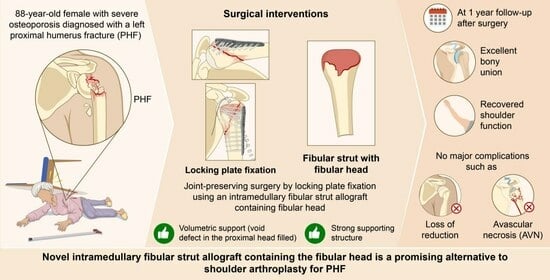
Novel Use of a Fibular Strut Allograft with Fibular Head in an Elderly Patient with Proximal Humeral Fracture and Severe Metaphyseal Comminution: An Alternative to Shoulder Arthroplasty
Abstract
:1. Introduction
2. Case Presentation
Surgical Technique
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iglesias-Rodríguez, S.; Domínguez-Prado, D.M.; García-Reza, A.; Fernández-Fernández, D.; Pérez-Alfonso, E.; García-Piñeiro, J.; Castro-Menéndez, M. Epidemiology of proximal humerus fractures. J. Orthop. Surg. Res. 2021, 16, 402. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Catalan, N. Conservative treatment of proximal Humerus fractures: When, how, and what to expect. Curr. Rev. Musculoskelet. Med. 2023, 16, 75–84. [Google Scholar] [CrossRef]
- Lopiz, Y.; Alcobía-Díaz, B.; Galán-Olleros, M.; García-Fernández, C.; Picado, A.L.; Marco, F. Reverse shoulder arthroplasty versus nonoperative treatment for 3-or 4-part proximal humeral fractures in elderly patients: A prospective randomized controlled trial. J. Shoulder Elb. Surg. 2019, 28, 2259–2271. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Weil, Y.; Barker, J.U.; Kelly, B.T.; Helfet, D.L.; Lorich, D.G. The importance of medial support in locked plating of proximal humerus fractures. J. Orthop. Trauma 2007, 21, 185–191. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, N.; Rui, B.; Chen, Y. The neck-shaft angle is the key factor for the positioning of calcar screw when treating proximal humeral fractures with a locking plate. Bone Jt. J. 2020, 102, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-B.; Moon, E.-S.; Kim, S.-K.; Kovacevic, D.; Kim, M.-S. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet. Disord. 2013, 14, 102. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Kim, T.-Y.; Hwang, J.-T. PHILOS plate fixation with polymethyl methacrylate cement augmentation of an osteoporotic proximal humerus fracture. Clin. Shoulder Elb. 2020, 23, 156. [Google Scholar] [CrossRef]
- Foruria, A.M.; Martinez-Catalan, N.; Valencia, M.; Morcillo, D.; Calvo, E. Proximal humeral fracture locking plate fixation with anatomic reduction, and a short-and-cemented-screws configuration, dramatically reduces the implant related failure rate in elderly patients. JSES Int. 2021, 5, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Lee, D.-H.; Chun, Y.-M.; Shin, S.-J. Which additional augmented fixation procedure decreases surgical failure after proximal humeral fracture with medial comminution: Fibular allograft or inferomedial screws? J. Shoulder Elb. Surg. 2018, 27, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Hyun, Y.-S.; Baek, S.-H. Strut support with tricortical iliac allografts in unstable proximal humerus fractures: Surgical indication and new definition of poor medial column support. Clin. Shoulder Elb. 2019, 22, 29. [Google Scholar] [CrossRef]
- Gardner, M.J.; Boraiah, S.; Helfet, D.L.; Lorich, D.G. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J. Orthop. Trauma 2008, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Chen, H.; Ma, B.; Fan, W.; Li, H. Fibular strut allograft influences reduction and outcomes after locking plate fixation of comminuted proximal humeral fractures in elderly patients: A retrospective study. BMC Musculoskelet. Disord. 2019, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.; Kim, S.-H. Comparison between MIPO and the deltopectoral approach with allogenous fibular bone graft in proximal humeral fractures. Clin. Shoulder Elb. 2020, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qi, Y.m.; Yang, L.; Wang, G.r.; Zheng, S.n.; Wang, Q.; Liang, B.; Jiang, C.z. Comparison of the effects of proximal humeral internal locking system (PHILOS) alone and PHILOS combined with fibular allograft in the treatment of Neer three-or four-part proximal Humerus fractures in the elderly. Orthop. Surg. 2019, 11, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.M.; Triplet, J.J.; Warmoth, P.J.; Passias, B.J.; McGowan, S.P.; Taylor, B.C. Improved outcomes using a fibular strut in proximal humerus fracture fixation. Orthopedics 2020, 43, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Tuerxun, M.; Tuxun, A.; Zeng, L.; Wang, Q.; Chen, Y. Locking plate combined with endosteal fibular allograft augmentation for medial column comminuted proximal humeral fracture. Orthopedics 2020, 43, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.J.; Myeroff, C.M. Reverse shoulder arthroplasty for proximal humerus fracture. Curr. Rev. Musculoskelet. Med. 2020, 13, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.N.; Bjørdal, J.; Wagle, T.M.; Karlberg, A.C.; Lien, O.A.; Eilertsen, L.; Mader, K.; Apold, H.; Larsen, L.B.; Madsen, J.E. Reverse shoulder arthroplasty is superior to plate fixation at 2 years for displaced proximal humeral fractures in the elderly: A multicenter randomized controlled trial. JBJS 2020, 102, 477–485. [Google Scholar] [CrossRef]
- Luciani, P.; Procaccini, R.; Rotini, M.; Pettinari, F.; Gigante, A. Angular stable plate versus reverse shoulder arthroplasty for proximal humeral fractures in elderly patient. Musculoskelet. Surg. 2020, 106, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Greiwe, R.M.; Kohrs, B.J.; Callegari, J.; Harm, R.G.; Hill, M.A.; Boyle, M.S. Open reduction internal fixation vs. reverse shoulder arthroplasty for the treatment of acute displaced proximal humerus fractures. JSES 2020, 30, 250–257. [Google Scholar] [CrossRef]
- Antonios, T.; Bakti, N.; Phadkhe, A.; Gulihar, A.; Singh, B. Outcomes following arthroplasty for proximal humeral fractures. J. Clin. Orthop. Trauma 2020, 11, S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Tabarestani, T.Q.; Levin, J.M.; Warren, E.; Boadi, P.; Twomey-Kozak, J.; Wixted, C.; Goltz, D.E.; Wickman, J.; Hurley, E.T.; Anakwenze, O. Preoperative glenoid bone density is associated with systemic osteoporosis in primary shoulder arthroplasty. JSES 2023, 33, 727–734. [Google Scholar] [CrossRef]
- Casp, A.J.; Montgomery Jr, S.R.; Cancienne, J.M.; Brockmeier, S.F.; Werner, B.C. Osteoporosis and implant-related complications after anatomic and reverse total shoulder arthroplasty. JAAOS-J. Am. Acad. Orthop. Surg. 2020, 28, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-C.; Rhee, K.-J.; Shin, H.-D. Tension band sutures using a washer for a proximal humerus fracture. J. Trauma Acute Care Surg. 2008, 64, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Jung, G.-H.; Song, K.-S. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics 2012, 35, 202–205. [Google Scholar] [CrossRef]
- Terrier, A.; Obrist, R.; Becce, F.; Farron, A. Cement stress predictions after anatomic total shoulder arthroplasty are correlated with preoperative glenoid bone quality. J. Shoulder Elb. Surg. 2017, 26, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Feltri, P.; Ferraro, S.; Ippolito, G.; Campopiano, G.; Previtali, D.; Filardo, G.; Marbach, F.; De Marinis, G.; Candrian, C. Impact of tuberosity treatment in reverse shoulder arthroplasty after proximal humeral fractures: A multicentre study. J. Orthop. Sci. 2023, 28, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Schmalzl, J.; Jessen, M.; Sadler, N.; Lehmann, L.-J.; Gerhardt, C. High tuberosity healing rate associated with better functional outcome following primary reverse shoulder arthroplasty for proximal humeral fractures with a 135° prosthesis. BMC Musculoskelet. Disord. 2020, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Yamada, S.; Kobori, Y.; Shiode, H. The clinical outcomes and tuberosity healing after reverse total shoulder arthroplasty for acute proximal humeral fracture using the turned stem tension band technique. J. Orthop. Sci. 2022, 27, 372–379. [Google Scholar] [CrossRef]
- Rivera, A.R.; Cardona, V. Reverse total shoulder arthroplasty for complex proximal humerus fracture in the elderly: Clinical and radiological results. JSES Rev. Rep. Technol. 2023, 3, 131–136. [Google Scholar] [CrossRef]
- Hackett Jr, D.J.; Hsu, J.E.; Matsen III, F.A. Primary shoulder hemiarthroplasty: What can be learned from 359 cases that were surgically revised? Clin. Orthop. Relat. Res. 2018, 476, 1031. [Google Scholar] [CrossRef] [PubMed]
- Solberg, B.D.; Moon, C.N.; Franco, D.P.; Paiement, G.D. Locked plating of 3-and 4-part proximal humerus fractures in older patients: The effect of initial fracture pattern on outcome. J. Orthop. Trauma 2009, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Harris, J.D.; Erickson, B.J.; Abrams, G.D.; Bruce, B.; McCormick, F.; Nicholson, G.P.; Romeo, A.A. Surgical management of complex proximal humerus fractures—A systematic review of 92 studies including 4500 patients. J. Orthop. Trauma 2015, 29, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sproul, R.C.; Iyengar, J.J.; Devcic, Z.; Feeley, B.T. A systematic review of locking plate fixation of proximal humerus fractures. Injury 2011, 42, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, Z.; Gu, F.; Xu, S.; Yue, Y.; Shao, A.; Sun, K. Effects of fibular strut augmentation for the open reduction and internal fixation of proximal humeral fractures: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Lim, J.-R.; Lee, K.-H.; An, H.; Yoon, T.-H.; Chun, Y.-M. The biomechanical effect of fibular strut grafts on humeral surgical neck fractures with lateral wall comminution. Sci. Rep. 2023, 13, 3744. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, B.M.; Erickson, B.J.; Harris, J.D.; Gupta, A.K.; Mighell, M.; Romeo, A.A. Fibular strut graft augmentation for open reduction and internal fixation of proximal humerus fractures: A systematic review and the authors’ preferred surgical technique. Orthop. J. Sports Med. 2016, 4, 2325967116656829. [Google Scholar] [CrossRef] [PubMed]
- Davids, S.; Allen, D.; Desarno, M.; Endres, N.K.; Bartlett, C.; Shafritz, A. Comparison of locked plating of varus displaced proximal humeral fractures with and without fibula allograft augmentation. J. Orthop. Trauma 2020, 34, 186–192. [Google Scholar] [CrossRef] [PubMed]









| Information | Details |
|---|---|
| Age at surgery | 88 |
| Sex | Female |
| Diagnoses | Severe osteoporosis (T-score −4.6 at the femoral neck) |
| Mild hypertension on medication | |
| Neer 4-part proximal humerus fracture on the left shoulder | |
| Physical Examination | Decreased painful range of motion in the left shoulder |
| Functional demand | Independent light household activities |
| Osteoporosis medication | None |
| Past medical history | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-H.; Ahn, Y.-S.; Kim, S.; Kim, M.-S. Novel Use of a Fibular Strut Allograft with Fibular Head in an Elderly Patient with Proximal Humeral Fracture and Severe Metaphyseal Comminution: An Alternative to Shoulder Arthroplasty. J. Clin. Med. 2024, 13, 2200. https://doi.org/10.3390/jcm13082200
Lim J-H, Ahn Y-S, Kim S, Kim M-S. Novel Use of a Fibular Strut Allograft with Fibular Head in an Elderly Patient with Proximal Humeral Fracture and Severe Metaphyseal Comminution: An Alternative to Shoulder Arthroplasty. Journal of Clinical Medicine. 2024; 13(8):2200. https://doi.org/10.3390/jcm13082200
Chicago/Turabian StyleLim, Jun-Hyuk, Yeong-Seub Ahn, Sungmin Kim, and Myung-Sun Kim. 2024. "Novel Use of a Fibular Strut Allograft with Fibular Head in an Elderly Patient with Proximal Humeral Fracture and Severe Metaphyseal Comminution: An Alternative to Shoulder Arthroplasty" Journal of Clinical Medicine 13, no. 8: 2200. https://doi.org/10.3390/jcm13082200