited utility of exercise stress tests in the evaluation of children with chest pain. Congenit. Heart Dis. 2012, 7, 455–459. [Google Scholar] [CrossRef]
- Massin, M.M. The role of exercise testing in pediatric cardiology. Arch. Cardiovasc. Dis. 2014, 107, 319–327. [Google Scholar] [CrossRef]
- Saleeb, S.F.; McLaughlin, S.R.; Graham, D.A.; Friedman, K.G.; Fulton, D.R. Resource reduction in pediatric chest pain: Standardized clinical assessment and management plan. Congenit. Heart Dis. 2018, 13, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Friedman, K.G.; Rathod, R.H.; Farias, M.; Graham, D.; Powell, A.J.; Fulton, D.R.; Newburger, J.W.; Colan, S.D.; Jenkins, K.J.; Lock, J.E. Resource utilization after introduction of a standardized clinical assessment and management plan. Congenit. Heart Dis. 2010, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Verghese, G.R.; Friedman, K.G.; Rathod, R.H.; Meiri, A.; Saleeb, S.F.; Graham, D.A.; Geggel, R.L.; Fulton, D.R. Resource Utilization Reduction for Evaluation of Chest Pain in Pediatrics Using a Novel Standardized Clinical Assessment and Management Plan (SCAMP). J. Am. Heart Assoc. 2012, 1, jah3-e000349. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A.; Friedman, K.G.; Fulton, D.R.; Geggel, R.L.; Saleeb, S.F. Needles in Hay II: Detecting Cardiac Pathology by the Pediatric Chest Pain Standardized Clinical Assessment and Management Plan. Congenit. Heart Dis. 2016, 11, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Bueno, F.; García-Pinilla, J.M.; Gómez-Doblas, J.J.; Montiel-Trujillo, A.; Rodríguez-Bailón, I.; de Teresa-Galván, E. Beta-blocker therapy for dynamic left ventricular outflow tract obstruction induced by exercise. Int. J. Cardiol. 2007, 117, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, C.; Lopes, L.R.; Almeida, A.R.; Miranda, R.; Ana, A.G.; Cotrim, H.; Andrade, J.P.; Picano, E.; Carrageta, M. Efficacy of beta-blocker therapy in symptomatic athletes with exercise-induced intra-ventricular gradients. Cardiovasc. Ultrasound. 2010, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Bueno, F.; Rodríguez Bailón, I.; López Salguero, R.; Doblas, J.J.G.; Cabeza, A.P.; Hernández, J.P.; Franco, A.D.; Hidalgo, L.M.; de Teresa Galván, E. Obstrucción dinámica intraventricular izquierda inducida por esfuerzo [Dynamic left ventricular outflow tract obstruction induced by exercise]. Rev. Esp. Cardiol. 2004, 57, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Zywica, K.; Jenni, R.; Pellikka, P.A.; Faeh-Gunz, A.; Seifert, B.; Attenhofer Jost, C.H. Dynamic left ventricular outflow tract obstruction evoked by exercise echocardiography: Prevalence and predictive factors in a prospective study. Eur. J. Echocardiogr. 2008, 9, 665–671. [Google Scholar] [CrossRef]
- Saeed, S.; Vegsundvåg, J. Usefulness of Stress Echocardiography in Assessment of Dynamic Left Ventricular Obstructions: Case Series and Review of the Literature. Cardiology 2021, 146, 441–450. [Google Scholar] [CrossRef]
- Alhaj, E.K.; Kim, B.; Cantales, D.; Uretsky, S.; Chaudhry, F.A.; Sherrid, M.V. Symptomatic exercise-induced left ventricular outflow tract obstruction without left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2013, 26, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Dores, H.; Mendes, L.; Ferreira, A.; Santos, J.F. Symptomatic Exercise-induced Intraventricular Gradient in Competitive Athlete. Arq. Bras. Cardiol. 2017, 109, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Wittlieb-Weber, C.A.; Cohen, M.S.; McBride, M.G.; Paridon, S.M.; Morrow, R.; Wasserman, M.; Wang, Y.; Stephens, P. Elevated left ventricular outflow tract velocities on exercise stress echocardiography may be a normal physiologic response in healthy youth. J. Am. Soc. Echocardiogr. 2013, 26, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.J. Left ventricular outflow tract velocities-all in context. J. Am. Soc. Echocardiogr. 2014, 27, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477, Erratum in Eur. Heart J. 2020, 41, 4242. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Dow, S.; Shah, K.; Henkin, S.; Taub, C. Complications of exercise and pharmacologic stress echocardiography. Front. Cardiovasc. Med. 2023, 10, 1228613. [Google Scholar] [CrossRef]
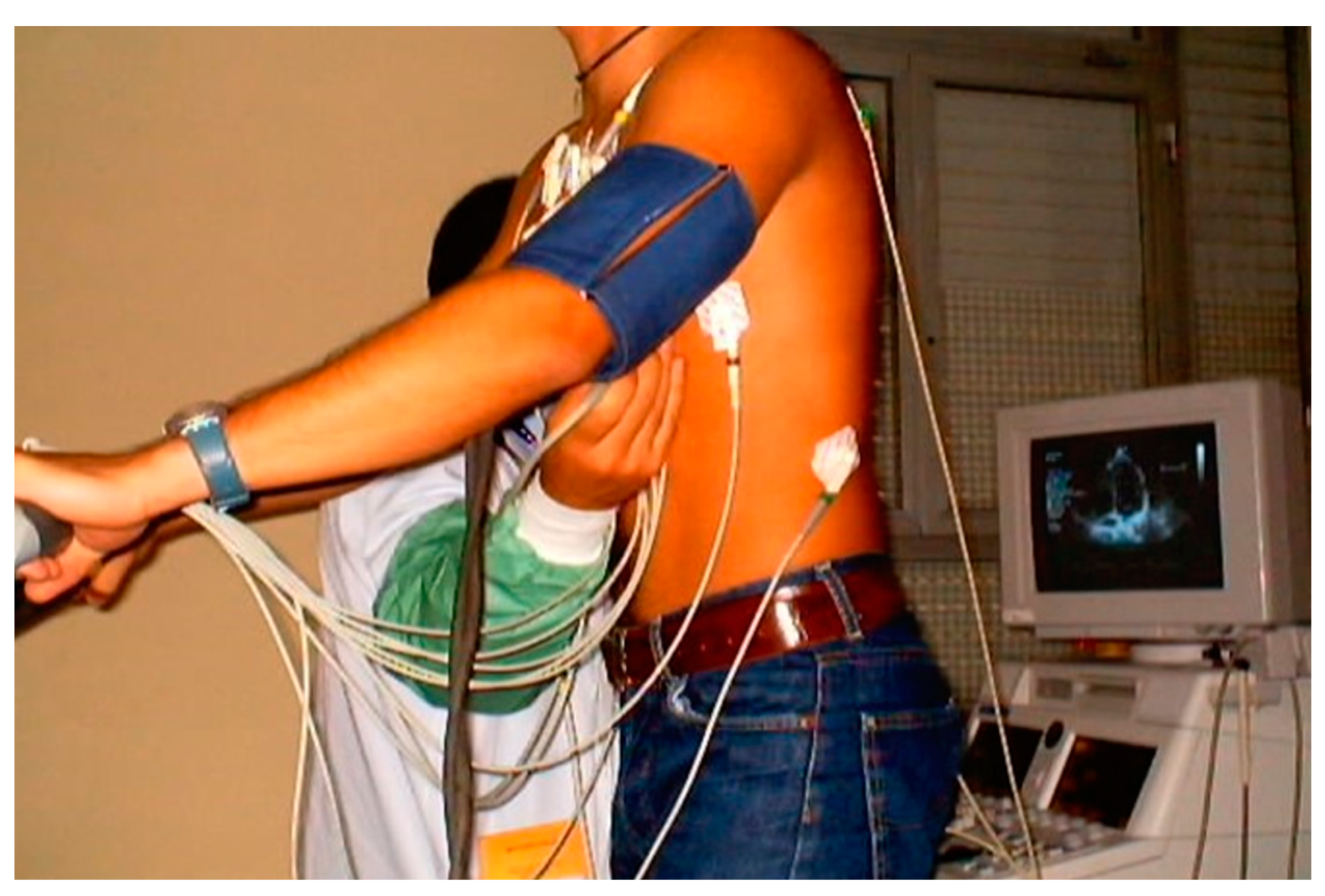
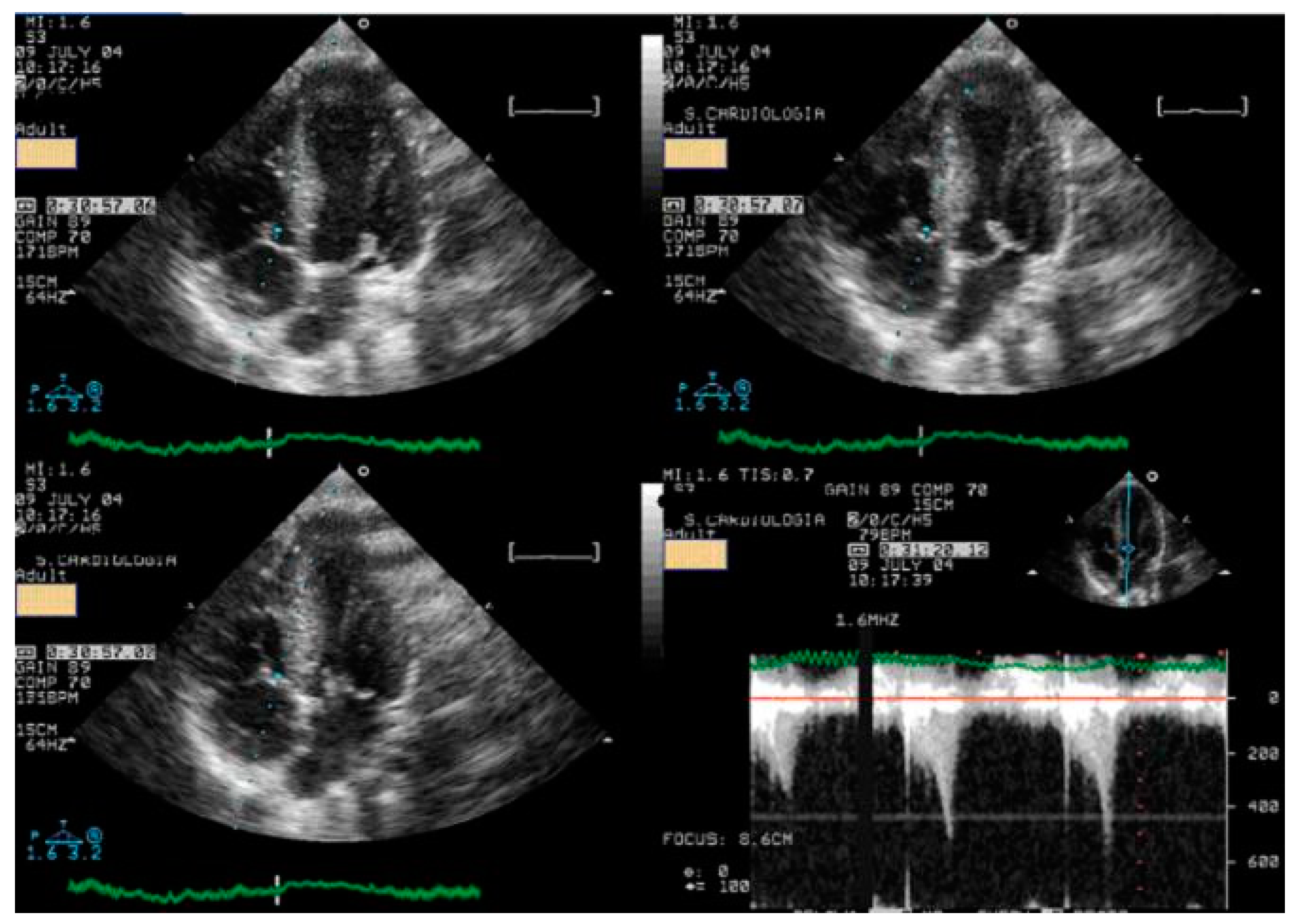
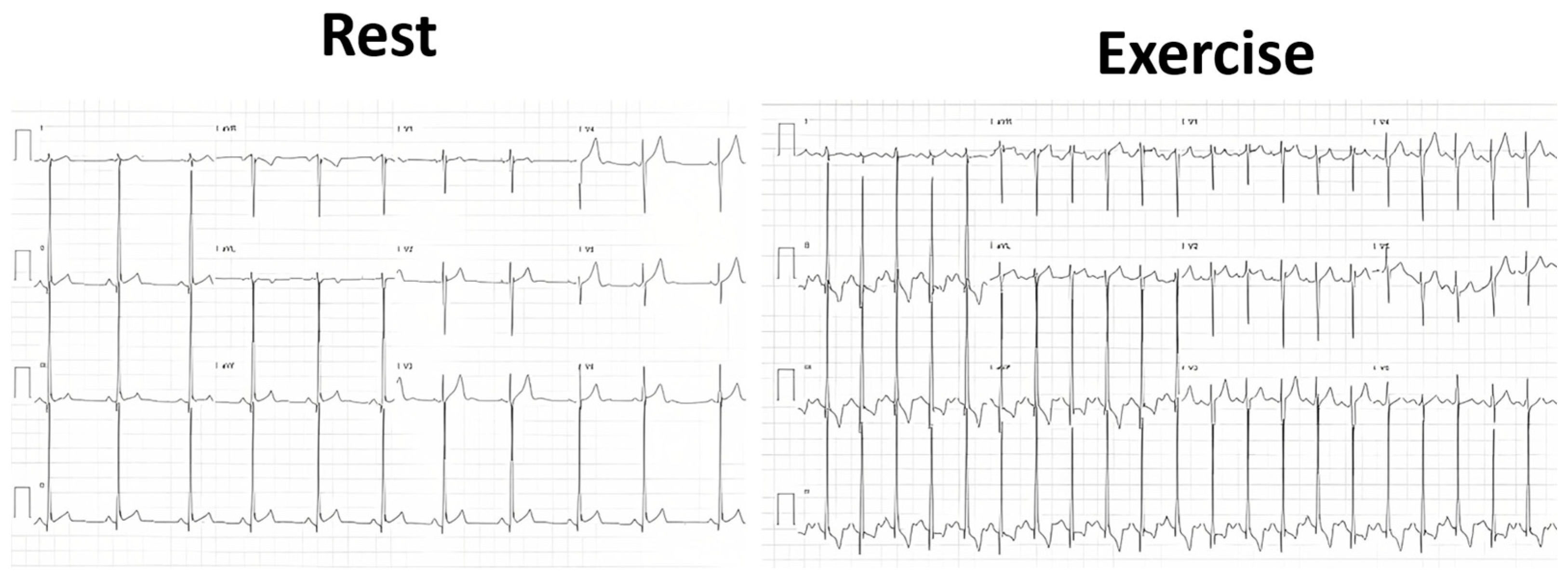
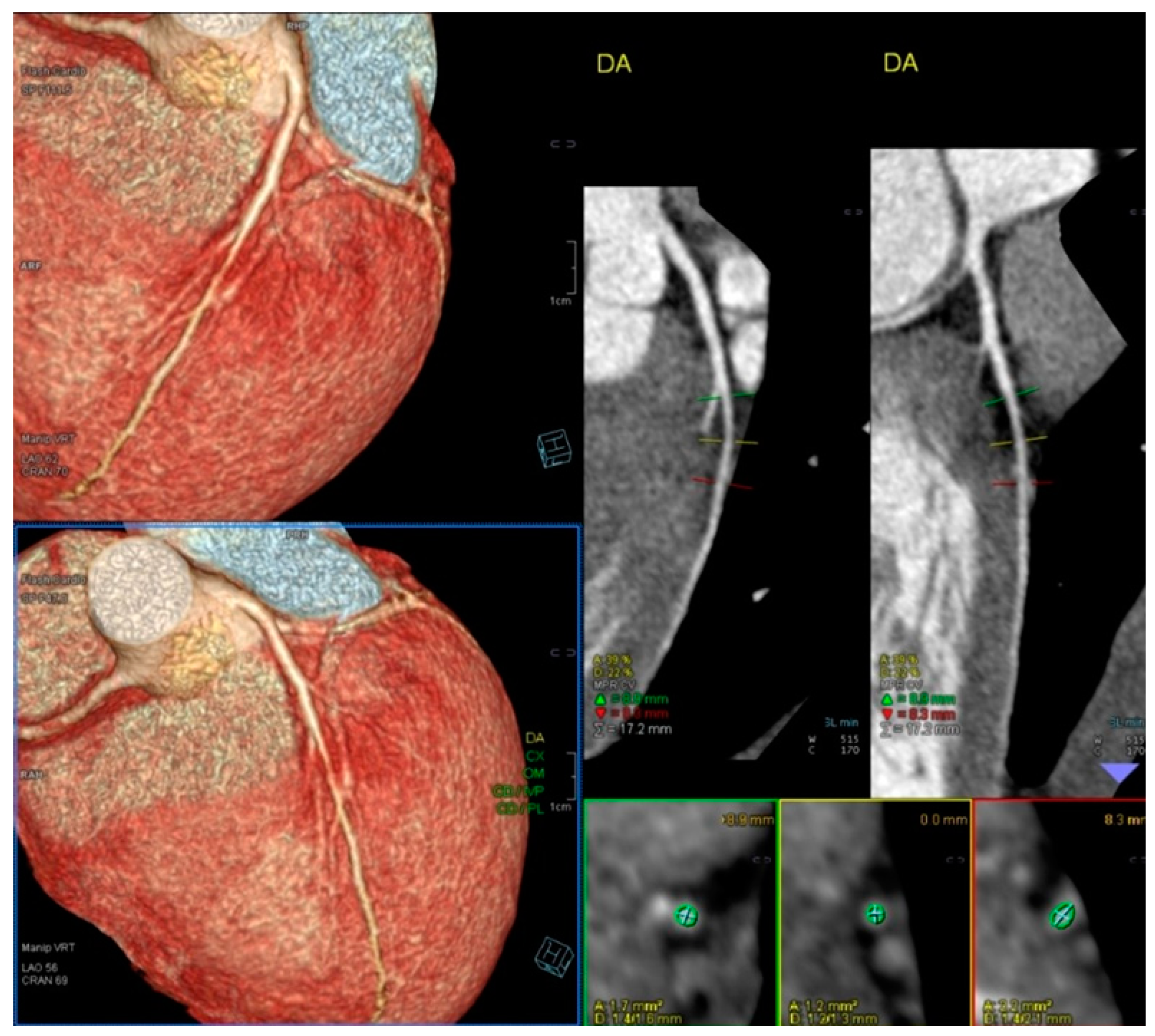
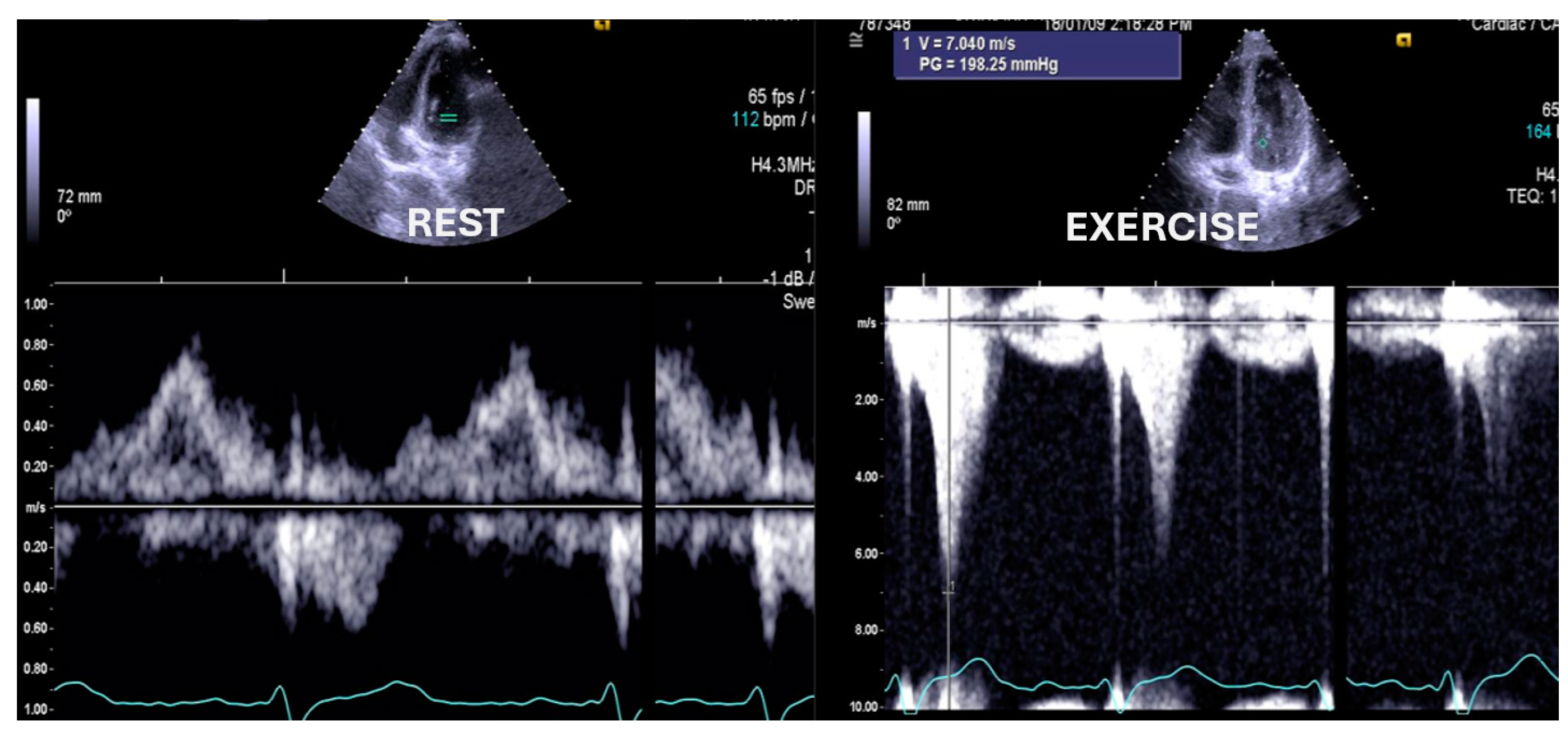
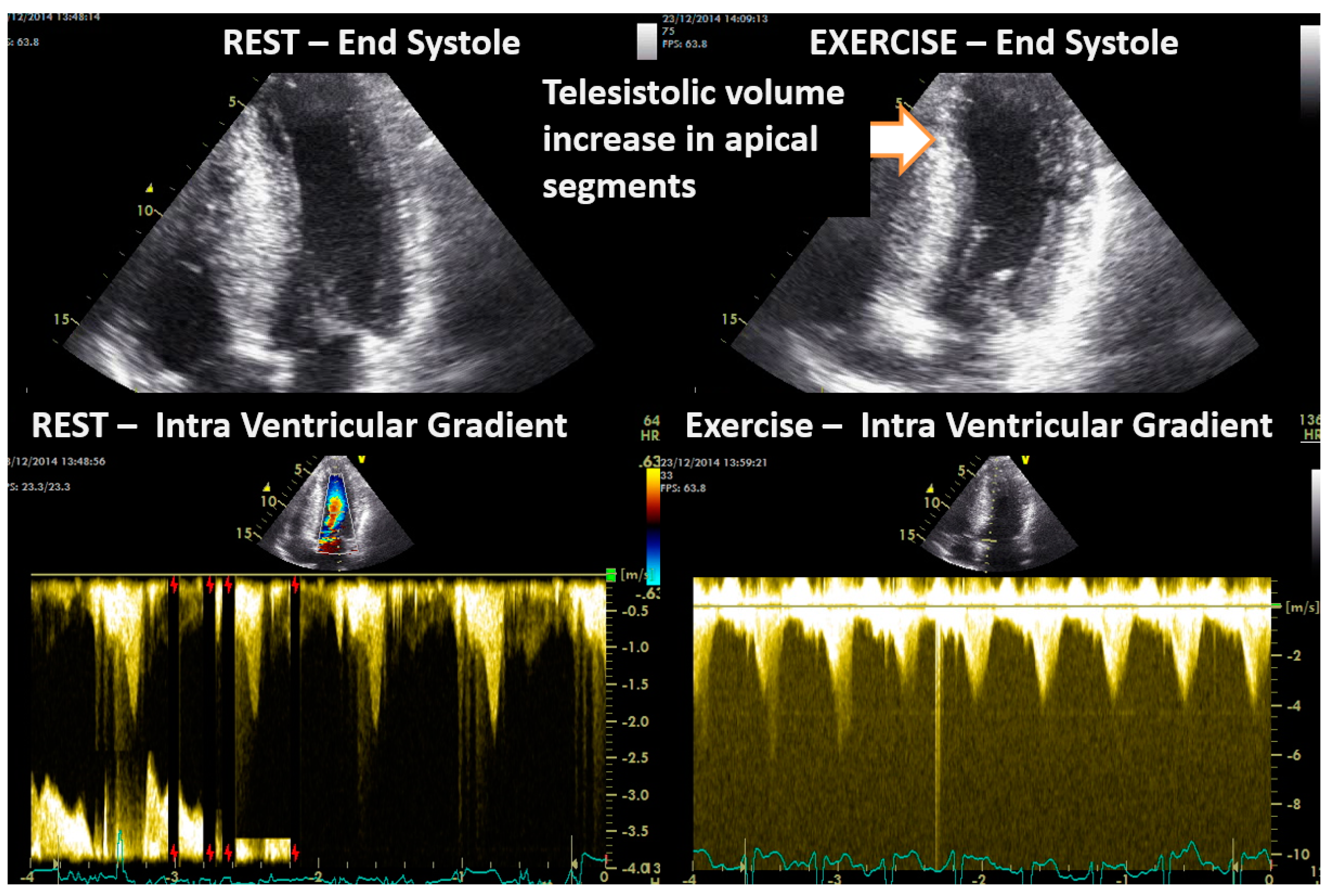
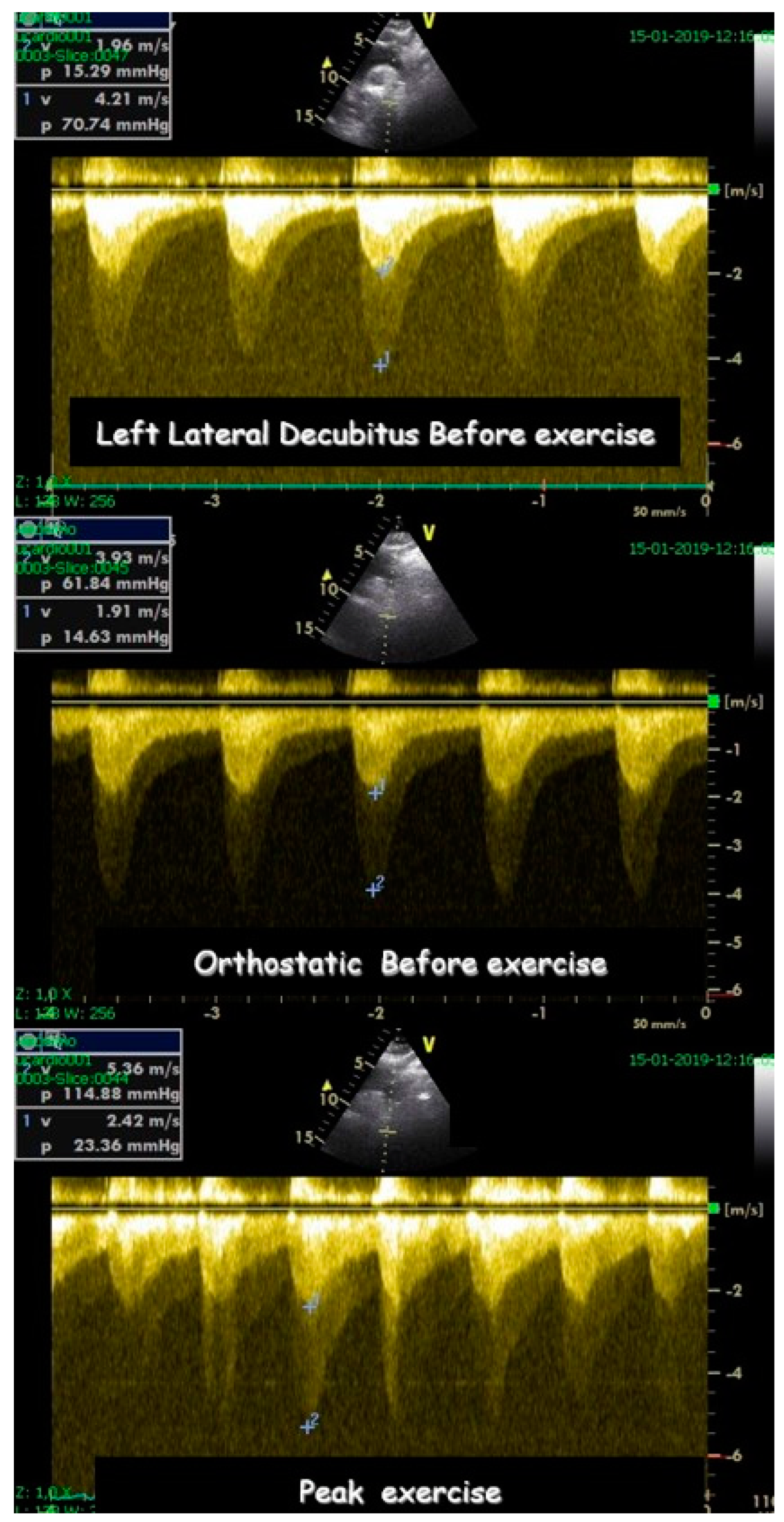
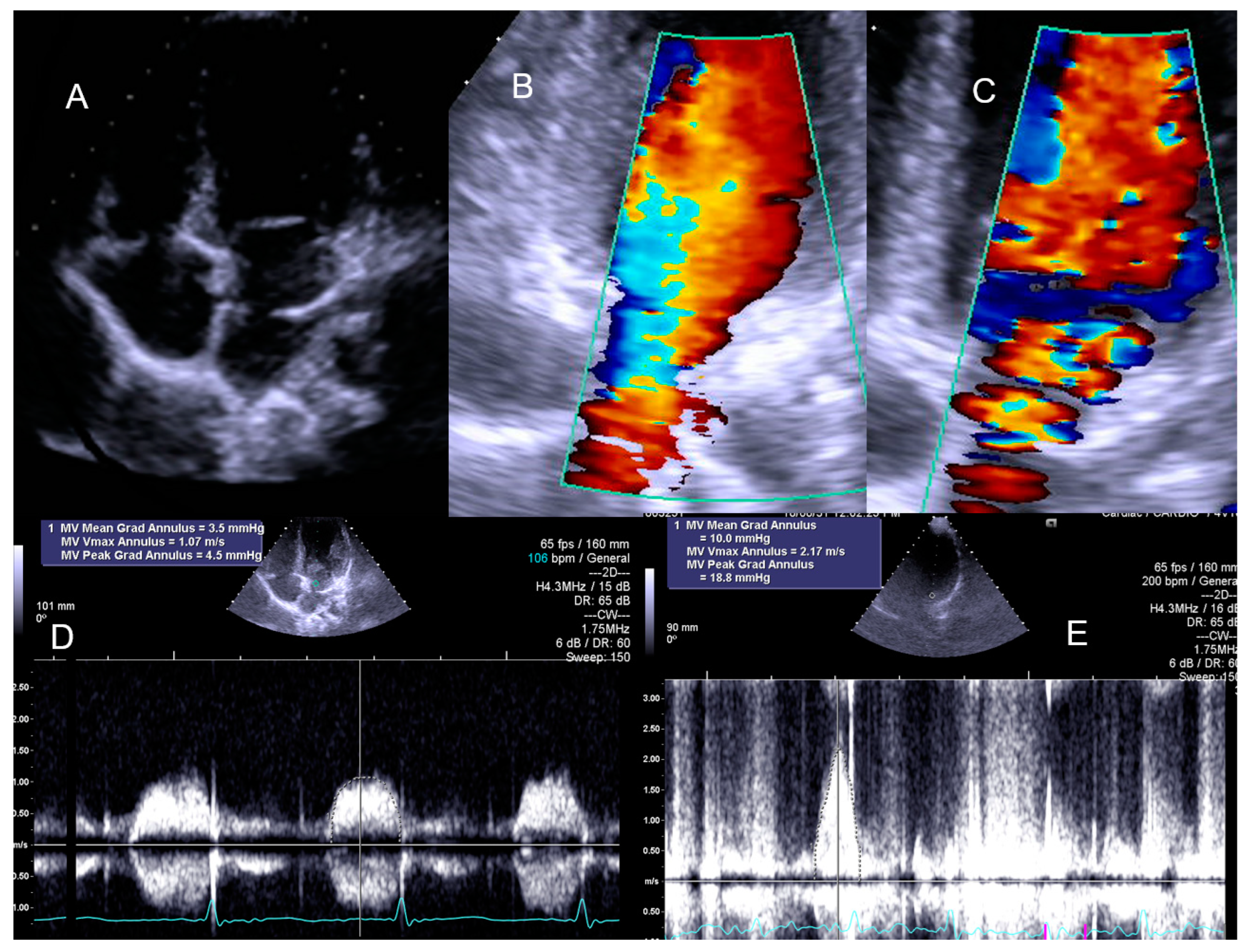
| Variables | Range | Value |
|---|---|---|
| Age | (6 to 17 year) | 14.13 ± 2.57 |
| Male | 199 (64.4%) | |
| Female | 110 (35.4%) | |
| Exercise symptoms are indication to ESE | 236 (76.4%) | |
| Abnormal ECG is motive for ESE | 16 (5.2%) | |
| Positive exercise stress ECG is the motive for ESE | 6 (1.9%) | |
| Other motives for ESE | 51 (16.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotrim, N.; Café, H.M.; Guardado, J.; Cordeiro, P.; Cotrim, H.; Martins, R.; Baquero, L.; Cotrim, C. Clinical Application of Exercise Stress Echocardiography in an Outpatient Pediatric Population. J. Clin. Med. 2024, 13, 2191. https://doi.org/10.3390/jcm13082191
Cotrim N, Café HM, Guardado J, Cordeiro P, Cotrim H, Martins R, Baquero L, Cotrim C. Clinical Application of Exercise Stress Echocardiography in an Outpatient Pediatric Population. Journal of Clinical Medicine. 2024; 13(8):2191. https://doi.org/10.3390/jcm13082191
Chicago/Turabian StyleCotrim, Nuno, Hugo M. Café, Jorge Guardado, Pedro Cordeiro, Hortense Cotrim, Rui Martins, Luís Baquero, and Carlos Cotrim. 2024. "Clinical Application of Exercise Stress Echocardiography in an Outpatient Pediatric Population" Journal of Clinical Medicine 13, no. 8: 2191. https://doi.org/10.3390/jcm13082191





