Evaluation of Motion Artifact Correction Technique for Cone-Beam Computed Tomography Image Considering Blood Vessel Geometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
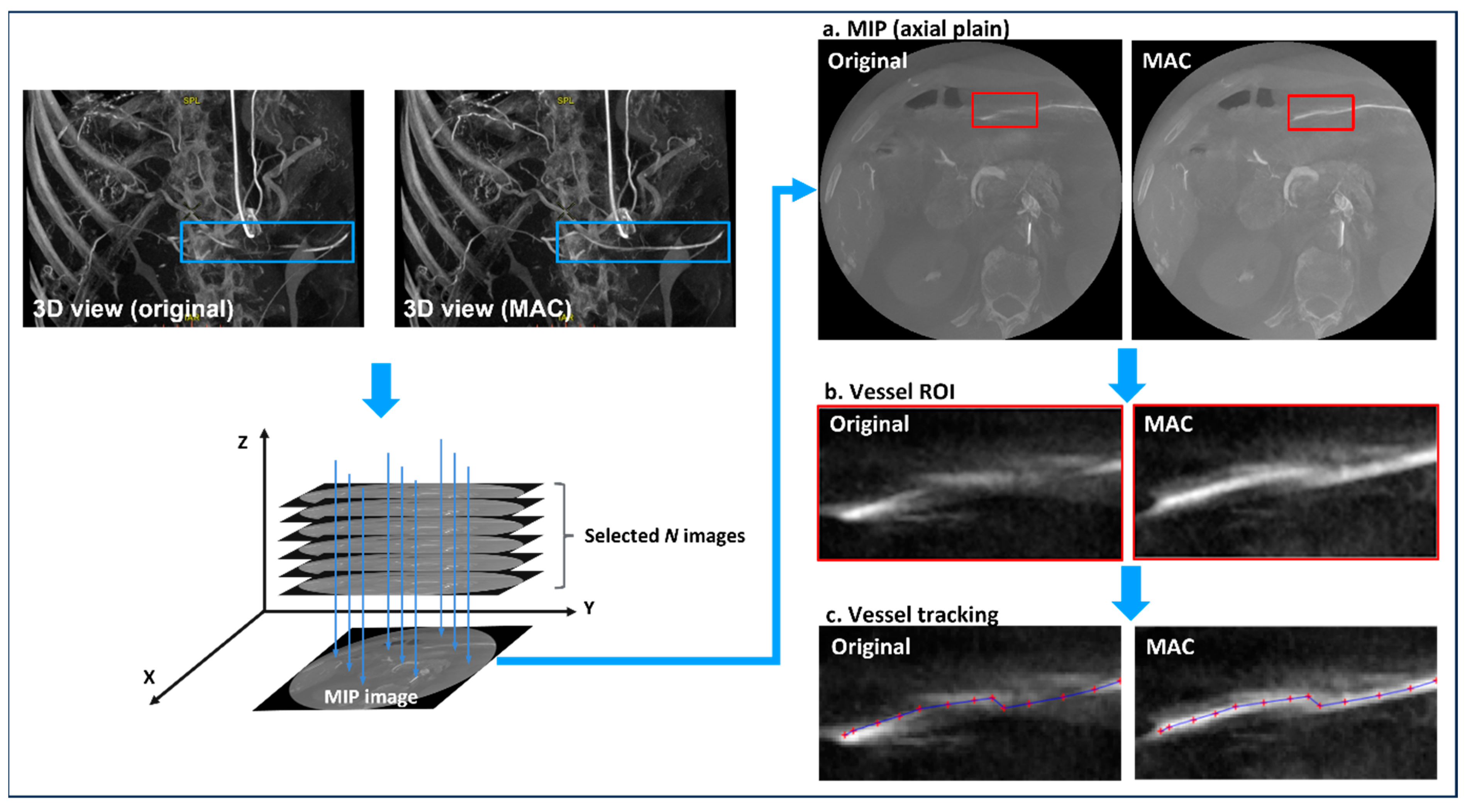
2.2. Motion-Corrected Index
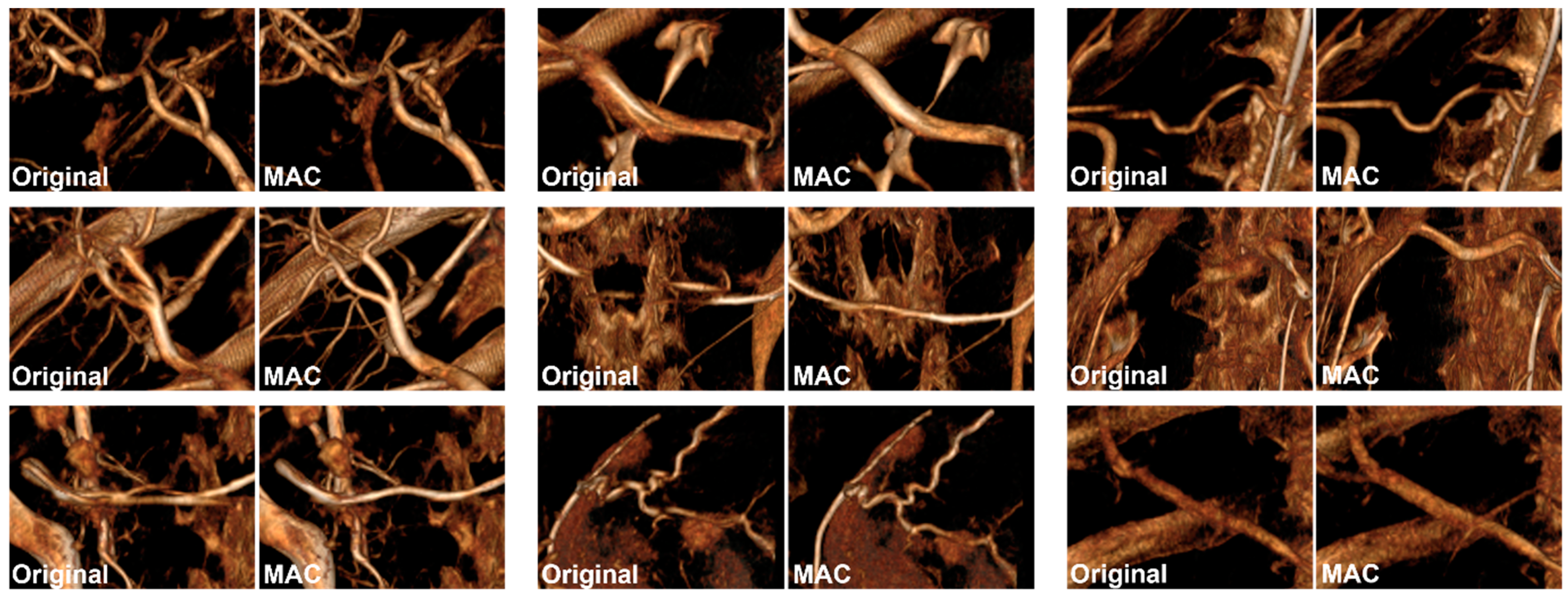
2.3. Experiment 1
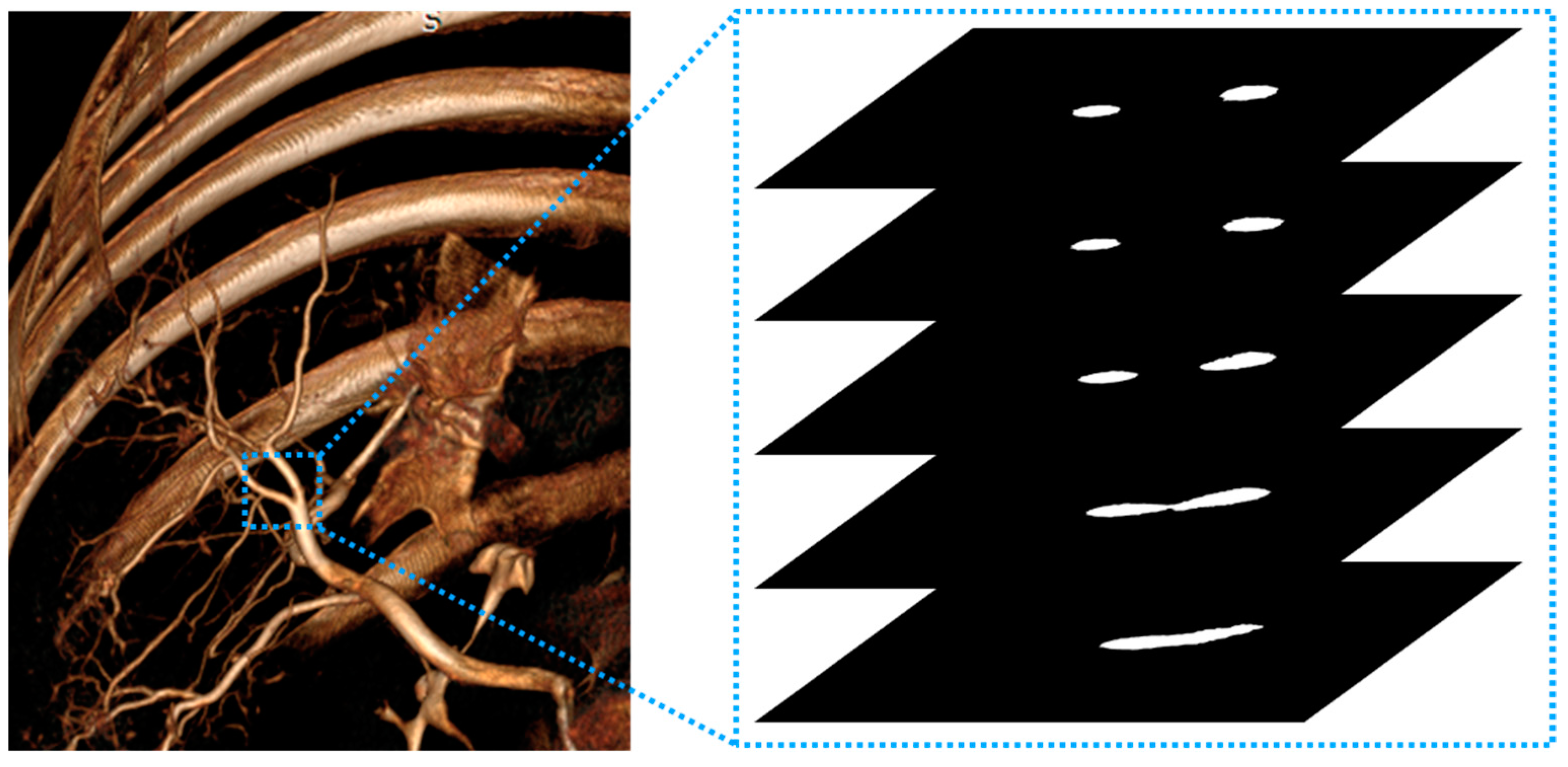
2.4. Experiment 2
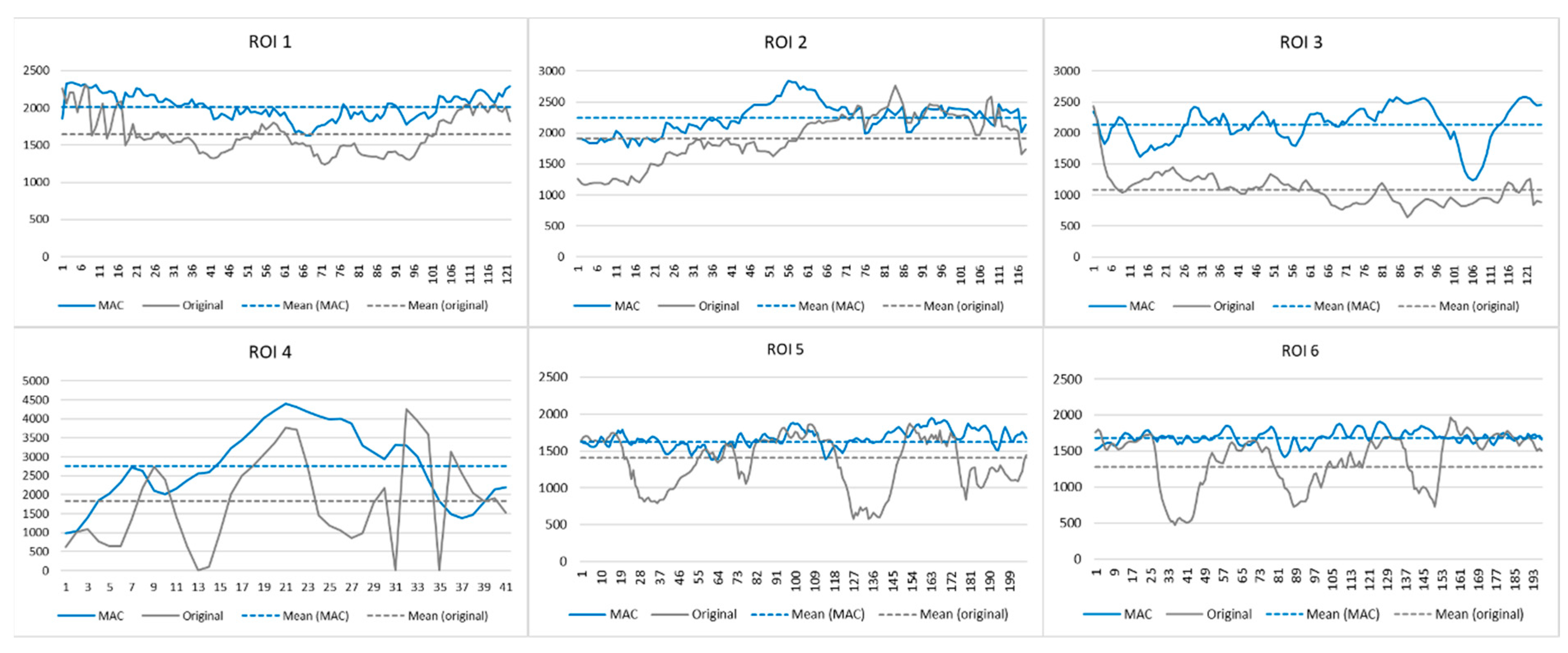
2.5. Experiment 3
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orth, R.C.; Wallace, M.J.; Kuo, M.D. C-arm Cone-beam CT: General Principles and Technical Considerations for Use in Interventional Radiology. J. Vasc. Interv. Radiol. 2009, 20, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Molteni, R.; Lorini, C.; Taliani, G.G.; Matteuzzi, B.; Mazzoni, E.; Colagrande, S. Motion artefacts in cone beam CT: An in vitro study about the effects on the images. Br. J. Radiol. 2015, 89, 20150687. [Google Scholar] [CrossRef] [PubMed]
- Tacher, V.; Radaelli, A.; Lin, M.; Geschwind, J.F. How I do it: Cone-Beam CT during transarterial chemoembolization for liver cancer. Radiology 2015, 274, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Kanayama, N.; Wada, K.; Ikawa, T.; Hirata, T.; Kishi, N.; Karino, T.; Washio, H.; Ueda, Y.; Miyazaki, M.; et al. Improvement of image quality and assessment of respiratory motion for hepatocellular carcinoma with portal vein tumor thrombosis using contrast-enhanced four-dimensional dual-energy computed tomography. PLoS ONE 2021, 16, e0244079. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Jacobs, R.; Pauwels, R.; Tijskens, E.; Fulton, R.; Nuyts, J. A motion correction approach for oral and maxillofacial cone-beam CT imaging. Phys. Med. Biol. 2021, 66, abfa38. [Google Scholar] [CrossRef] [PubMed]
- Marchant, T.E.; Price, G.J.; Matuszewski, B.J.; Moore, C.J. Reduction of motion artefacts in on-board cone beam CT by warping of projection images. Br. J. Radiol. 2011, 84, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Bae, W.; Ye, J.C. Translational motion correction algorithm for truncated cone-beam CT using opposite projections. J. Xray Sci. Technol. 2017, 25, 927–944. [Google Scholar] [CrossRef]
- Kyme, A.Z.; Fulton, R.R. Motion estimation and correction in SPECT, PET and CT. Phys. Med. Biol. 2021, 66, ac093b. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.C.; Liu, F.; Goodman, K.; Rosenzweig, K.E.; Mageras, G.S. Correction of motion artifacts in cone-beam CT using a patient-specific respiratory motion model. Med. Phys. 2010, 37, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Eldib, M.E.; Hegazy, M.A.A.; Cho, M.H.; Cho, M.H.; Lee, S.Y. A head-motion estimation algorithm for motion artifact correction in dental CT imaging. Phys. Med. Biol. 2018, 63, 065014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L. A rigid motion artifact reduction method for CT based on blind deconvolution. Algorithms 2019, 12, 155. [Google Scholar] [CrossRef]
- Kincaid, R.E.; Hertanto, A.E.; Hu, Y.C.; Wu, A.J.; Goodman, K.A.; Pham, H.D.; Yorke, E.D.; Zhang, Q.; Chen, Q.; Mageras, G.S. Evaluation of respiratory motion-corrected cone-beam CT at end expiration in abdominal radiotherapy sites: A prospective study. Acta Oncol. 2018, 57, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Adams, W.H.; Thomsen, B.; Ashraf, U.; Vasilopoulos, V. Validation of second-generation motion-correction software for computed tomography coronary angiography with novel quantitative approach. J. Comput. Assist. Tomogr. 2021, 45, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Jeong, Y.J.; Lee, G.; Lee, N.K.; Kim, J.Y.; Lee, J.W. Influence of Heart Rate and Innovative Motion-Correction Algorithm on Coronary Artery Image Quality and Measurement Accuracy Using 256-Detector Row Computed Tomography Scanner: Phantom Study. Korean J. Radiol. 2021, 20, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Shuai, T.; Li, W.; You, Y.; Deng, L.; Li, J.; Peng, L.; Li, Z. Combined Coronary CT Angiography and Evaluation of Access Vessels for TAVR Patients in Free-Breathing with Single Contrast Medium Injection Using a 16-cm-Wide Detector CT. Acad. Radiol. 2021, 28, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Kwon, L.M.; Hwang, J.S.; Lee, Y.; Kim, H.C.; Chung, J.W.; Choi, J.W. A Motion Artifact Correction Algorithm for cone-beam CT in Patients with Hepatic Malignancies Treated with Transarterial Chemoembolization. J. Vasc. Interv. Radiol. 2022, 22, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.; Chang, C.Y.; Thomas, B.J.; MacMahon, P.J.; Palmer, W.E. Using cone-beam CT as a low-dose 3D imaging technique for the extremities: Initial experience in 50 subjects. Skelet. Radiol. 2015, 44, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ouadah, S.; Jacobson, M.; Stayman, J.W.; Ehtiati, T.; Weiss, C.; Siewerdsen, J.H. Correction of patient motion in cone-beam CT using 3D-2D registration. Phys. Med. Biol. 2017, 62, 8813–8831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P.; Simoncelli, E.P. Image quality assessment: From error visibility to structural similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Full Width at Half Maximum. Available online: https://en.wikipedia.org/wiki/Full_width_at_half_maximum (accessed on 24 August 2023).
- Wei, C.; Albrecht, J.; Rit, S.; Laurendeau, M.; Thummerer, A.; Corradini, S.; Belka, C.; Steininger, P.; Ginzinger, F.; Kurz, C.; et al. Reduction of cone-beam CT artifacts in a robotic CBCT device using saddle trajectories with integrated infrared tracking. Med. Phys. 2024, 51, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- Sisniega, A.; Stayman, J.W.; Cao, Q.; Yorkston, J.; Siewerdsen, J.H.; Zbijewski, W. Image-based motion compensation for high-resolution extremities cone-beam CT. Proc. SPIE Int. Soc. Opt. Eng. 2016, 9783, 156–162. [Google Scholar]
- Vipin, K.; Bhurchandi, K.M. No-reference image quality assessment algorithms: A survey. Optik 2015, 126, 1090–1097. [Google Scholar]
- Prokop, M.; Shin, H.O.; Schanz, A.; Schaefer-Prokop, C.M. Use of maximum intensity projections in CT angiography: A basic review. Radiographics 1997, 17, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, E.; Richter, A.; Frame, M. Diagnostic imaging-evaluating image quality using visual grading characteristic (VGC) analysis. Vet. Res. Commun. 2010, 34, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Floridi, C.; Radaelli, A.; Abi-Jaoudeh, N.; Grass, M.; Lin, M.; Chiaradia, M.; Geschwind, J.F.; Kobeiter, H.; Squillaci, E.; Maleux, G.; et al. C-arm cone-beam computed tomography in interventional oncology: Technical aspects and clinical applications. Radiol. Med. 2014, 119, 521–532. [Google Scholar] [CrossRef]
- Jonczyk, M.; Collettini, F.; Geisel, D.; Schnapauff, D.; Böning, G.; Wieners, G.; Gebauer, G. Radiation exposure during TACE procedures using additional cone-beam CT (CBCT) for guidance: Safety and precautions. Acta Radiol. 2018, 59, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Kothary, N.; Abdelmaksoud, M.H.; Tognolini, A.; Fahrig, R.; Rosenberg, J.; Hovsepian, D.M.; Ganguly, A.; Louie, J.D.; Kuo, W.T.; Hwang, G.L.; et al. Imaging guidance with C-arm CT: Prospective evaluation of its impact on patient radiation exposure during transhepatic arterial chemoembolization. J. Vas. Interv. Radiol. 2011, 22, 1535–1543. [Google Scholar] [CrossRef]
- Tognolini, A.; Louie, J.; Hwang, G.; Hofmann, L.; Sze, D.; Kothary, N. C-arm computed tomography for hepatic interventions: A practical guide. J. Vasc. Interv. Radiol. 2010, 21, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Schaal, D.; Curry, H.; Clark, R.; Magliari, A.; Kupelian, P.; Khuntia, D.; Beriwal, S. Review of cone beam computed tomography based online adaptive radiotherapy: Current trend and future direction. Radiat. Oncol. J. 2023, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, B.; Hassan, G.M.; Reynolds, M.; Sabet, M.; Kendrick, J.; Rowshanfarzad, P.; Ebert, M. Deep learning methods for enhancing cone-beam CT image quality toward adaptive radiation therapy: A systematic review. Med. Phys. 2022, 49, 6019–6054. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, F.L.; Lo, Y.C. Improving Image Quality of On-Board Cone-Beam CT in Radiation Therapy Using Image Information Provided by Planning Multi-Detector CT: A Phantom Study. PLoS ONE 2016, 11, e0157072. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.J.; Mao, W.; Liu, C.; Aref, I.; Elshaikh, M.; Lee, J.K.; Pradhan, D.; Movsas, B.; Chetty, I.J.; Siddiqui, F. Improvements in CBCT Image Quality Using a Novel Iterative Reconstruction Algorithm: A Clinical Evaluation. Adv. Radiat. Oncol. 2019, 4, 390–400. [Google Scholar] [CrossRef] [PubMed]
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| MAC | C | UC | C | UC | C | UC | UC | C | C | C | C | C | C |
| Num. of ROI * | 1 | None | 4 | None | 1 | None | None | 1 | 5 | 1 | 3 | 1 | 1 |
| Patient | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| MAC | C | UC | C | C | C | UC | C | C | C | C | UC | UC | C |
| Num. of ROI | 3 | None | 3 | 2 | 3 | None | 5 | 3 | 3 | 1 | None | None | 2 |
| Patient | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | - | - |
| MAC | C | C | UC | UC | UC | UC | C | C | UC | UC | C | - | - |
| Num. of ROI | 2 | 3 | None | None | None | None | 1 | 3 | None | None | 2 | - | - |
| ROI | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| VGA (R1) | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 1 | 2 | 2 | 1 |
| VGA (R2) | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 0 |
| MCI * | 0.426 | 0.402 | 0.782 | 0.628 | 0.712 | 0.865 | 0.827 | 0.912 | 0.534 | 0.548 | 0.812 | 0.485 | 0.646 | 0.817 |
| ROI | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| VGA (R1) | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 1 | 0 | 2 | 1 | 2 | 2 | 2 |
| VGA (R2) | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| MCI | 0.698 | 0.751 | 0.675 | 0.586 | 0.705 | 0.712 | 0.531 | 0.794 | 0.912 | 0.698 | 0.698 | 0.582 | 0.575 | 0.586 |
| ROI | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 |
| VGA (R1) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 2 |
| VGA (R2) | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 2 | 2 | 2 |
| MCI | 0.711 | 0.548 | 0.651 | 0.742 | 0.531 | 0.648 | 0.705 | 0.771 | 0.802 | 0.641 | 0.698 | 0.658 | 0.575 | 0.546 |
| ROI | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | - | - |
| VGA (R1) | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | - | - |
| VGA (R2) | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | - | - |
| MCI | 0.752 | 0.711 | 0.695 | 0.652 | 0.512 | 0.623 | 0.721 | 0.771 | 0.692 | 0.755 | 0.646 | 0.511 | - | - |
| Uncorrected | Corrected † | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean | Mean |
| MCI * | −0.008 | 0.007 | 0.006 | 0.015 | −0.002 | 0.009 | −0.013 | 0.002 | 0.008 | 0.011 | 0.004 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Lee, H.; Jun, H.; Cho, S. Evaluation of Motion Artifact Correction Technique for Cone-Beam Computed Tomography Image Considering Blood Vessel Geometry. J. Clin. Med. 2024, 13, 2253. https://doi.org/10.3390/jcm13082253
Jung Y, Lee H, Jun H, Cho S. Evaluation of Motion Artifact Correction Technique for Cone-Beam Computed Tomography Image Considering Blood Vessel Geometry. Journal of Clinical Medicine. 2024; 13(8):2253. https://doi.org/10.3390/jcm13082253
Chicago/Turabian StyleJung, Yunsub, Ho Lee, Hoyong Jun, and Soobuem Cho. 2024. "Evaluation of Motion Artifact Correction Technique for Cone-Beam Computed Tomography Image Considering Blood Vessel Geometry" Journal of Clinical Medicine 13, no. 8: 2253. https://doi.org/10.3390/jcm13082253





