Evaluating the Efficacy of the Erector Spinae Plane Block as a Supplementary Approach to Cardiac Anesthesia during Off-Pump Coronary Bypass Graft Surgery via Median Sternotomy: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization and Blinding
2.4. Perioperative Management
2.5. Erector Spinae Plane Block
2.6. Outcome Measures
2.7. Sample Size Calculation
2.8. Statistical Analysis
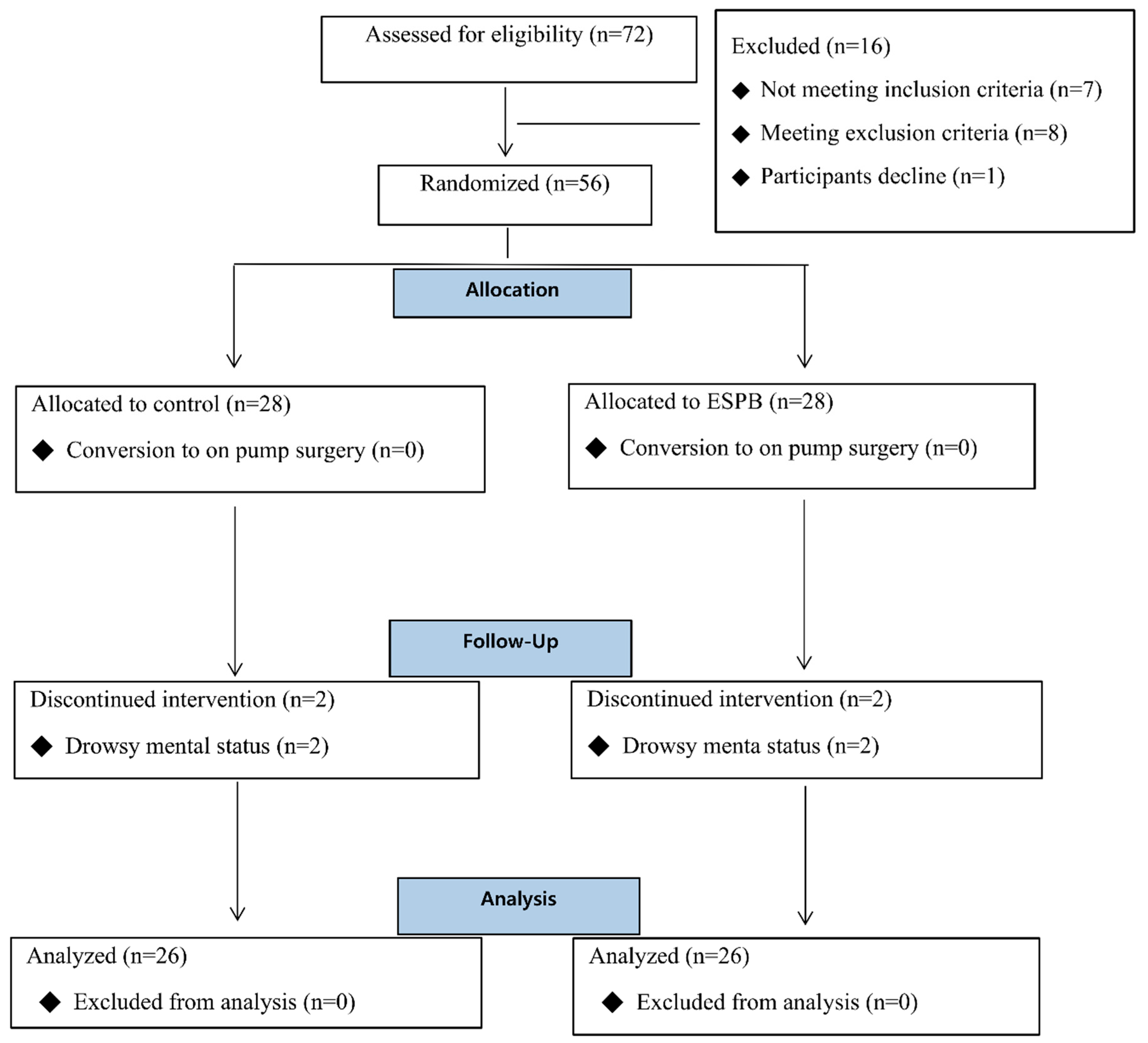
3. Results
4. Discussion
4.1. Discussion
4.2. Strengths and Limitations
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaefi, S.; Mittel, A.; Loberman, D.; Ramakrishna, H. Off-pump versus on-pump coronary artery bypass grafting-a systematic review and analysis of clinical outcomes. J. Cardiothorac. Vasc. Anesth. 2019, 33, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Ofoegbu, C.K.P.; Manganyi, R.M. Off-Pump Coronary Artery Bypass Grafting; is it Still Relevant? Curr. Cardiol. Rev. 2022, 18, e271021197431. [Google Scholar] [CrossRef] [PubMed]
- Cogan, J. Pain management after cardiac surgery. Semin. Cardiothorac. Vasc. Anesth. 2010, 14, 201–204. [Google Scholar] [CrossRef]
- Korsik, E.; Meineri, M.; Zakhary, W.Z.A.; Balga, I.; Jawad, K.; Ender, J.; Flo Forner, A. Persistent and acute postoperative pain after cardiac surgery with anterolateral thoracotomy or median sternotomy: A prospective observational study. J. Clin. Anesth. 2022, 77, 110577. [Google Scholar] [CrossRef]
- Chin, K.J.; El-Boghdadly, K. Mechanisms of action of the erector spinae plane (ESP) block: A narrative review. Can. J. Anaesth. 2021, 68, 387–408. [Google Scholar] [CrossRef]
- Imantalab, V.; Mirmansouri, A.; Mohammadzadeh Jouryabi, A.; Naderi Nabi, B.; Kanani, G.; Nassiri Sheikhani, N.; Atrkarroushan, Z.; Ghazanfar Tehran, S.; Samadpour, N. Comparing the Effectiveness of Patient Control Analgesia Pump and Bolus Morphine in Controlling Pain After Cardiopulmonary Bypass Graft Surgery. Anesth. Pain Med. 2017, 7, e12756. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Kokki, H.; Hynynen, M. Pain after cardiac surgery: A prospective cohort study of 1-year incidence and intensity. Anesthesiology 2006, 105, 794–800. [Google Scholar] [CrossRef]
- Roncada, G.; Dendale, P.; Linsen, L.; Hendrikx, M.; Hansen, D. Reduction in pulmonary function after CABG surgery is related to postoperative inflammation and hypercortisolemia. Int. J. Clin. Exp. Med. 2015, 8, 10938–10946. [Google Scholar]
- Zubrzycki, M.; Liebold, A.; Skrabal, C.; Reinelt, H.; Ziegler, M.; Perdas, E.; Zubrzycka, M. Assessment and pathophysiology of pain in cardiac surgery. J. Pain Res. 2018, 11, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Kalso, E.; Mennander, S.; Tasmuth, T.; Nilsson, E. Chronic post-sternotomy pain. Acta Anaesthesiol. Scand. 2001, 45, 935–939. [Google Scholar] [CrossRef]
- Kwanten, L.E.; O’Brien, B.; Anwar, S. Opioid-Based Anesthesia and Analgesia for Adult Cardiac Surgery: History and Narrative Review of the Literature. J. Cardiothorac. Vasc. Anesth. 2019, 33, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Landino, S.; Cote, C.; Lee, J.; Orhurhu, V.; Shah, R.M.; McGurk, S.; Kaneko, T.; Shekar, P.; Pelletier, M.P. Chronic opioid use after coronary bypass surgery. J. Card. Surg. 2019, 34, 67–73. [Google Scholar] [CrossRef]
- Maeßen, T.; Korir, N.; Van de Velde, M.; Kennes, J.; Pogatzki-Zahn, E.; Joshi, G.P. Pain management after cardiac surgery via median sternotomy: A systematic review with procedure-specific postoperative pain management (PROSPECT) recommendations. Eur. J. Anaesthesiol. 2023, 40, 758–768. [Google Scholar] [CrossRef] [PubMed]
- De Cassai, A.; Bonvicini, D.; Correale, C.; Sandei, L.; Tulgar, S.; Tonetti, T. Erector spinae plane block: A systematic qualitative review. Minerva Anestesiol. 2019, 85, 308–319. [Google Scholar] [CrossRef]
- Saha, S.K.; Ranjan, R.; Adhikary, A.B. Comparison of traditional and upper thoracic epidural analgesia after off-pump coronary artery bypass graft surgery: A Quasi-experimental study. Health Sci. Rep. 2022, 5, e774. [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.; Khelemsky, Y. Poststernotomy pain: A clinical review. J. Cardiothorac. Vasc. Anesth. 2011, 25, 1163–1178. [Google Scholar] [CrossRef]
- Landoni, G.; Isella, F.; Greco, M.; Zangrillo, A.; Royse, C.F. Benefits and risks of epidural analgesia in cardiac surgery. Br. J. Anaesth. 2015, 115, 25–32. [Google Scholar] [CrossRef]
- Caruso, T.J.; Lawrence, K.; Tsui, B.C.H. Regional anesthesia for cardiac surgery. Curr. Opin. Anaesthesiol. 2019, 32, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.N.; Chauhan, S.; Bhoi, D.; Kaushal, B.; Hasija, S.; Sangdup, T.; Bisoi, A.K. Bilateral Erector Spinae Plane Block for Acute Post-Surgical Pain in Adult Cardiac Surgical Patients: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2019, 33, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Mueller, X.M.; Tinguely, F.; Tevaearai, H.T.; Revelly, J.P.; Chioléro, R.; von Segesser, L.K. Pain location, distribution, and intensity after cardiac surgery. Chest 2000, 118, 391–396. [Google Scholar] [CrossRef]
- Oğur, L.; Akesen, S.; Gören, S.; Kan, İ.; Başağan Moğol, E.; Gurbet, A. Comparison of Intra- and postoperative effectiveness of erector spinae plane block and patient controlled analgesia in patients undergoing coronary artery bypass grafting surgery. Am. J. Transl. Res. 2022, 14, 2469–2479. [Google Scholar] [PubMed]
- Saadawi, M.; Layera, S.; Aliste, J.; Bravo, D.; Leurcharusmee, P.; Tran, Q. Erector spinae plane block: A narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J. Clin. Anesth. 2021, 68, 110063. [Google Scholar] [CrossRef]
- Pawa, A.; King, C.; Thang, C.; White, L. Erector spinae plane block: The ultimate ‘plan A’ block? Br. J. Anaesth. 2023, 130, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Coşarcan, S.K.; Doğan, A.T.; Gurkan, Y.; Erçelen, Ö. Analgesic Effect of Dual Injection Technique for the Erector Spinae Plane Block in Beating Heart Coronary By-Pass Surgeries. Cureus 2021, 13, e14122. [Google Scholar] [CrossRef]
- Wiech, M.; Żurek, S.; Kurowicki, A.; Horeczy, B.; Czuczwar, M.; Piwowarczyk, P.; Widenka, K.; Borys, M. Erector Spinae Plane Block Decreases Chronic Postoperative Pain Severity in Patients Undergoing Coronary Artery Bypass Grafting. J. Clin. Med. 2022, 11, 5949. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.; Alabd, A.S.; Ahmed, A.M.M.; Deghidy, E.A. Erector spinae versus paravertebral plane blocks in modified radical mastectomy: Randomised comparative study of the technique success rate among novice anaesthesiologists. Indian J. Anaesth. 2020, 64, 49–54. [Google Scholar] [CrossRef] [PubMed]

| Control Group (n = 26) | ESPB Group (n = 26) | p Value | |
|---|---|---|---|
| Age (years) | 64.58 ± 11.07 [43, 83] | 63.65 ± 10.83 [47, 85] | 0.602 |
| Female | 6 (23.1) | 4 (15.4) | 0.482 |
| Height (cm) | 163.75 ± 9.30 [144.0, 179.1] | 164.79 ± 7.97 [145.0, 183.0] | 0.667 |
| Weight (kg) | 66.82 ± 13.63 [44.42, 94.40] | 70.33 ± 11.11 [49.42, 90.10] | 0.314 |
| Hypertension | 20 (76.9) | 21 (80.8) | 0.734 |
| Diabetes mellitus | 16 (61.5) | 16 (61.5) | 1.000 |
| Cerebral vascular disease | 5 (19.2) | 2 (7.7) | 0.223 |
| Chronic kidney disease | 4 (15.4) | 6 (23.1) | 0.482 |
| NYHA class | 2.19 ± 0.98 [1, 4] | 2.00 ± 0.80 [1, 4] | 0.442 |
| CCS score | 2.19 ± 0.98 [1, 4] | 2.04 ± 0.72 [1, 4] | 0.522 |
| EuroSCORE | 1.73 ± 1.50 [0.50, 6.29] | 1.45 ± 0.73 [0.50, 3.01] | 0.397 |
| Heart failure | 10 (38.5) | 10 (38.5) | 1.000 |
| Ejection fraction (%) | 49.19 ± 12.67 [26, 67] | 49.73 ± 11.54 [27, 72] | 0.873 |
| Surgery time (min) | 243.31 ± 33.27 [175, 325] | 239.54 ± 31.14 [171, 315] | 0.675 |
| Anesthesia time (min) | 327.31 ± 35.92 [270, 420] | 331.73 ± 33.67 [250, 420] | 0.649 |
| Control Group (n = 26) | ESPB Group (n = 26) | p Value | Mean Difference (95% CI) | |
|---|---|---|---|---|
| Primary Outcomes | ||||
| VAS 6 h | 5.96 ± 1.84 [2, 9] | 4.46 ± 1.98 [0, 9] | 0.020 * | −1.27 (−2.33, −0.20) |
| VAS 12 h | 4.58 ± 2.25 [2, 9] | 3.85 ± 1.87 [0, 9] | 0.208 | −0.73 (−1.88, 0.42) |
| VAS 24 h | 3.73 ± 2.07 [1, 9] | 3.62 ± 1.75 [1, 8] | 0.829 | −0.12 (−1.18, 0.95) |
| VAS 48 h | 2.85 ± 2.39 [0, 10] | 2.00 ± 1.62 [0, 6] | 0.143 | −0.85 (−1.99, 0.29) |
| VAS (7–10) | 13 (50) | 4 (15.4) | 0.008 * | |
| Secondary outcomes | ||||
| Number of rescue analgesic events | 1.27 ± 1.25 [0, 4] | 0.73 ± 0.92 [0, 3] | 0.083 | −0.54 (−1.15, 0.07) |
| Rescue analgesic dose (MME) | 19.04 ± 18.76 [0, 60.0] | 9.83 ± 12.84 [0, 45.0] | 0.044 * | −9.21 (−18.16, −0.25) |
| Number of antiemetic agents required | 0.19 ± 0.49 [0, 2] | 0.15 ± 0.54 [0, 2] | 0.790 | −0.04 (−0.33, 0.25) |
| New onset atrial fibrillation, n (%) | 3 (11.5) | 3 (11.5) | 1.000 | |
| Pneumothorax | 0 (0) | 0 (0) | 1.000 | |
| Wound infection | 1 (3.8) | 0 (0) | 0.313 | |
| Mortality | 0 (0) | 0 (0) | 1.000 | |
| ICU duration (day) | 1.27 ± 0.83 [1, 4] | 1.12 ± 0.33 [1, 2] | 0.382 | −0.15 (−0.50, 0.20) |
| Hospital day (day) | 12.50 ± 12.50 [6, 68] | 9.73 ± 2.41 [7, 16] | 0.277 | −2.78 (−7.89, 2.36)- |
| Time to extubation (min) | 671.15 ± 528.07 [185, 2835] | 561.15 ± 263.26 [185, 1125] | 0.346 | −110.00 (−342.43, 122.43) |
| Variable | Regression Coefficient | T Value | p Value |
|---|---|---|---|
| ESPB | −1.386 | −2.331 | 0.025 * |
| Sex | −0.602 | −0.754 | 0.455 |
| Age | 0.001 | 0.028 | 0.978 |
| DM | −0.117 | −0.182 | 0.978 |
| EuroSCORE | −0.362 | −0.182 | 0.856 |
| Operation time | −0.008 | −1.187 | 0.242 |
| Graft number | 0.084 | 0.170 | 0.866 |
| Ejection fraction | 0.021 | 0.712 | 0.481 |
| Dose of Norepinephrine | −0.001 | −0.096 | 0.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Song, S.W.; Jeon, Y.-G.; Song, S.A.; Hong, S.; Park, J.-H. Evaluating the Efficacy of the Erector Spinae Plane Block as a Supplementary Approach to Cardiac Anesthesia during Off-Pump Coronary Bypass Graft Surgery via Median Sternotomy: A Randomized Clinical Trial. J. Clin. Med. 2024, 13, 2208. https://doi.org/10.3390/jcm13082208
Kim S, Song SW, Jeon Y-G, Song SA, Hong S, Park J-H. Evaluating the Efficacy of the Erector Spinae Plane Block as a Supplementary Approach to Cardiac Anesthesia during Off-Pump Coronary Bypass Graft Surgery via Median Sternotomy: A Randomized Clinical Trial. Journal of Clinical Medicine. 2024; 13(8):2208. https://doi.org/10.3390/jcm13082208
Chicago/Turabian StyleKim, Sujin, Seung Woo Song, Yeong-Gwan Jeon, Sang A. Song, Soonchang Hong, and Ji-Hyoung Park. 2024. "Evaluating the Efficacy of the Erector Spinae Plane Block as a Supplementary Approach to Cardiac Anesthesia during Off-Pump Coronary Bypass Graft Surgery via Median Sternotomy: A Randomized Clinical Trial" Journal of Clinical Medicine 13, no. 8: 2208. https://doi.org/10.3390/jcm13082208
APA StyleKim, S., Song, S. W., Jeon, Y.-G., Song, S. A., Hong, S., & Park, J.-H. (2024). Evaluating the Efficacy of the Erector Spinae Plane Block as a Supplementary Approach to Cardiac Anesthesia during Off-Pump Coronary Bypass Graft Surgery via Median Sternotomy: A Randomized Clinical Trial. Journal of Clinical Medicine, 13(8), 2208. https://doi.org/10.3390/jcm13082208






