Sexual Dysfunction in Postural Orthostatic Tachycardia Syndrome (POTS): A Cross-Sectional, Case-Control Study
Abstract
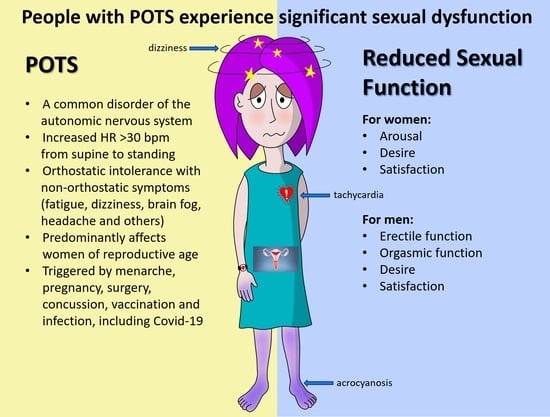
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Surveys
2.3. Additional Cohort
2.4. Statistical Analyses
3. Results
3.1. Online Patient Characteristics
3.2. Specialty Clinic Characteristics
3.3. Depression Scores
3.4. Female Sexual Function Scores
3.5. Male Sexual Function Scores
3.6. Predictors of Sexual Dysfunction in POTS
4. Discussion
4.1. Main Findings
4.2. Sexual Function and Autonomic Nervous System
4.3. Sexual Dysfunction and Neurologic Disorders
4.4. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, K.; Smith, A.; Blitshteyn, S. POTS and Pregnancy: A Review of Literature and Recommendations for Evaluation and Treatment. Int. J. Womens Health 2022, 14, 1831–1847. [Google Scholar] [CrossRef]
- Bourne, K.M.; Nerenberg, K.A.; Stiles, L.E.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Peltier, A.; Diedrich, A.; Biaggioni, I.; et al. Symptoms of postural orthostatic tachycardia syndrome in pregnancy: A cross-sectional, community-based survey. BJOG 2023, 130, 1120–1127. [Google Scholar] [CrossRef]
- Peggs, K.J.; Nguyen, H.; Enayat, D.; Keller, N.R.; Al-Hendy, A.; Raj, S.R. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int. J. Gynaecol. Obstet. 2012, 118, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Basson, R.; Rees, P.; Wang, R.; Montejo, A.L.; Incrocci, L. Sexual function in chronic illness. J. Sex. Med. 2010, 7, 374–388. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory—II. Psychological Assessment; APA: New York, NY, USA, 1996. [Google Scholar]
- Pederson, C.L.; Wagner, B.M. The depressing truth about depression scales for people with chronic invisible illness. J. Health Sci. Educ. 2022, 6, 1–6. [Google Scholar]
- Rosen, C.; Brown, J.; Heiman, S.; Leiblum, C.; Meston, R.; Shabsigh, D.; Ferguson, R.; D’Agostino, R. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex. Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31, a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef]
- Meston, C.M.; Frohlich, P.F. The Neurobiology of Sexual Function. Arch. Gen. Psychiatry 2000, 57, 1012–1030. [Google Scholar] [CrossRef]
- Yang, C.C.; Jiang, X. Clinical Autonomic Neurophysiology and the Male Sexual Response: An Overview. J. Sex. Med. 2009, 6, 221–228. [Google Scholar] [CrossRef]
- De Tejada, I.S.; Angulo, J.; Cellek, S.; González-Cadavid, N.; Heaton, J.; Pickard, R.; Simonsen, U. Pathophysiology of Erectile Dysfunction. J. Sex. Med. 2005, 2, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Donatucci, C.F. Etiology of Ejaculation and Pathophysiology of Premature Ejaculation. J. Sex. Med. 2006, 3, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Raccagni, C.; Indelicato, E.; Sidoroff, V.; Daniaux, M.; Bader, A.; Toth, B.; Jelisejevas, L.A.; Hochleitner, M.; Fanciulli, A.; Leys, F.; et al. Female sexual dysfunction in multiple system atrophy: A prospective cohort study. Clin. Auton. Res. 2021, 31, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Ghajarzadeh, M.; Jalilian, R.; Azimi, A.; Togha, M.; Sahraian, M.A.; Mohammadifar, M. Comparison of Sexual Dysfunction in Women with Migraine and Multiple Sclerosis (MS). Maedica 2016, 11, 44–47. [Google Scholar] [PubMed]
- Glayzer, J.E.; McFarlin, B.L.; Castori, M.; Suarez, M.L.; Meinel, M.C.; Kobak, W.H.; Steffen, A.D.; Schlaeger, J.M. High rate of dyspareunia and probable vulvodynia in Ehlers-Danlos syndromes and hypermobility spectrum disorders: An online survey. Am. J. Med. Genet. C Semin. Med. Genet. 2021, 187, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Minopoulou, I.; Pyrgidis, N.; Tishukov, M.; Sokolakis, I.; Baniotopoulos, P.; Kefas, A.; Doumas, M.; Hatzichristodoulou, G.; Dimitroulas, T. Sexual dysfunction in women with systemic autoimmune rheumatic disorders: A systematic review and meta-analysis. Rheumatology 2023, 62, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, M.; Mahdi, A.; Johansson, M.; Fedorowski, A.; Olshansky, B. Cardiovascular dysautonomia in postacute sequelae of SARS-CoV-2 infection. J. Cardiovasc. Electrophysiol. 2024, 35, 608–617. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Harirugsakul, K.; Wainipitapong, S.; Phannajit, J.; Paitoonpong, L.; Tantiwongse, K. Erectile dysfunction after COVID-19 recovery: A follow-up study. PLoS ONE 2022, 17, e0276429. [Google Scholar] [CrossRef]
- Shaw, B.H.; Stiles, L.E.; Bourne, K.; Green, E.A.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Diedrich, A.; Raj, V.; et al. The face of postural tachycardia syndrome—insights from a large cross-sectional online community-based survey. J. Intern. Med. 2019, 286, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Toghae, M.; Raisi, F.; Saffari, E. The prevalence of female sexual dysfunction among migraine patients. Iran. J. Neurol. 2015, 14, 8–11. [Google Scholar] [PubMed]
- Ifergane, G.; Ben-Zion, I.Z.; Plakht, Y.; Regev, K.; Wirguin, I. Not only headache: Higher degree of sexual pain symptoms among migraine sufferers. J. Headache Pain 2008, 9, 113–117. [Google Scholar] [CrossRef]
- Pradeep, R.; Sundarmurthy, H.; Karan, V.; Kulkarni, P. Prevalence and Predictors of Female Sexual Dysfunction in Migraine. Ann. Indian Acad. Neurol. 2019, 22, 291–294. [Google Scholar] [PubMed]
- Blitshteyn, S. Dysautonomia, hypermobility spectrum disorder and mast cell activation syndrome as migraine comorbidities. Curr. Neurol. Neurosci. Rep. 2023, 23, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, D.; Yalınay Dikmen, P.; Ilgaz Aydınlar, E.; Incesu, C. The relation of sexual function to migraine-related disability, depression and anxiety in patients with migraine. J. Headache Pain 2014, 15, 32. [Google Scholar] [CrossRef]
- Aksoy, D.; Solmaz, V.; Cevik, B.; Gencten, Y.; Erdemir, F.; Kurt, S.G. The evaluation of sexual dysfunction in male patients with migraine and tension type headache. J. Headache Pain 2013, 14, 46. [Google Scholar] [CrossRef]



| POTS Patients n = 160 | Controls n = 62 | p | Cohen’s d | |
|---|---|---|---|---|
| Age | 30.2 ± 7.9 | 29.1 ± 4.6 | 0.5572 | 0.18 |
| COMPASS-31 | 55.3 ± 11.8 | |||
| 26.2 ± 7.0 | |||
| 2.4 ± 1.6 | |||
| 9.2 ± 2.9 | |||
| 11.9 ± 4.4 | |||
| 2.7 ± 2.1 | |||
| 2.8 ± 1.0 | |||
| 22.9 ± 10.7 | 8.2 ± 6.8 | <0.0001 | 1.50 |
| 15.1 ± 8.6 | 5.9 ± 5.4 | <0.0001 | 1.17 |
| 1.1 ± 1.0 | 0.4 ± 0.7 | <0.0001 | 0.77 |
| 22.4 ± 6.9 | 24.8 ± 5.7 | 0.0085 | 0.36 |
| 3.1 ± 1.4 | 4.6 ± 1.1 | <0.0001 | −1.18 |
| 3.8 ± 1.5 | 4.9 ± 1.2 | <0.0001 | −0.78 |
| 4.0 ± 1.6 | 4.0 ± 1.4 | 0.9640 | 0.01 |
| 3.8 ± 1.6 | 4.2 ± 1.3 | 0.0986 | 0.23 |
| 4.0 ± 1.5 | 4.5 ± 1.2 | 0.0061 | −0.40 |
| 3.8 ± 1.8 | 2.7 ± 1.9 | 0.0001 | 0.60 |
| POTS Patients n = 29 | Controls n = 27 | p | Cohen’s d | |
|---|---|---|---|---|
| Age | 30.1 ± 6.0 | 29.8 ± 5.6 | 0.5583 | 0.16 |
| COMPASS-31 | 46.4 ± 11.5 | |||
| 21.4 ± 6.5 | |||
| 1.7 ± 1.7 | |||
| 8.8 ± 3.0 | |||
| 9.1 ± 4.2 | |||
| 3.0 ± 2.1 | |||
| 2.5 ± 0.9 | |||
| 22.7 ± 9.8 | 11.0 ± 11.6 | 0.0002 | 1.09 |
| 16.2 ± 7.5 | 7.6 ± 5.9 | 0.0001 | 1.09 |
| 1.2 ± 1.0 | 0.6 ± 0.8 | 0.0173 | 0.65 |
| 39.7 ± 11.8 | 54.7 ± 24.6 | 0.0007 | −0.99 |
| 16.6 ± 6.0 | 22.0 ± 11.6 | 0.0011 | −0.93 |
| 5.7 ± 2.7 | 7.6 ± 3.0 | 0.0170 | −0.66 |
| 4.4 ± 1.5 | 7.3 ± 2.2 | <0.0001 | −1.48 |
| 7.1 ± 2.8 | 9.1 ± 4.8 | 0.0620 | −0.52 |
| 5.2 ± 2.0 | 6.9 ± 2.7 | 0.0118 | −0.71 |
| POTS Mean (SD) n = 11 | Control Mean (SD) n = 7 | p | Cohen’s d | |
|---|---|---|---|---|
| Age | 40.18 (9.37) | 36.00 (13.81) | 0.45 | −0.37 |
| COMPASS-31 | 47.55 ± 13.91 | |||
| Orthostatic | 5.54 ± 2.37 | |||
| Vasomotor | 3.69 ± 1.31 | |||
| Secretomotor | 2.61 ± 2.50 | |||
| GI | 11.15 ± 5.44 | |||
| Bladder | 1.15 ± 1.57 | |||
| Pupillomotor | 8.46 ± 3.78 | |||
| BDII | 15.45 (10.15) | 10.57 (9.54) | 0.32 | −0.49 |
| FSF | 19.47 (6.65) | 27.08 (5.58) | 0.023 | 1.22 |
| Desire | 2.51 (0.84) | 3.34 (1.24) | 0.11 | 0.82 |
| Arousal | 3.30 (1.59) | 4.11 (0.94) | 0.24 | 0.59 |
| Lubrication | 3.95 (1.91) | 5.34 (0.96) | 0.10 | 0.86 |
| Orgasm | 3.35 (1.86) | 4.11 (1.42) | 0.37 | 0.45 |
| Satisfaction | 3.35 (1.20) | 4.74 (1.49) | 0.04 | 1.06 |
| Pain | 3.02 (2.21) | 5.43 (0.89) | 0.01 | 1.33 |
| COMPASS-31 | BDI-II | Sexual Function Scores (FSF/IIEF) | ||
|---|---|---|---|---|
| Age | Female | −0.04, p = 0.5408 | −0.08, p = 0.2700 | −0.02, p = 0.6240 |
| Male | 0.09, p = 0.6394 | 0.12, p = 0.5395 | 0.43, p = 0.0186 | |
| COMPASS-31 | Female | 1 | 0.34, p < 0.0001 | −0.28, p = 0.0003 |
| Male | 1 | 0.37, p = 0.0454 | 0.26, p = 0.1679 | |
| BDI-II | Female | 1 | −0.21, p = 0.0082 | |
| Male | 1 | 0.08, p = 0.6691 | ||
| Sexual function scores | Female (FSF) | 1 | ||
| Male (IIEF) | 1 |
| B | SE | 95% CI | p | ||
|---|---|---|---|---|---|
| COMPASS-31 | Female | −0.076 | 0.048 | (−0.170, 0.019) | 0.1155 |
| Male | 0.254 | 0.193 | (−0.144, 0.653) | 0.2000 | |
| Age | Female | −0.058 | 0.067 | (−0.190, 0.074) | 0.3851 |
| Male | 0.827 | 0.347 | (0.113, 1.541) | 0.0250 | |
| BDI-II | Female | −0.160 | 0.053 | (−0.264, −0.055) | 0.0030 |
| Male | −0.072 | 0.227 | (−0.540, 0.397) | 0.7550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blitshteyn, S.; Lange, A.; Corinaldi, C.; Guy, P.; Brook, J. Sexual Dysfunction in Postural Orthostatic Tachycardia Syndrome (POTS): A Cross-Sectional, Case-Control Study. J. Clin. Med. 2024, 13, 2274. https://doi.org/10.3390/jcm13082274
Blitshteyn S, Lange A, Corinaldi C, Guy P, Brook J. Sexual Dysfunction in Postural Orthostatic Tachycardia Syndrome (POTS): A Cross-Sectional, Case-Control Study. Journal of Clinical Medicine. 2024; 13(8):2274. https://doi.org/10.3390/jcm13082274
Chicago/Turabian StyleBlitshteyn, Svetlana, Anna Lange, Chelsea Corinaldi, Paige Guy, and Jill Brook. 2024. "Sexual Dysfunction in Postural Orthostatic Tachycardia Syndrome (POTS): A Cross-Sectional, Case-Control Study" Journal of Clinical Medicine 13, no. 8: 2274. https://doi.org/10.3390/jcm13082274
APA StyleBlitshteyn, S., Lange, A., Corinaldi, C., Guy, P., & Brook, J. (2024). Sexual Dysfunction in Postural Orthostatic Tachycardia Syndrome (POTS): A Cross-Sectional, Case-Control Study. Journal of Clinical Medicine, 13(8), 2274. https://doi.org/10.3390/jcm13082274