Reinforcement Techniques in Arthroscopic Repair of Large-to-Massive Rotator Cuff Tears: A Comparative Study of Superior Capsule Reconstruction and Patch Graft Augmentation
Abstract
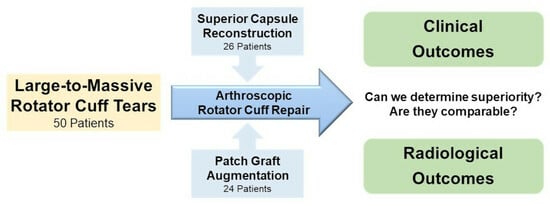
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Range of Motion Evaluation
2.3. Functional and Clinical Satisfaction Evaluation
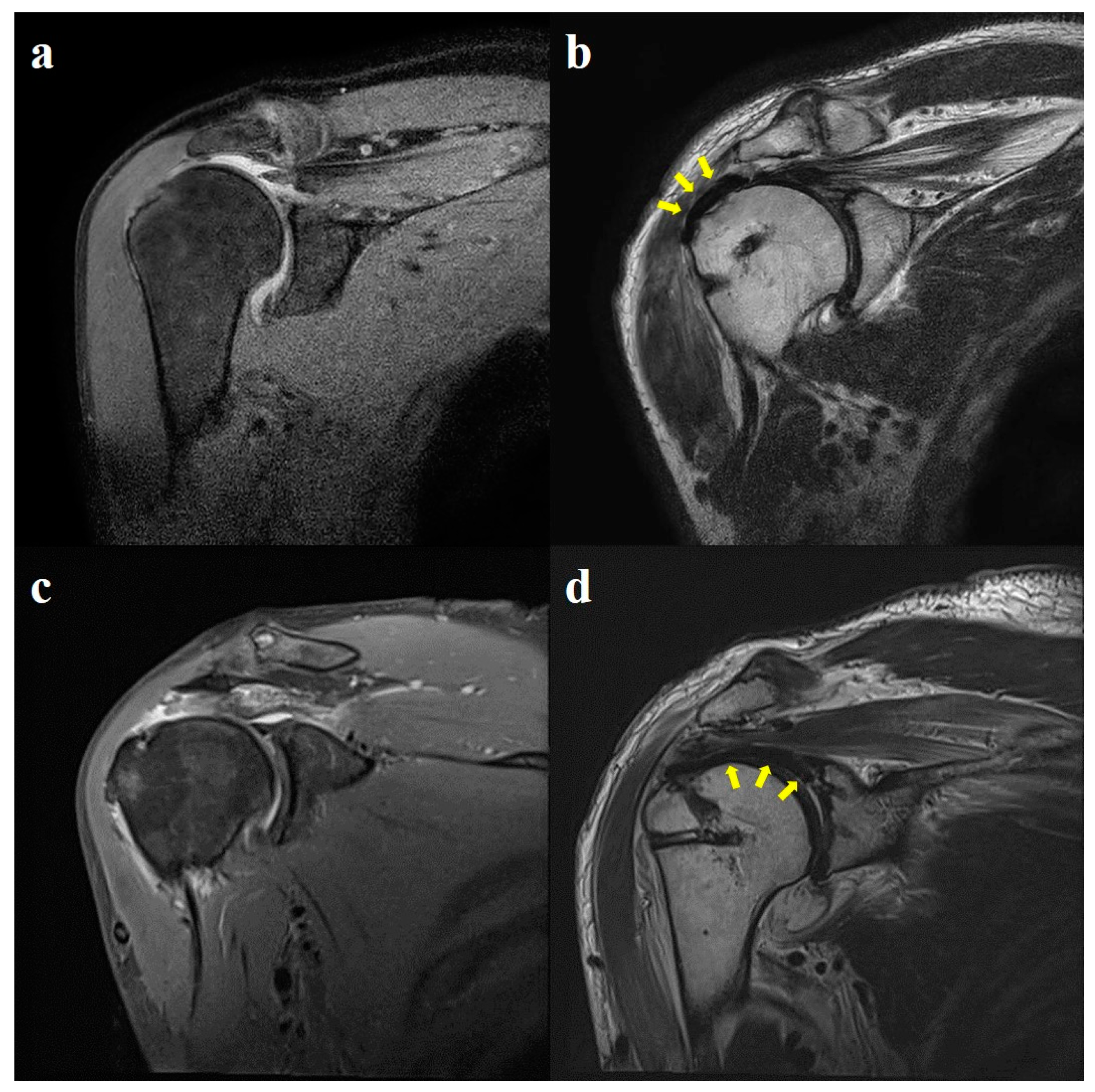
2.4. Radiological Evaluation
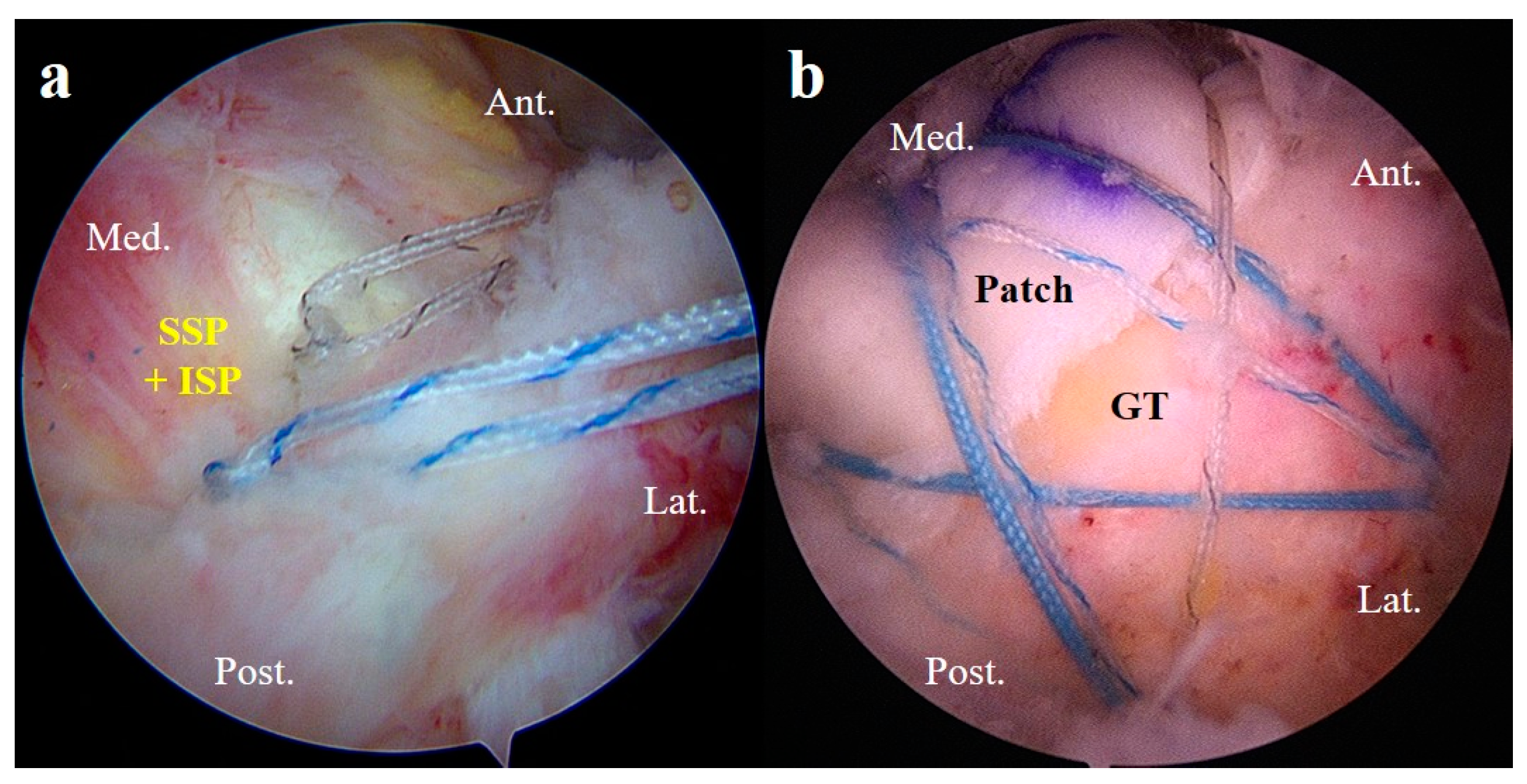
2.5. Surgical Technique
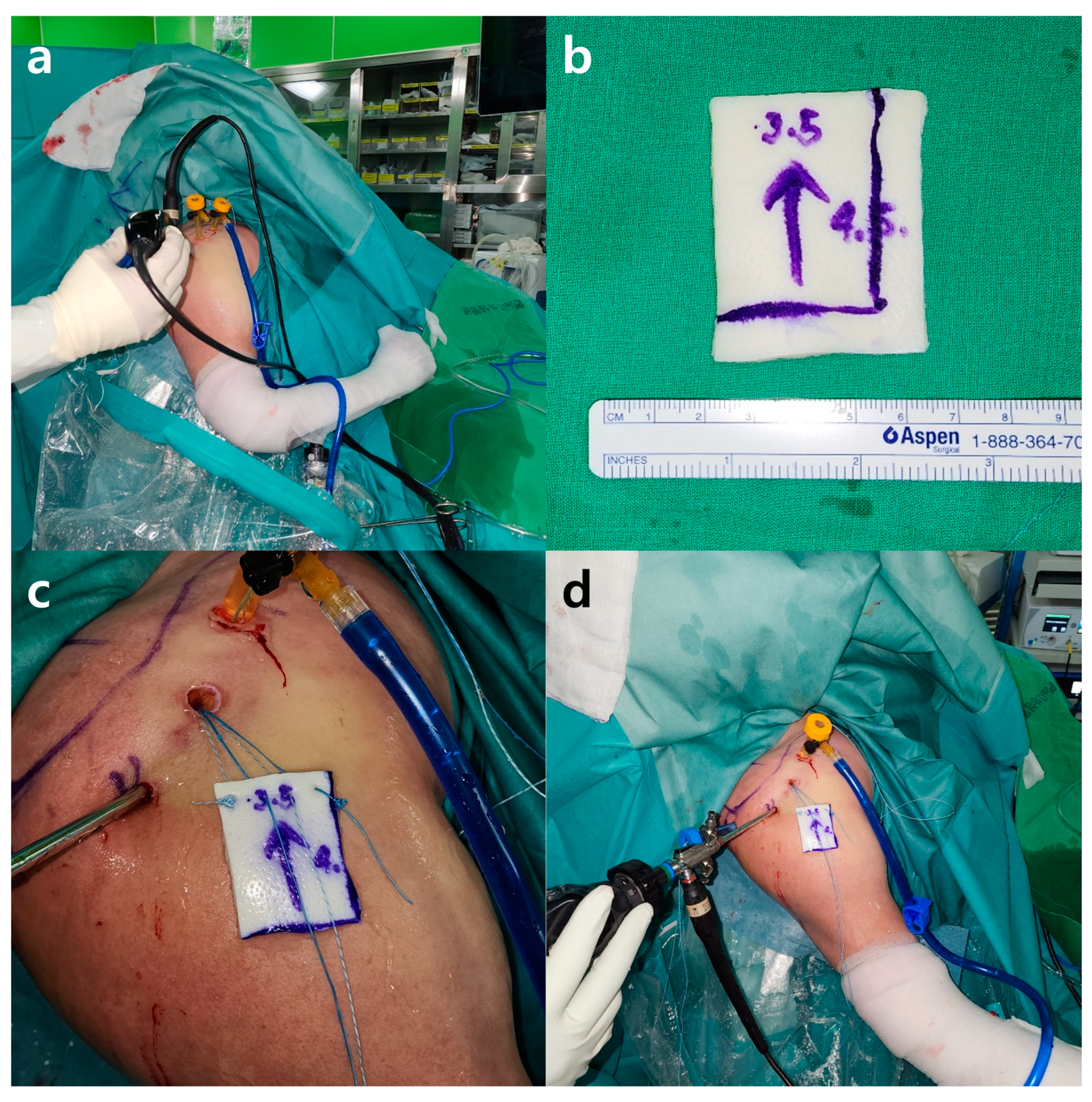
2.5.1. Preparation Procedure
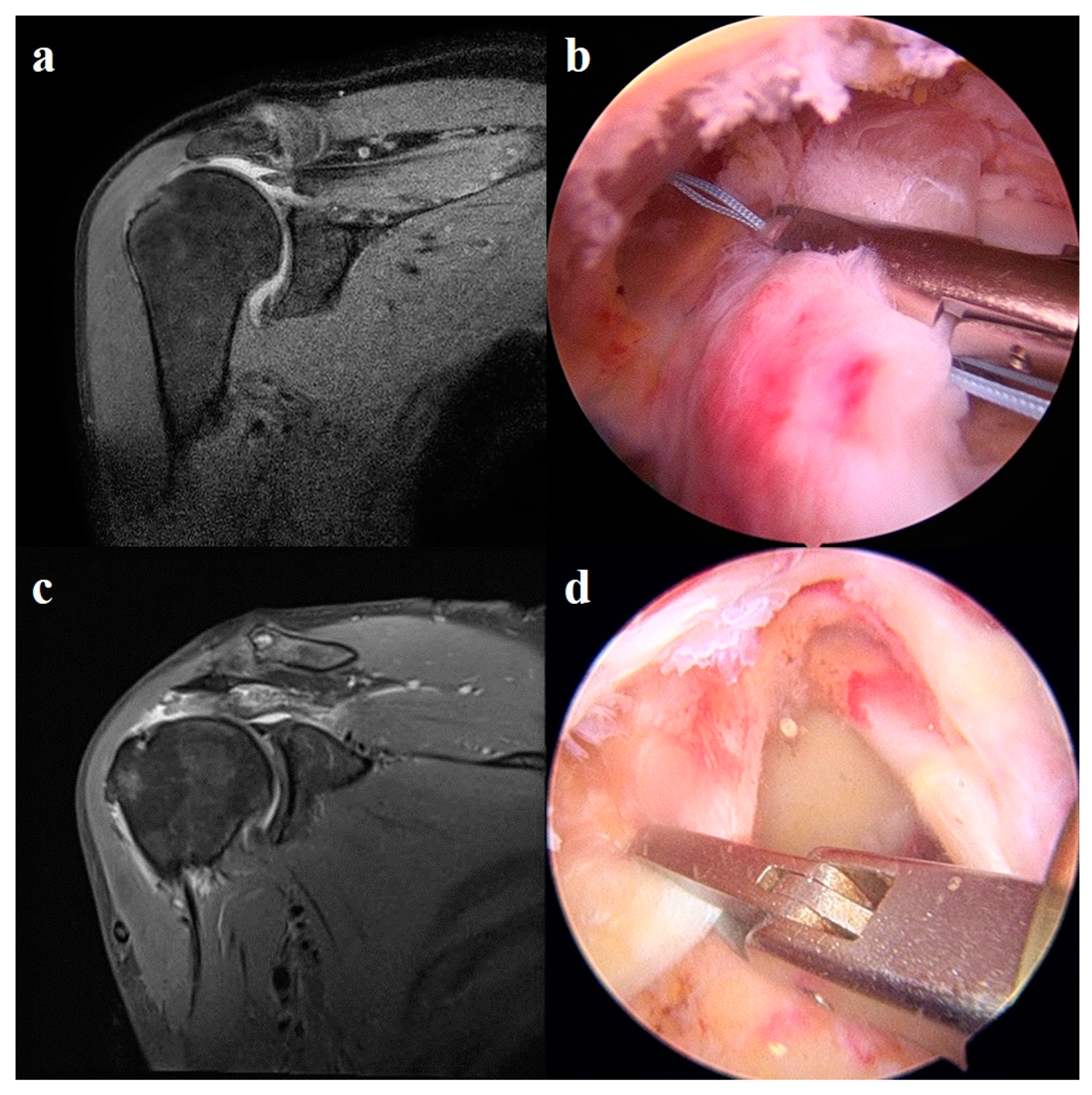
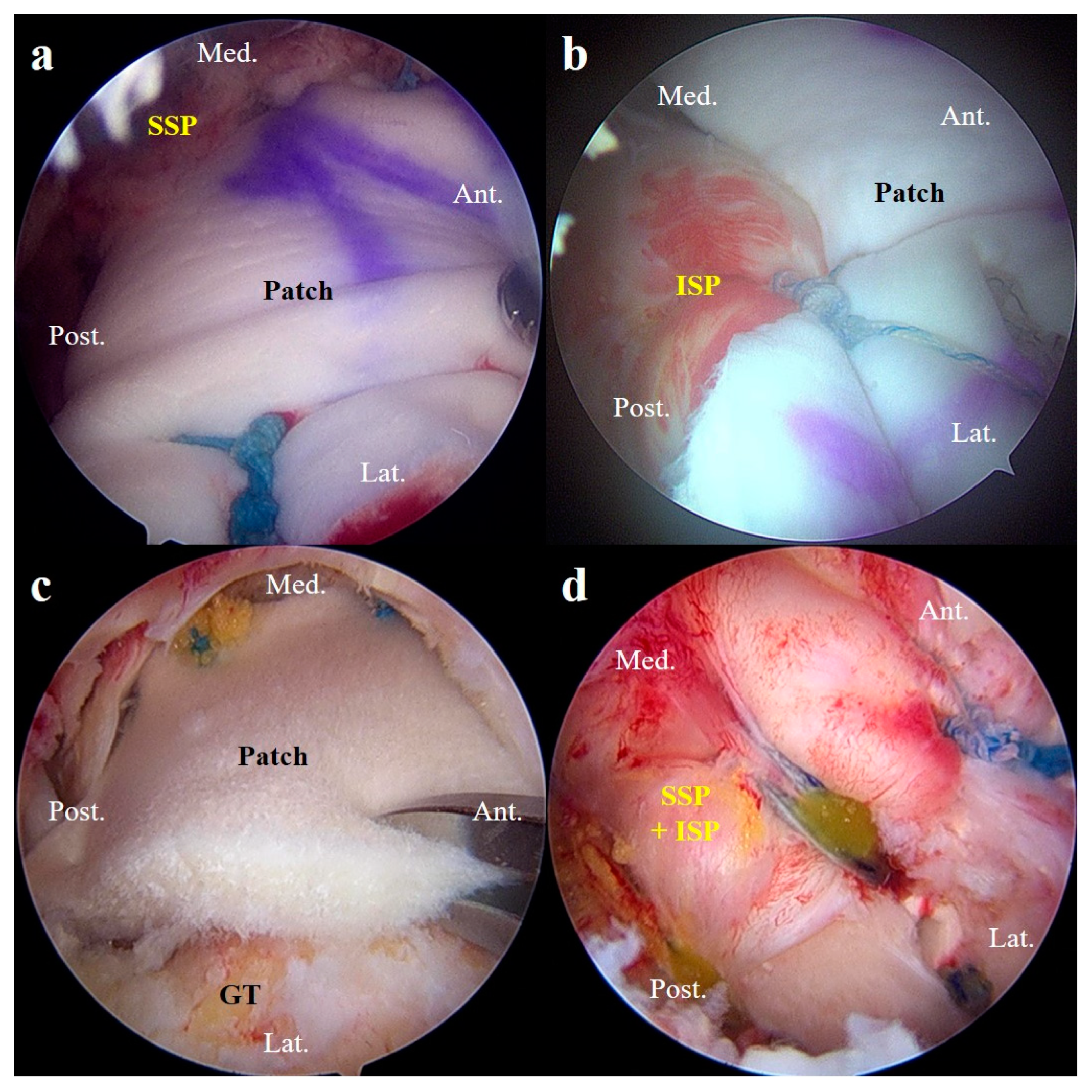
2.5.2. Superior Capsule Reconstruction
2.5.3. Patch Graft Augmentation
2.6. Postoperative Rehabilitation
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Range of Motion Outcomes
3.3. Functional and Clinical Satisfaction Outcomes
3.4. Radiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, S.H.; Kang, J.Y.; Oh, C.H.; Gong, H.S. Effect of age on functional and structural outcome after rotator cuff repair. Am. J. Sports Med. 2010, 38, 672–678. [Google Scholar] [PubMed]
- Mihata, T.; McGarry, M.H.; Pirolo, J.M.; Kinoshita, M.; Lee, T.Q. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: A biomechanical cadaveric study. Am. J. Sports Med. 2012, 40, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.M.; Oh, J.H. Bridging graft in irreparable massive rotator cuff tears: Autogenic biceps graft versus allogenic dermal patch graft. Clin. Orthop. Surg. 2017, 9, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Neviaser, J.S.; Neviaser, R.J.; Neviaser, T.J. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J. Bone Jt. Surg. Am. 1978, 60, 681–684. [Google Scholar] [CrossRef]
- Encalada-Diaz, I.; Cole, B.J.; MacGillivray, J.D.; Ruiz-Suarez, M.; Kercher, J.S.; Friel, N.A.; Valero-Gonzalez, F. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: Preliminary results at 12 months’ follow-up. J. Shoulder Elbow Surg. 2011, 20, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.A.; Gidwani, S.; Curtis, M.J. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta Orthop. Belg. 2007, 73, 432–436. [Google Scholar] [PubMed]
- Gilot, G.J.; Alvarez-Pinzon, A.M.; Barcksdale, L.; Westerdahl, D.; Krill, M.; Peck, E. Outcome of large to massive rotator cuff tears repaired with and without extracellular matrix augmentation: A prospective comparative study. Arthroscopy 2015, 31, 1459–1465. [Google Scholar] [CrossRef]
- Mihata, T.; Lee, T.Q.; Hasegawa, A.; Fukunishi, K.; Kawakami, T.; Fujisawa, Y.; Neo, M. Superior capsule reconstruction for reinforcement of arthroscopic rotator cuff repair improves cuff integrity. Am. J. Sports Med. 2019, 47, 379–388. [Google Scholar] [CrossRef]
- Barber, F.A.; Burns, J.P.; Deutsch, A.; Labbé, M.R.; Litchfield, R.B. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 2012, 28, 8–15. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, J.Y.; Lee, Y.B. The use of intravenous zoledronate may reduce retear rate after rotator cuff repair in older female patients with osteoporosis: A first in-human prospective study. J. Clin. Med. 2022, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, J.P.; Deutsch, A.; Green, A.; Rudicel, S.; Christensen, J.; Marraffino, S.; Rodeo, S. Time to failure after rotator cuff repair: A prospective imaging study. J. Bone Jt. Surg. 2013, 95, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, G.-H.; Cha, D.-H.; Lee, J.-H.; Lee, Y.-B. A Comparison of Clinical Outcomes in Rotator Cuff Re-Tear Patients Who Had Either an Arthroscopic Primary Repair or Arthroscopic Patch Augmentation for Large-to-Massive Rotator Cuff Tears. Diagnostics 2023, 13, 1961. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, K.I. The SMC knot—A new slip knot with locking mechanism. Arthroscopy 2000, 16, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Fiebig, D.; Kieser, B.; Albrecht, B.; Schill, A.; Scheibel, M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.M.; Cvetanovich, G.; Savin, D.; Romeo, A.A. Superior capsular reconstruction. JBJS Rev. 2018, 6, e10. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Park, M.S.; Rhee, S.M. Treatment strategy for irreparable rotator cuff tears. Clin. Orthop. Surg. 2018, 10, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Ek, E.T.H.; Neukom, L.; Catanzaro, S.; Gerber, C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J. Shoulder Elbow Surg. 2013, 22, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Duralde, X.A.; Bair, B. Massive rotator cuff tears: The result of partial rotator cuff repair. J. Shoulder Elbow Surg. 2005, 14, 121–127. [Google Scholar] [CrossRef]
- Moursy, M.; Forstner, R.; Koller, H.; Resch, H.; Tauber, M. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: A modified technique to improve tendon transfer integrity. J. Bone Jt. Surg. Am. 2009, 91, 1924–1931. [Google Scholar] [CrossRef]
- Werner, C.M.L.; Steinmann, P.A.; Gilbart, M.; Gerber, C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J. Bone Jt. Surg. Am. 2005, 87, 1476–1486. [Google Scholar]
- Favard, L.; Levigne, C.; Nerot, C.; Gerber, C.; de Wilde, L.; Mole, D. Reverse prostheses in arthropathies with cuff tear: Are survivorship and function maintained over time? Clin. Orthop. Relat. Res. 2011, 469, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Badylak, S.F.; Steinmann, S.P.; Iannotti, J.P. Extracellular matrix scaffold devices for rotator cuff repair. J. Bone Jt. Surg. Am. 2010, 19, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Cain, E.L.; Schwartz, M.L.; Clancy, W.G., Jr. Allograft reconstruction for massive, irreparable rotator cuff tears. Am. J. Sports Med. 2006, 34, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Sclamberg, S.G.; Tibone, J.E.; Itamura, J.M.; Kasraeian, S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J. Shoulder Elbow Surg. 2004, 13, 538–541. [Google Scholar] [CrossRef]
- di Benedetto, P.; Mancuso, F.; Tosolini, L.; Buttironi, M.M.; Beltrame, A.; Causero, A. Treatment options for massive rotator cuff tears: A narrative review. Acta Biomed. 2021, 92, e2021026. [Google Scholar]
- McCarron, J.A.; Milks, R.A.; Mesiha, M.; Aurora, A.; Walker, E.; Iannotti, J.P.; Derwin, K.A. Reinforced fascia patch limits cyclic gapping of rotator cuff repairs in a human cadaveric model. J. Shoulder Elbow Surg. 2012, 21, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, S.S.; Barth, J.R.H.; Richards, D.P.; Zlatkin, M.B.; Larsen, M. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthroscopy 2007, 23, 347–354. [Google Scholar] [CrossRef]
- Gupta, A.K.; Hug, K.; Berkoff, D.J.; Boggess, B.R.; Gavigan, M.; Malley, P.C.; Toth, A.P. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am. J. Sports Med. 2012, 40, 141–147. [Google Scholar] [CrossRef]
- Nimura, A.; Kato, A.; Yamaguchi, K.; Mochizuki, T.; Okawa, A.; Sugaya, H.; Akita, K. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J. Shoulder Elbow Surg. 2012, 21, 867–872. [Google Scholar] [CrossRef]
- Ishihara, Y.; Mihata, T.; Tamboli, M.; Nguyen, L.; Park, K.J.; McGarry, M.H.; Lee, T.Q. Role of the superior shoulder capsule in passive stability of the glenohumeral joint. J. Shoulder Elbow Surg. 2014, 23, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, J.P.; Codsi, M.J.; Kwon, Y.W.; Derwin, K.; Ciccone, J.; Brems, J.J. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J. Bone Jt. Surg. 2006, 88, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, P.; Scotti, C.; Nonis, A.; Vitali, M.; Di Serio, C.; Peretti, G.M.; Fraschini, G. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: A 3-year follow-up study. Am. J. Sports Med. 2014, 42, 1169–1175. [Google Scholar] [CrossRef] [PubMed]


| Variables | Group 1 (n = 26) | Group 2 (n = 24) | p Value |
|---|---|---|---|
| Age (years) | 68.96 ± 7.55 | 65.50 ± 7.69 | 0.117 |
| Sex (Male/Female) | 13/13 | 10/14 | 0.555 |
| Side of involvement (Dominant/Non-dominant) | 22/4 | 18/6 | 0.396 |
| Duration of symptom (months) | 13.56 ± 25.31 | 14.63 ± 26.50 | 0.696 |
| Smoking (Yes/No) | 3/23 | 3/21 | 0.917 |
| Traumatic event (Yes/No) | 13/13 | 10/14 | 0.555 |
| Same-side operation history (Yes/No) | 2/24 | 3/21 | 0.571 |
| Duration of operation (minutes) | 90.38 ± 20.09 | 83.13 ± 15.17 | 0.021 † |
| Concomitant procedures | 0.739 | ||
| Only acromioplasty (n) | 10 (38.5%) | 4 (16.7%) | |
| Acromioplasty and bicep tenotomy (n) | 12 (46.1%) | 14 (58.3%) | |
| Acromioplasty and bicep tenodesis (n) | 4 (15.4%) | 6 (25.0%) |
| Factors | Group 1 (n = 26) | Group 2 (n = 24) | p Value |
|---|---|---|---|
| Forward elevation | |||
| Pre-operation (°) | 106.54 ± 60.92 | 129.17 ± 67.49 | 0.112 |
| Post-operation ‡ (°) | 157.31 ± 28.36 | 168.75 ± 19.18 | 0.099 |
| Internal rotation | |||
| Pre-operation (point) | 2.50 ± 3.16 | 4.13 ± 3.43 | 0.024 § |
| Post-operation (point) | 5.92 ± 4.91 | 6.71 ± 4.54 | 0.518 |
| IR level difference (point) | 3.42 ± 5.17 | 2.58 ± 4.31 | 0.625 |
| Factors | Group 1 (n = 26) | Group 2 (n = 24) | p Value |
|---|---|---|---|
| P-VAS | |||
| Pre-operation | 6.46 ± 1.65 | 6.08 ± 2.43 | 0.776 |
| Post-operation † | 2.58 ± 1.30 | 2.63 ± 1.31 | 0.904 |
| SST | |||
| Pre-operation | 3.85 ± 2.49 | 4.08 ± 2.10 | 0.543 |
| Post-operation | 6.73 ± 2.14 | 8.08 ± 2.14 | 0.034 ‡ |
| UCLA | |||
| Pre-operation | 13.12 ± 4.61 | 11.38 ± 4.22 | 0.114 |
| Post-operation | 23.15 ± 5.38 | 32.50 ± 10.88 | 0.001 ‡ |
| ASES | |||
| Pre-operation | 44.43 ± 16.93 | 44.24 ± 16.84 | 0.816 |
| Post-operation | 74.50 ± 9.98 | 71.61 ± 12.47 | 0.448 |
| CSS | |||
| Pre-operation | 38.12 ± 18.85 | 36.00 ± 15.61 | 0.778 |
| Post-operation | 48.46 ± 14.69 | 54.50 ± 10.56 | 0.150 |
| Retear | Group 1 (n = 26) | Group 2 (n = 24) | p Value |
|---|---|---|---|
| No | 18 (69.2%) | 16 (66.7%) | 0.846 |
| Yes | 8 (30.8%) | 8 (33.3%) | 0.590 |
| Partial-thickness | 6 | 5 | |
| Full-thickness | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, J.-S.; Choi, J.-K.; Kim, K.-T.; Lee, H.-W.; Lee, Y.-B. Reinforcement Techniques in Arthroscopic Repair of Large-to-Massive Rotator Cuff Tears: A Comparative Study of Superior Capsule Reconstruction and Patch Graft Augmentation. J. Clin. Med. 2024, 13, 2276. https://doi.org/10.3390/jcm13082276
Yee J-S, Choi J-K, Kim K-T, Lee H-W, Lee Y-B. Reinforcement Techniques in Arthroscopic Repair of Large-to-Massive Rotator Cuff Tears: A Comparative Study of Superior Capsule Reconstruction and Patch Graft Augmentation. Journal of Clinical Medicine. 2024; 13(8):2276. https://doi.org/10.3390/jcm13082276
Chicago/Turabian StyleYee, Jae-Sung, Jin-Kwan Choi, Ki-Tae Kim, Ho-Won Lee, and Yong-Beom Lee. 2024. "Reinforcement Techniques in Arthroscopic Repair of Large-to-Massive Rotator Cuff Tears: A Comparative Study of Superior Capsule Reconstruction and Patch Graft Augmentation" Journal of Clinical Medicine 13, no. 8: 2276. https://doi.org/10.3390/jcm13082276
APA StyleYee, J.-S., Choi, J.-K., Kim, K.-T., Lee, H.-W., & Lee, Y.-B. (2024). Reinforcement Techniques in Arthroscopic Repair of Large-to-Massive Rotator Cuff Tears: A Comparative Study of Superior Capsule Reconstruction and Patch Graft Augmentation. Journal of Clinical Medicine, 13(8), 2276. https://doi.org/10.3390/jcm13082276