Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications
Abstract
:1. Introduction
2. Epidemiological Surveillance: Data Availability, Management, and Modeling
3. Molecular Aspects of Viral Replication and Immune Response
3.1. Molecular Mechanisms of Viral Replication
3.2. Molecular Mechanism of Host Immune Response
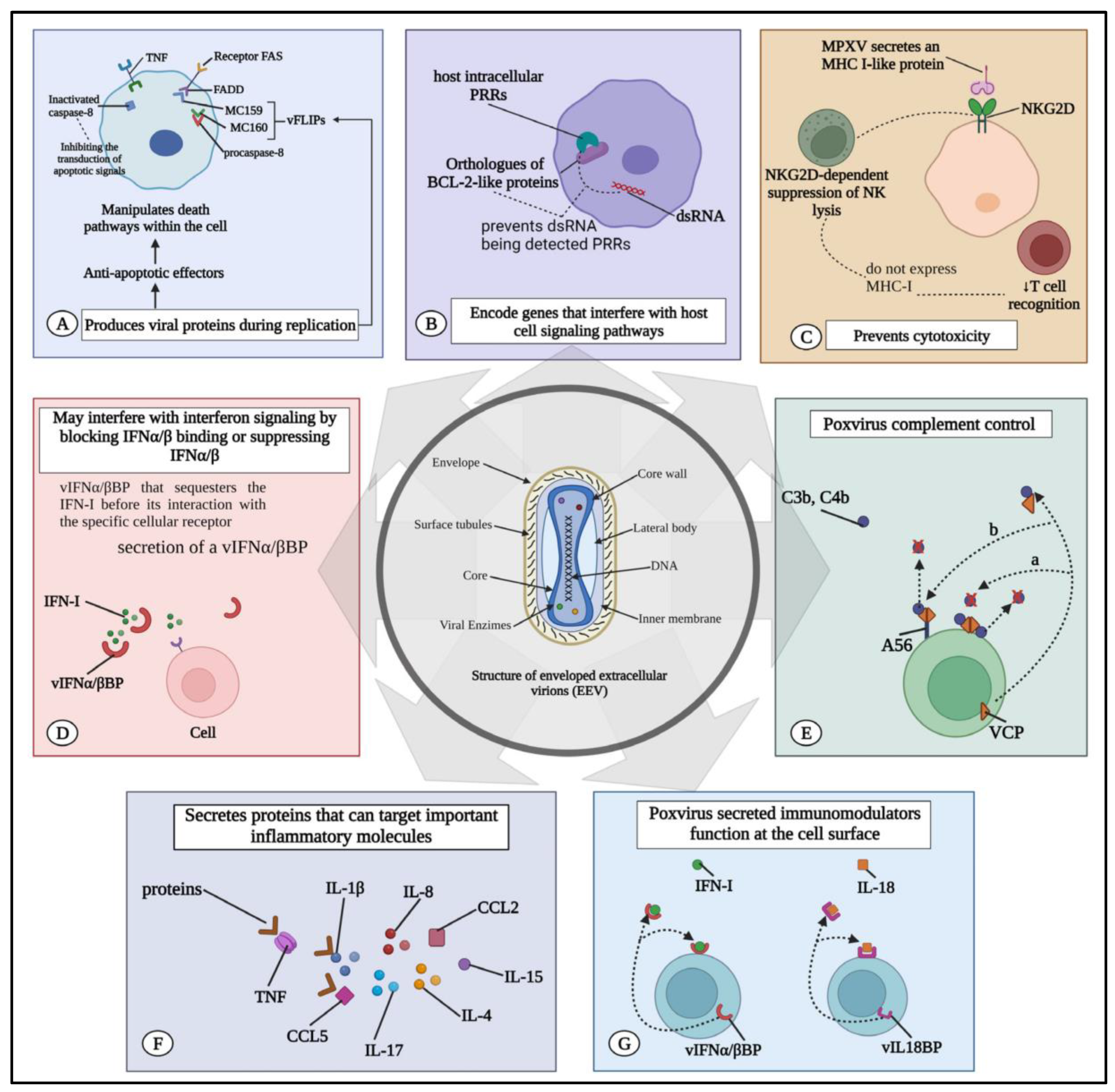
3.3. Evasion Strategies of the Virus
4. Diagnostic Methods for MPXV
4.1. Mpox Laboratory-Based Testing Methods
4.2. Nucleic Acid Amplification Testing (NAAT)
4.3. Electron Microscopy
4.4. Virus Isolation and Culture
4.5. Serology
4.6. Whole-Genome Sequencing (WGS)
5. Public Health Implications
- Supporting outbreak response efforts: International partners can assist affected countries in deploying rapid response teams, establishing isolation and treatment facilities, and implementing control measures such as case detection, contact tracing, and vaccination campaigns [54].
- Strengthening laboratory capacity: Enhancing laboratory diagnostics and surveillance capabilities is crucial for the timely detection and confirmation of Mpox cases. International collaboration can support the establishment of laboratory networks, training of personnel, and procurement of diagnostic reagents and equipment [55].
- Conducting research: Research efforts should focus on advancing the understanding of Mpox epidemiology, transmission dynamics, and host–pathogen interactions. International collaboration enables the sharing of research findings, data analysis, and coordination of multicenter studies to address knowledge gaps and inform evidence-based interventions [56].
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banuet-Martinez, M.; Yang, Y.; Jafari, B.; Kaur, A.; Butt, Z.A.; Chen, H.H.; Yanushkevich, S.; Moyles, I.R.; Heffernan, J.M.; Korosec, C.S. Monkeypox: A review of epidemiological modelling studies and how modelling has led to mechanistic insight. Epidemiol. Infect. 2023, 151, e121. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Cripps, S.; Tanner, M.A. Forecasting for COVID-19 has failed. Int. J. Forecast. 2022, 38, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.; Ioannidis, J.P.; Tanner, M.A.; Cripps, S. Effect estimates of COVID-19 non pharmaceutical interventions are non-robust and highly model-dependent. J. Clin. Epidemiol. 2021, 136, 96–132. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, K.; Gruson, H.; Johnson, H.; Niehus, R.; Prasse, B.; Sandmann, F.; Deuschel, J.; Wolffram, D.; Abbott, S.; Ullrich, A.; et al. Predictive performance of multi-model ensemble forecasts of COVID-19 across European nations. Elife 2023, 12, e81916. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Pierini, M.; Mazzoli, S. Monkeypox: Early estimation of basic reproduction number R0 in Europe. J. Med. Virol. 2023, 95, e28270. [Google Scholar] [CrossRef] [PubMed]
- Jona Lasinio, G.; Divino, F.; Lovison, G.; Mingione, M.; Alaimo Di Loro, P.; Farcomeni, A.; Maruotti, A. Two years of COVID-19 pandemic: The Italian experience of Statgroup-19. Environmetrics 2022, 33, e2768. [Google Scholar] [CrossRef] [PubMed]
- Mingione, M.; Ciccozzi, M.; Falcone, M.; Maruotti, A. Short-term forecasts of Monkeypox cases in multiple countries: Keep calm and don’t panic. J. Med. Virol. 2023, 95, e28159. [Google Scholar] [CrossRef] [PubMed]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Virus replication. In Fenner and White’s Medical Virology; Academic Press: Cambridge, MA, USA, 2017; p. 39. [Google Scholar]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlovic, S.; Cordic, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: A reemergent threat to humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Abas, A.H.; Tallei, T.E.; Al-Zaher, M.A.; Al-Sheef, N.M.; Fatimawali; Al-Nass, E.Z.; Al-Ebrahim, E.A.; Effendi, Y.; Idroes, R.; et al. Monkeypox outbreak 2022: What we know so far and its potential drug targets and management strategies. J. Med. Virol. 2023, 95, e28306. [Google Scholar] [CrossRef]
- Zandi, M.; Shafaati, M.; Hosseini, F. Mechanisms of immune evasion of monkeypox virus. Front. Microbiol. 2023, 14, 1106247. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Fiorino, C.; Aqeel, A.; Liu, Y.; Henao, R.; Ko, E.R.; Burke, T.W.; Bodinayake, C.K.; Woods, C.W.; Ginsburg, G.S.; et al. The host response to viral infections reveals common and virus-specific signatures in the peripheral blood. Front. Immunol. 2021, 12, 741837. [Google Scholar] [CrossRef]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, G.; Tahiliani, V.; Eldi, P.; Karupiah, G. Vaccine-induced protection against or-thopoxvirus infection is mediated through the combined functions of CD4 T cell-dependent antibody and CD8 T cell responses. J. Virol. 2015, 89, 1889–1899. [Google Scholar] [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Alcamí, A.; Hernáez, B. New insights into the immunomodulatory properties of poxvirus cytokine decoy receptors at the cell surface. F1000Res 2018, 7, PMC5998005. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Yu, F.; Cheng, R.; Zhang, Y.; Zhou, D.; Ren, X.; Deng, Z.; Zhao, H. Structural and functional insights into the modulation of T cell costimulation by monkeypox virus protein M2. Nat. Commun. 2023, 14, 5186. [Google Scholar] [CrossRef] [PubMed]
- Qudus, M.S.; Cui, X.; Tian, M.; Afaq, U.; Sajid, M.; Qureshi, S.; Liu, S.; Ma, J.; Wang, G.; Faraz, M.; et al. The prospective outcome of the monkeypox outbreak in 2022 and characterization of monkeypox disease immunobiology. Front. Cell. Infect. Microbiol. 2023, 13, 1284014. [Google Scholar]
- He, Y.; Tang, Y.; Wang, C.; Zhou, Z.; Li, W.; Tian, M. The Global Health Threat of Monkey-pox Virus: Understanding Its Biology, Transmission, and Potential Therapeutic Interventions. Infect. Drug Resist. 2023, 16, 7759–7766. [Google Scholar] [CrossRef]
- Lucena-Neto, F.D.; Falcão, L.F.M.; Vieira-Junior, A.S.; Moraes, E.C.S.; David, J.P.F.; Silva, C.C.; Sousa, J.R.; Duarte, M.I.S.; Vasconcelos, P.F.C.; Quaresma, J.A.S. Monkeypox Virus Immune Evasion and Eye Manifestation: Beyond Eyelid Implications. Viruses 2023, 15, 2301. [Google Scholar] [CrossRef]
- Giovanetti, M.; Cella, E.; Moretti, S.; Scarpa, F.; Ciccozzi, A.; Slavov, S.N.; Benedetti, F.; Zella, D.; Ceccarelli, G.; Ciccozzi, M.; et al. Monitoring monkeypox: Safeguarding global health through rapid response and global surveillance. Pathogens 2023, 12, 1153. [Google Scholar] [CrossRef]
- 2022 Monkeypox Outbreak: Global Trends; World Health Organization; Geneva, Switzerland, 2022; Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 27 June 2023).
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, H.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods 2010, 169, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Testing for the Monkeypox Virus: Interim Guidance. Available online: https://iris.who.int/handle/10665/354488 (accessed on 5 December 2022).
- Scarpa, F.; Sanna, D.; Azzena, I.; Cossu, P.; Locci, C.; Angeletti, S.; Maruotti, A.; Ceccarelli, G.; Casu, M.; Fiori, P.L.; et al. Genetic Variability of the Monkeypox Virus Clade IIb B.1. J. Clin. Med. 2022, 11, 6388. [Google Scholar] [CrossRef]
- Gentile, M.; Gelderblom, H.R. Rapid viral diagnosis: Role of electron microscopy. New Microbiol. 2005, 28, 1–12. [Google Scholar] [PubMed]
- Müller, M.; Ingold-Heppner, B.; Stocker, H.; Heppner, F.L.; Dittmayer, C.; Laue, M. Electron microscopy images of monkeypox virus infection in 24-year-old man. Lancet 2022, 400, 1618. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980. [Google Scholar] [CrossRef]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C.; et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. MSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell 2016, 167, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of peptide-based ELISA for serological diagnostics: A retrospective study of human monkeypox infection. Vector-Borne Zoonotic Dis. 2012, 12, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Farlow, J.; Ichou, M.A.; Huggins, J.; Ibrahim, S. Comparative whole genome sequence analysis of wild-type and cidofovir-resistant monkeypoxvirus. Virol. J. 2010, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Fonseca, V.; de la Fuente, A.G.; Gonzalez, S.; Fleitas, F.; Lima, M.; Guimarães, N.R.; Iani, F.C.; Rojas, A.; Alfonso, T.; et al. Exploring the Genomic Dynamics of the Monkeypox Epidemic in Paraguay. Viruses 2024, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Malekani, J.; Kabamba, J.; Bomponda, P.L.; et al. Introduction of monkeypox into a community and household: Risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Guo, X.; Hong, D.; Gong, Q.; Xie, W.; Li, T.; Wang, J.; Chuai, X.; Chiu, S. Duration of humoral immunity from smallpox vaccination and its cross-reaction with Mpox virus. Signal Transduct. Target. Ther. 2023, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Goldstein, J. Adverse events occurring after smallpox vaccination. In Seminars in Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2003; Volume 14, pp. 189–195. [Google Scholar]
- McQuiston, J.H. The CDC Domestic Mpox Response—United States, 2022–2023; U.S. Department of Health & Human Services: Washington, DC, USA, 2023; p. 72.
- Branda, F.; Pierini, M.; Mazzoli, S. Monkeypox: EpiMPX surveillance system and open data with a special focus on European and Italian epidemic. J. Clin. Virol. Plus 2022, 2, 100114. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Managing Epidemics: Key Facts about Major Deadly Diseases; World Health Organization: Geneve, Switzerland, 2018.
- Williams, B.A.; Jones, C.H.; Welch, V.; True, J.M. Outlook of pandemic preparedness in a post-COVID-19 world. NPJ Vaccines 2023, 8, 178. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhao, Y. Multi-mitigation strategies in medical supplies for epidemic outbreaks. Socio-Econ. Plan. Sci. 2023, 87, 101516. [Google Scholar] [CrossRef]
- Mutch, C.P.; Tiernan, J.; Koch, O.; Poller, B. The PATH to PPE Mastery-Programme for Assessment and Training in HCID (High Consequence Infectious Diseases) PPE (Personal Protective Equipment), Mastery. Infect. Prev. Pract. 2023, 5, 100308. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, S.E.; Abolade, S.A.; Ofeh, A.S.; Awoyinka, T.B.; Okolo, B.O.; Ayeni, E.T.; Kolawole, E.O. Knowledge, attitude, and perception of monkeypox among medical/health students across media space in Nigeria. Int. J. Community Med. Public Health 2022, 9, 4391. [Google Scholar] [CrossRef]
- Ben-Enukora, C.; Oyero, O.; Okorie, N.; Odiboh, O.O.; Adeyeye, B.K. Analysis of 2017 risk communication on Human Monkey Pox outbreak in Nigeria’s News Media. Int. J. Educ. Inf. Technol. 2020, 14, 69–75. [Google Scholar] [CrossRef]
- Roess, A.A.; Monroe, B.P.; Kinzoni, E.A.; Gallagher, S.; Ibata, S.R.; Badinga, N.; Molouania, T.M.; Mabola, F.S.; Mombouli, J.V.; Carroll, D.S.; et al. Assessing the effectiveness of a community intervention for monkeypox prevention in the Congo basin. PLoS Neglected Trop. Dis. 2011, 5, e1356. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, R.M.; Yazbek, S.; Gebreal, A.; Hussein, M.; Addai, S.A.; Mensah, E.; Sarfo, M.; Kofi, A.; Al-Ahdal, T.; Eshun, G. Monkeypox vaccine acceptance among Ghanaians: A call for action. Vaccines 2023, 11, 240. [Google Scholar] [CrossRef]
- Gesser-Edelsburg, A.; Shir-Raz, Y.; Walter, N.; Mordini, E.; Dimitriou, D.; James, J.J.; Green, M.S. The public sphere in emerging infectious disease communication: Recipient or active and vocal partner? Disaster Med. Public Health Prep. 2015, 9, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Hoff, N.A. Utilization Assessment of Infectious Disease Surveillance Data to Enhance Methods for Better Understanding Disease Occurrence, Trends and Gaps in Disease Reporting in a Resource Limited Setting: Monkeypox in the Democratic Republic of Congo; University of California: Los Angeles, CA, USA, 2014. [Google Scholar]
- Fitzmaurice, A.G.; Mahar, M.; Moriarty, L.F.; Bartee, M.; Hirai, M.; Li, W.; Gerber, A.R.; Tappero, J.W.; Bunnell, R.; Group, G.I. Contributions of the US Centers for Disease Control and Prevention in implementing the Global Health Security Agenda in 17 partner countries. Emerg. Infect. Dis. 2017, 23, S15. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, D.; Ihekweazu, C. Nigeria’s efforts to strengthen laboratory diagnostics-why access to reliable and affordable diagnostics is key to building resilient laboratory systems. Afr. J. Lab. Med. 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- White, L.A.; Forester, J.D.; Craft, M.E. Disease outbreak thresholds emerge from interactions between movement behavior, landscape structure, and epidemiology. Proc. Natl. Acad. Sci. USA 2018, 115, 7374–7379. [Google Scholar] [CrossRef]
- Laurenson-Schafer, H.; Sklenovská, N.; Hoxha, A.; Kerr, S.M.; Ndumbi, P.; Fitzner, J.; Almiron, M.; de Sousa, L.A.; Briand, S.; Cenciarelli, O.; et al. Description of the first global outbreak of mpox: An analysis of global surveillance data. Lancet 2023, 11, e1012-23. [Google Scholar] [CrossRef]
- Ward, T.; Overton, C.E.; Paton, R.S.; Christie, R.; Cumming, F.; Fyles, M. Understanding the infection severity and epidemiological characteristics of mpox in the UK. Nat. Commun. 2024, 15, 2199. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.F.; Farinacci, D.; Lombardi, F.; Ciccullo, A.; Tamburrini, E.; Santangelo, R.; Borghetti, A.; Di Giambenedetto, S. Clinical presentation of human monkeypox virus infection during the 2022 outbreak: Descriptive case series from a large italian Research Hospital. Virol. J. 2023, 20, 214. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branda, F.; Romano, C.; Ciccozzi, M.; Giovanetti, M.; Scarpa, F.; Ciccozzi, A.; Maruotti, A. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. J. Clin. Med. 2024, 13, 2234. https://doi.org/10.3390/jcm13082234
Branda F, Romano C, Ciccozzi M, Giovanetti M, Scarpa F, Ciccozzi A, Maruotti A. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. Journal of Clinical Medicine. 2024; 13(8):2234. https://doi.org/10.3390/jcm13082234
Chicago/Turabian StyleBranda, Francesco, Chiara Romano, Massimo Ciccozzi, Marta Giovanetti, Fabio Scarpa, Alessandra Ciccozzi, and Antonello Maruotti. 2024. "Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications" Journal of Clinical Medicine 13, no. 8: 2234. https://doi.org/10.3390/jcm13082234






