In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sperm Preparation
2.3. Chemicals
2.4. Experimental Design
2.5. Flow Cytometry Analysis
2.6. Assessment of the Degree of Chromatin Compactness
2.7. Evaluation of Sperm Apoptosis/Vitality
2.8. Evaluation of the Mitochondrial Membrane Potential
2.9. Evaluation of Sperm DNA Fragmentation
2.10. Detection of Lipoperoxidation
2.11. Measurement of Mitochondrial Superoxide Levels
2.12. Statistical Analysis
3. Results
3.1. Sperm Parameters
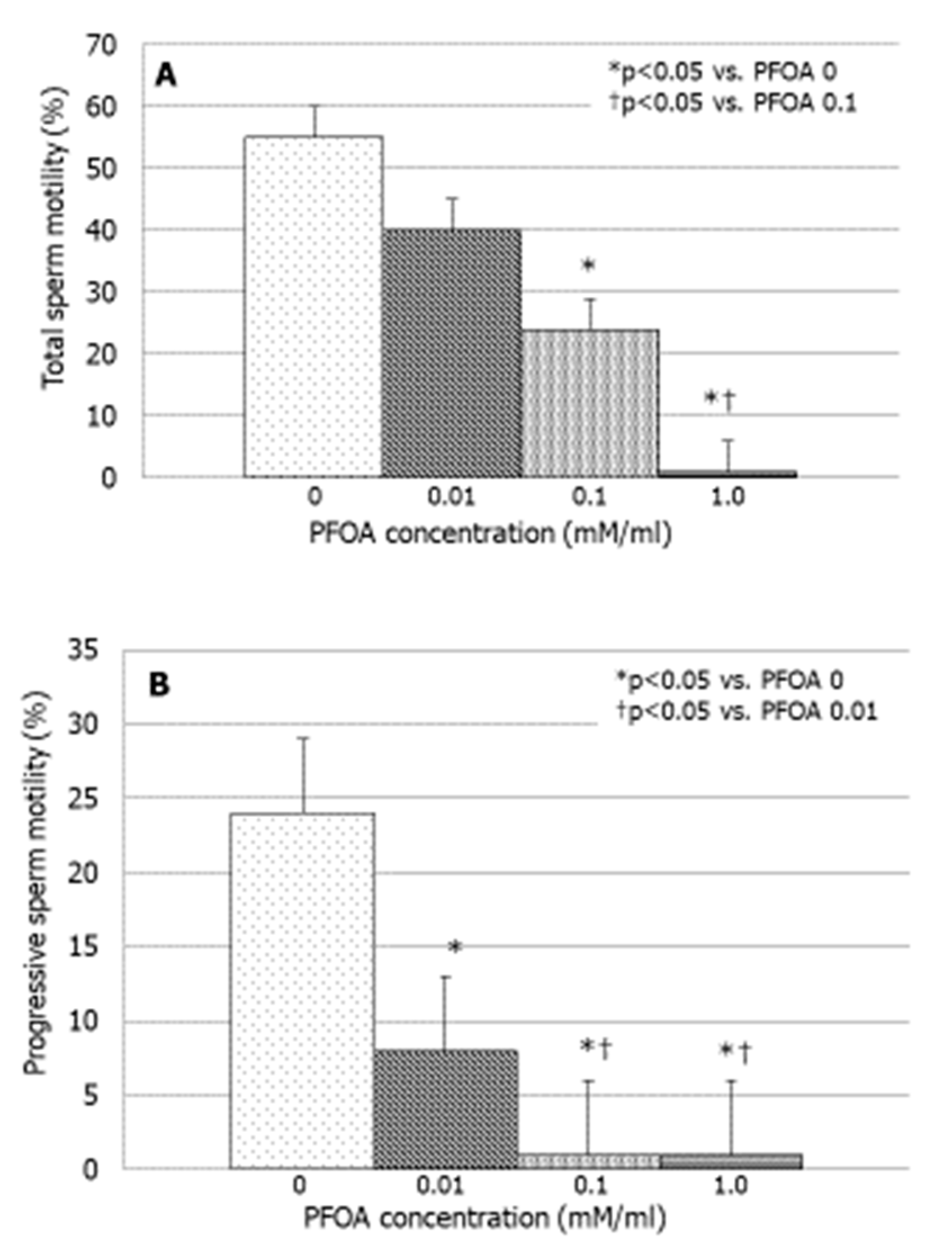
3.2. Effects of PFOA on Sperm Motility
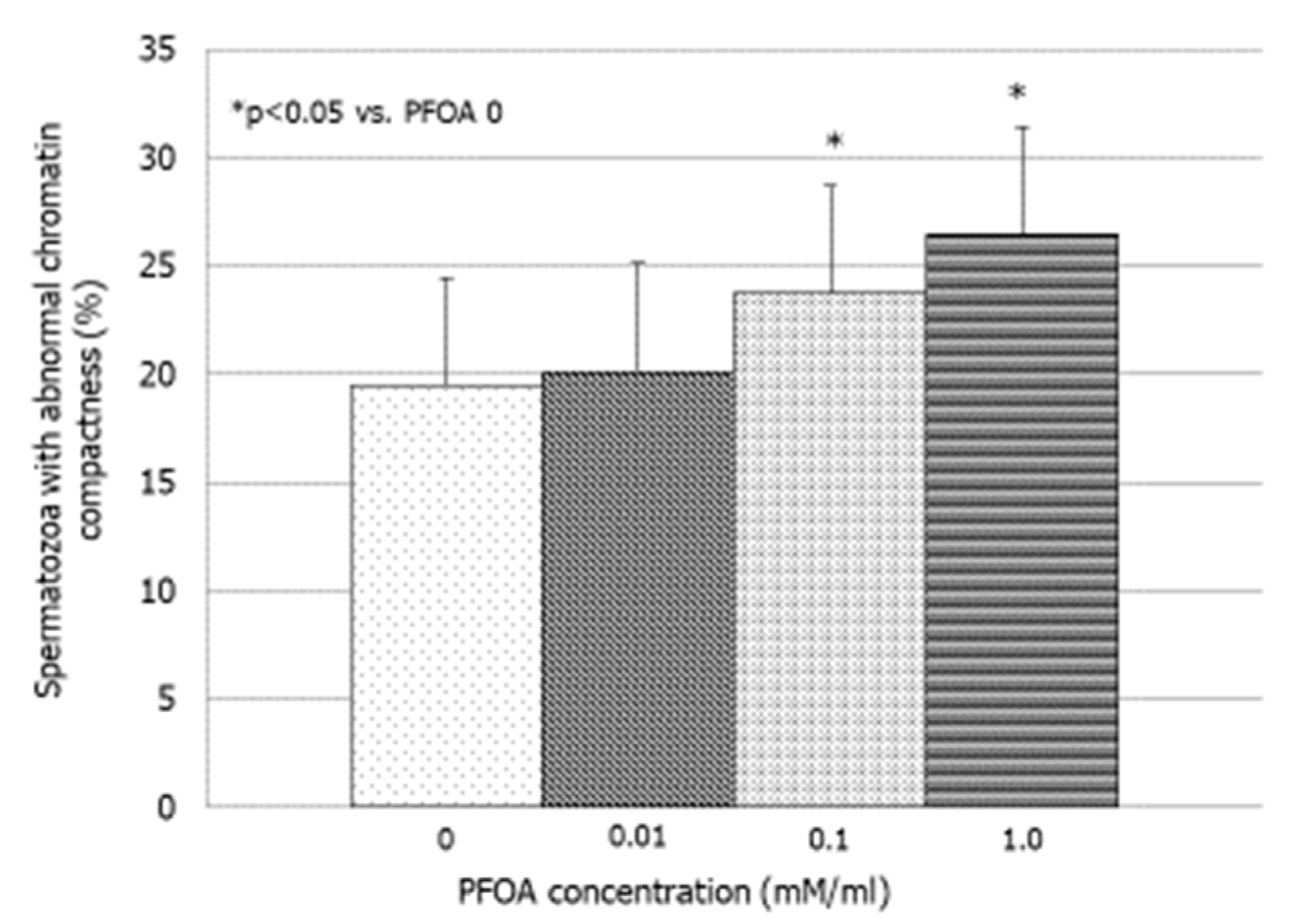
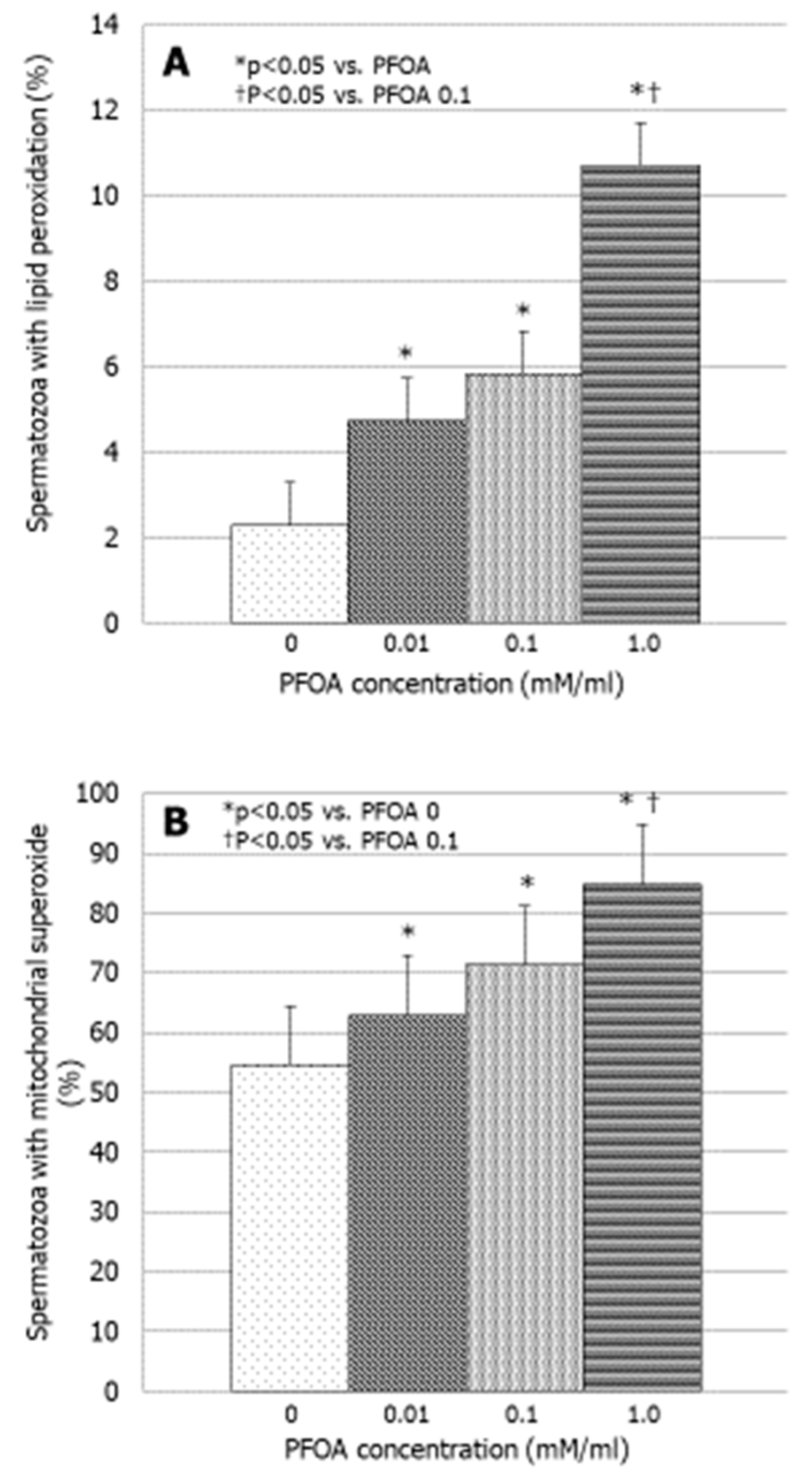
3.3. Effects of PFOA on Bio-Functional Sperm Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurewicz, J.; Radwan, M.; Sobala, W.; Ligocka, D.; Radwan, P.; Bochenek, M.; Hawuła, W.; Jakubowski, L.; Hanke, W. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod. Toxicol. 2013, 42, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Corona, G.; Vitale, P.; Maseroli, E.; Rossi, M.; Fino, M.G.; Maggi, M. Current smoking is associated with lower seminal vesicles and ejaculate volume, despite higher testosterone levels, in male subjects of infertile couples. Hum. Reprod. 2015, 30, 590–602. [Google Scholar] [CrossRef]
- Calogero, A.E.; La Vignera, S.; Condorelli, R.A.; Perdichizzi, A.; Valenti, D.; Asero, P.; Carbone, U.; Boggia, B.; De Rosa, N.; Lombardi, G.; et al. Environmental car exhaust pollution damages human sperm chromatin and DNA. J. Endocrinol. Investig. 2011, 34, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Martenies, S.E.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A systematic review. Toxicology 2013, 10, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rago, R.; Salacone, P.; Caponecchia, L.; Sebastianelli, A.; Marcucci, I.; Calogero, A.E.; Condorelli, R.; Vicari, E.; Morgia, G.; Favilla, V.; et al. The semen quality of the mobile phone users. J. Endocrinol. Investig. 2013, 36, 970–974. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, F.; Chen, Q.; Yang, H.; Zhou, N.; Sun, L.; Zou, P.; Yang, W.; Cao, J.; Zhou, Z.; et al. Associations of ambient air pollutant exposure with seminal plasma MDA, sperm mtDNA copy number, and mtDNA integrity. Environ. Int. 2020, 136, 105483. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Mongioì, L.M.; Li Volti, G.; Barbagallo, I.; Avola, R.; Calogero, A.E. Nicotine effects and receptor expression on human spermatozoa: Possible neuroendocrine mechanism. Front. Physiol. 2017, 28, 177. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Calogero, A.E.; Giacone, F.; Fiore, M.; Barchitta, M.; Agodi, A.; Ferrante, M. B(a)P adduct levels and fertility: A cross-sectional study in a Sicilian population. Mol. Med. Rep. 2017, 15, 3398–3404. [Google Scholar] [CrossRef]
- Kantiani, L.; Llorca, M.; Sanchís, J.; Farré, M.; Barceló, D. Emerging food contaminants: A review. Anal. Bioanal. Chem. 2010, 398, 2413–2427. [Google Scholar] [CrossRef]
- Steenland, K.; Fletcher, T.; Savitz, D.A. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect. 2010, 118, 1100–1108. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Tarone, R.E.; Olsen, J. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Lange, C.C.; Ellefson, M.E.; Mair, D.C.; Church, T.R.; Goldberg, C.L.; Herron, R.M.; Medhdizadehkashi, Z.; Nobiletti, J.B.; Rios, J.A.; et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ. Sci. Technol. 2012, 46, 6330–6338. [Google Scholar] [CrossRef]
- Raymer, J.H.; Michael, L.C.; Studabaker, W.B.; Olsen, G.W.; Sloan, C.S.; Wilcosky, T.; Walmer, D.K. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) and their associations with human semen quality measurements. Reprod. Toxicol. 2012, 33, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Renner, R. Growing concern over perfluorinated chemicals. Environ. Sci. Technol. 2001, 35, 154A–160A. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risks to humans. In Some Chemicals Used as Solvents and in Polymer Manufacture; WHO: Geneva, Switzerland, 2017; Volume 110. [Google Scholar]
- Di Nisio, A.; Sabovic, I.; Valente, U.; Tescari, S.; Rocca, M.S.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; Pozzi, N.; Plebani, M.; et al. Endocrine disruption of androgenic activity by perfluoroalkyl substances: Clinical and experimental evidence. J. Clin. Endocrinol. Metab. 2019, 6, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Seacat, A.M.; Thomford, P.J.; Hansen, K.J.; Olsen, G.W.; Case, M.T.; Butenhoff, J.L. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 2002, 68, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef]
- Petersen, M.S.; Halling, J.; Jørgensen, N.; Nielsen, F.; Grandjean, P.; Jensen, T.K.; Weihe, P. Reproductive Function in a Population of Young Faroese Men with Elevated Exposure to Polychlorinated Biphenyls (PCBs) and Perfluorinated Alkylate Substances (PFAS). Int. J. Environ. Res. Public Health 2018, 15, 1880. [Google Scholar] [CrossRef]
- Vested, A.; Giwercman, A.; Bonde, J.P.; Toft, G. Persistent organic pollutants and male reproductive health. Asian J. Androl. 2014, 16, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Vested, A.; Jørgensen, K.T.; Bonde, J.P.; Henriksen, T.B.; Toft, G. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: A systematic review. Crit. Rev. Toxicol. 2016, 46, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Tescari, S.; Di Nisio, A. Impact of perfluorochemicals on human health and reproduction: A male’s perspective. J. Endocrinol. Investig. 2018, 41, 639–645. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2010 WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010.
- Emerce, E.; Çetin, Ö. Genotoxicity assessment of perfluoroalkyl substances on human sperm. Toxicol. Ind. Health 2018, 16, 748233718799191. [Google Scholar] [CrossRef] [PubMed]
- Šabović, I.; Cosci, I.; De Toni, L.; Ferramosca, A.; Stornaiuolo, M.; Di Nisio, A.; Dall’Acqua, S.; Garolla, A.; Foresta, C. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J. Endocrinol. Investig. 2020, 43, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M. Guidelines for the use of flow cytometry. Immun. Inflamm. Dis. 2017, 5, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Guerranti, C.; De Leo, V.; Boschi, L.; Luddi, A.; Gori, M.; Orvieto, R.; Piomboni, P. Chromosomal aneuploidies and DNA fragmentation of human spermatozoa from patients exposed to perfluorinated compounds. Andrologia 2015, 47, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Joensen, U.N.; Bossi, R.; Leffers, H.; Jensen, A.A.; Skakkebaek, N.E.; Jørgensen, N. Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect. 2009, 117, 923–927. [Google Scholar] [CrossRef]
- Vested, A.; Ramlau-Hansen, C.H.; Olsen, S.F.; Bonde, J.P.; Stovring, H.; Kristensen, S.L.; Halldorsson, T.I.; Rantakokko, P.; Kiviranta, H.; Ernst, E.H.; et al. In utero exposure to persistent organochlorine pollutants and reproductive health in the human male. Reproduction 2014, 148, 635–646. [Google Scholar] [CrossRef]
- Toft, G.; Jönsson, B.A.G.; Lindh, C.H.; Giwercman, A.; Spano, M.; Heederik, D.; Lenters, V.; Vermeulen, R.; Rylander, L.; Pedersen, H.S.; et al. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum. Reprod. 2012, 27, 2532–2540. [Google Scholar] [CrossRef]
- Calogero, A.; Polosa, R.; Perdichizzi, A.; Guarino, F.; La Vignera, S.; Scarfia, A.; Fratantonio, E.; Condorelli, R.A.; Bonanno, O.; Barone, N.; et al. Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod. Biomed. Online 2009, 19, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and male fertility: Effects of benzo-α-pyrene and resveratrol on human sperm function in vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Effects of bisphenols on testicular steroidogenesis. Front. Endocrinol. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Shafeeque, C.M.; Sharma, S.K.; Pandey, N.K.; Singh, R.; Mohan, J.; Kolluri, G.; Saxena, M.; Sharma, B.; Sastry, K.V.H.; et al. Bisphenol A reduces fertilizing ability and motility by compromising mitochondrial function of sperm. Environ. Toxicol. Chem. 2015, 34, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; Raaschou-Nielsen, O.; Sørensen, M.; Roursgaard, M.; Loft, S.; Møller, P. Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutat. Res. 2010, 700, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Specht, I.O.; Hougaard, K.S.; Spanò, M.; Bizzaro, D.; Manicardi, G.C.; Lindh, C.H.; Toft, G.; Jönsson, B.A.G.; Giwercman, A.; Bonde, J.P.E. Sperm DNA integrity in relation to exposure to environmental perfluoroalkyl substances—A study of spouses of pregnant women in three geographical regions. Reprod. Toxicol. 2012, 33, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S. Differential toxicity between perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). J. Toxicol. Sci. 2016, 41, SP27–SP36. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Sai, K.; Umemura, T.; Hasegawa, R.; Kurokawa, Y. Short-term exposure to the peroxisome proliferators, perfluorooctanoic acid and perfluorodecanoic acid, causes significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett. 1991, 57, 55–60. [Google Scholar] [CrossRef]
- Hu, X.Z.; Hu, D.C. Effects of perfluorooctanoate and perfluorooctane sulfonate exposure on hepatoma Hep G2 cells. Arch. Toxicol. 2009, 83, 851–861. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Ye, J.S.; Long, Y.; Qin, H.M. Effect of PFOA on Oxidative Stress and Membrane Damage of Escherichia coli. Huan Jing Ke Xue 2017, 38, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Khosrowbeygi, A.; Zarghami, N.; Farzadi, L. Fatty acid composition in normozoospermic, asthenozoospermic, asthenoteratozoospermic and oligoasthenoteratozoospermic ejaculates. Iran. J. Reprod. Med. 2008, 6, 39–43. [Google Scholar]
- Aksoy, Y.; Aksoy, H.; Altinkaynak, K.; Aydin, H.R.; Ozkan, A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Štramová, X.; Čegan, A.; Hampl, R.; Kanďár, R. Effects of smoking on fatty acid composition of phospholipid sperm membrane and the malondialdehyde levels in human seminal plasma. Andrologia 2015, 47, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jones, P.D.; DeCoen, W.; King, L.; Fraker, P.; Newsted, J.; Giesy, J.P. Alterations in cell membrane properties caused by perfluorinated compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 77–88. [Google Scholar] [CrossRef]
- Suh, K.S.; Choi, E.M.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; et al. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol. Med. Rep. 2017, 15, 3871–3878. [Google Scholar] [CrossRef]
| Sperm Parameter | Values |
|---|---|
| Concentration (million/mL) | 74 ± 8.5 |
| Total count (million/ejaculate) | 260 ± 30.8 |
| Progressive motility (%) | 30.6 ± 1.16 |
| Total motility (%) | 60 ± 6.5 |
| Normal forms (%) | 6.1 ± 0.47 |
| Leukocytes (million/mL) | 0.7 ± 0.04 |
| PFOA Concentration (mM/mL) | 0 | 0.01 | 0.1 | 1.0 |
|---|---|---|---|---|
| Spermatozoa alive (%) | 44.0 ± 3.3 | 34.9 ± 3.6 | 28.2 ± 8.4 | 27.1 ± 4.7 |
| Spermatozoa in early apoptosis (%) | 2.4 ± 1.6 | 2.7 ± 4.4 | 1.3 ± 3.5 | 4.8 ± 3.4 |
| Spermatozoa in late apoptosis (%) | 26.2 ± 6.9 | 32.4 ± 10.0 | 22.3 ± 3.7 | 29.9 ± 9.0 |
| Spermatozoa with low mitochondrial membrane potential (%) | 10.6 ± 1.7 | 14.6 ± 4.4 | 11.6± 4.1 | 20.0 ± 7.2 |
| Spermatozoa with DNA fragmentation (%) | 8.5 ± 3.4 | 9.1 ± 5.4 | 9.9 ± 1.2 | 10.7 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamo, A.; La Vignera, S.; Mogioì, L.M.; Crafa, A.; Barbagallo, F.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences? J. Clin. Med. 2024, 13, 2201. https://doi.org/10.3390/jcm13082201
Alamo A, La Vignera S, Mogioì LM, Crafa A, Barbagallo F, Cannarella R, Aversa A, Calogero AE, Condorelli RA. In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences? Journal of Clinical Medicine. 2024; 13(8):2201. https://doi.org/10.3390/jcm13082201
Chicago/Turabian StyleAlamo, Angela, Sandro La Vignera, Laura M. Mogioì, Andrea Crafa, Federica Barbagallo, Rossella Cannarella, Antonio Aversa, Aldo E. Calogero, and Rosita A. Condorelli. 2024. "In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences?" Journal of Clinical Medicine 13, no. 8: 2201. https://doi.org/10.3390/jcm13082201
APA StyleAlamo, A., La Vignera, S., Mogioì, L. M., Crafa, A., Barbagallo, F., Cannarella, R., Aversa, A., Calogero, A. E., & Condorelli, R. A. (2024). In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences? Journal of Clinical Medicine, 13(8), 2201. https://doi.org/10.3390/jcm13082201














