Validation of an Inhaled Therapy Beliefs Questionnaire in Patients with Chronic Obstructive Pulmonary Disease
Abstract
:1. Introduction
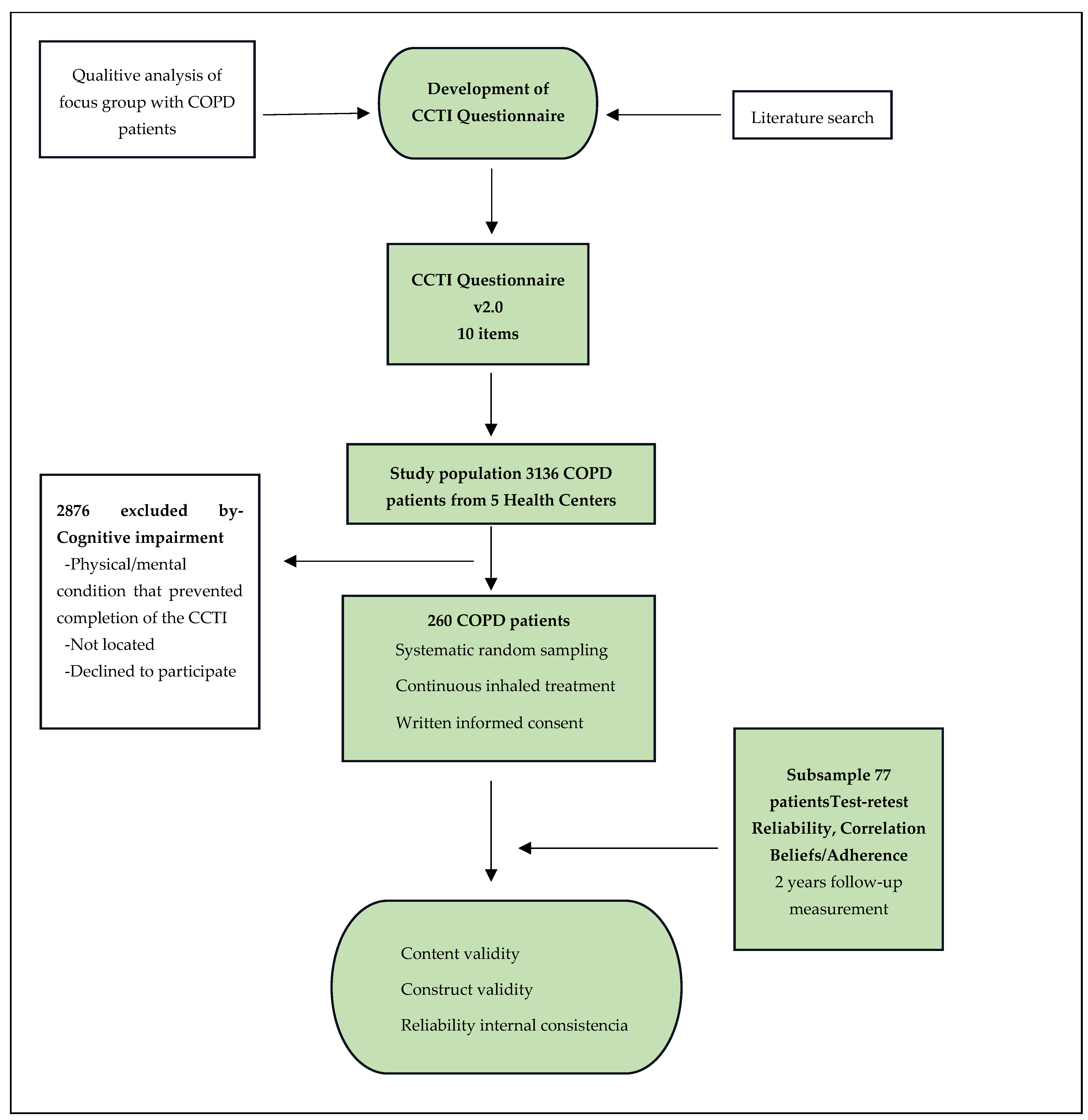
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogelmeir, J.F.; Criner, G.J.; Martinez, F.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for diagnosis, management and prevention of Chronic Obstructive Lung Disease 2017 Report Gold executive summary. Am. J. Resp. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Donner, C.F.; Amaducci, S.; Bacci, E.; Baldacci, S.; Bartoli, M.L.; Beghi, G.M.; Benfante, A.; Brighindi, S.; Casali, L.; Castiglia, D.; et al. Inhalation Therapy in the next decade: Determinants of adherence to treatment in asthma and COPD. Monaldi Arch. Chest Dis. 2018, 88, 886. [Google Scholar] [CrossRef] [PubMed]
- Braido, F.; Lavorini, F.; Blasi, F.; Baiardini, I.; Canonica, G.W. Switching treatments in COPD: Implications for costs and treatment adherence. Int. J. Chron. Obs. Pulmon Dis. 2015, 10, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Bosnic-Anticevich, S.; Chrystyn, H.; Costello, R.W.; Dolovich, M.B.; Fletcher, M.J.; Lavorini, F.; Rodríguez-Roisin, R.; Ryan, D.; Ming, S.W.Y.; Price, D.B. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int. J. Chron. Obs. Pulmon Dis. 2016, 12, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Tottenborg, S.S.; Lange, P.; Johnsen, S.P.; Nielsen, H.; Ingebrigtsen, T.S.; Thomsen, R.W. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir. Med. 2016, 119, 160–167. [Google Scholar] [CrossRef]
- Rogliani, P.; Ora, J.; Puxeddu, E.; Matera, M.D.; Cazzola, M. Adherence to COPD treatment: Myth and reality. Respir. Med. 2017, 129, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Harb, N.; Foster, J.M.; Dobler, C.C. Patient-perceived treatment burden of chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon Dis. 2017, 12, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.P. Motivación y Salud en Emoción y Motivación. La Adaptación Humana; Centro de Estudios Ramón Areces SA: Madrid, Spain, 2003; pp. 831–854. [Google Scholar]
- Insa, L.L.; Benito Monleón, M.A.; Piqueras Espallargas, A. El enfermo de cáncer: Una aproximación a su representación social. Psicol. Soc. 2010, 22, 318–327. [Google Scholar] [CrossRef]
- Conde Gutiérrez del Álamo, F.; Santoro Domingo, P. Grupo de Asesores en Adherencia al Tratamiento Antirretrovírico de Seisida. Tipología; Valores y Preferencias de las personas con VIH e imaginarios de la infección: Resultados de un estudio cualitativo. Rev. Esp. Salud Pública 2012, 86, 139–152. [Google Scholar] [CrossRef]
- Ofman, S.D. Aproximaciones al estudio de las representaciones sociales de la salud y enfermedad: El caso de la diabetes mellitus. Rev. Virtual De La Fac. De Psicol. Y Psicopedag. De La Univ. De El Salvador. 2012, 27, 34–42. [Google Scholar]
- Muñoz Cobos, F.; Acero Guasch, N.; Cuenca del Moral, R.; Barnestein Fonseca, P.; Leiva Fernández, F.; García Ruiz, A. Cómo vivir con EPOC: Percepción de los pacientes. An. De Psicol. 2016, 32, 18–31. [Google Scholar] [CrossRef]
- Calleja Cartón, L.A.; Muñoz Cobos, F.; Aguiar Leiva, V.; Navarro Guitart, C.; Barnestein Fonseca, P.; Leiva Fernández, F. Factores asociados al cumplimiento terapéutico en pacientes con EPOC. Análisis de la perspectiva de los pacientes. Med. Fam. Andal. 2021, 22, 11–24. [Google Scholar]
- Kaptein, A.; Scharloo, M.; Fischer, M.; Snoei, L.; Hughes, B.; Weinman, J.; Kaplan, R.M.; Rabe, K.F. 50 years of psychological research on patients with COPD-road to ruin or highway to heaven? Respir. Med. 2009, 103, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Manary, M.P.; Boulding, W.; Staelin, R.; Glickman, S.W. The patient experience and health outcomes. N. Engl. J. Med. 2013, 368, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Lennox, L.; Bell, D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Lee, J.Y.; Jo, M.W.; Do, Y.K. Does heterogeneity in reporting patient experience matter? An anchoring vignette approach. Int. J. Qual. Health Care 2018, 30, 40–41. [Google Scholar] [CrossRef]
- Wolf, J.; Niederhauser, V.; Marshburn, D.; LaVela, S. Defining patient experience. Patient Exp. J. 2014, 1, 7–19. [Google Scholar] [CrossRef]
- Youseff, A.; Chaudhary, Z.K.; Wiljer, D.; Mylopoulos, M.; Sockalingam, S. Mapping Evidence of Patients’ Experiencies in Integrated Care: A Scoping Review. Gen. Hosp. Psichiatr. 2019, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shahin, W.; Kennedy, G.A.; Stupans, I. The impact of personal and cultural beliefs on medication adherence of patients with chronic illnesses: A systematic review. Patient Prefer. Adherence 2019, 13, 1019–1035. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Horne, R.; Chapman, S.C.E.; Parham, R.; Freemantle, N.; Forbes, A.; Cooper, V. Understanding Patients’ Adherence-Related Beliefs about Medicines Prescribed for Long-Term Conditions: A Meta-Analytic Review of the Necessity-Concerns Framework. PLoS ONE 2013, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.; Barber, N.; Horne, R. Understanding different beliefs held by adherers; unintentional nonadherers; and intentional nonadherers: Application of the Necessity-Concerns Framework. J. Psychosom. Res. 2008, 64, 41–46. [Google Scholar] [CrossRef]
- Homętowska, H.; Świątoniowska-Lonc, N.; Klekowski, J.; Chabowski, M.; Jankowska-Polańska, B. Treatment Adherence in Patients with Obstructive Pulmonary Diseases. Int. J. Environ. Res. Public. Health 2022, 19, 11573. [Google Scholar] [CrossRef] [PubMed]
- Huume, K.K.; Brusse-Keizer, M.; Van der Valk, P.; Movig, K.; Van der Palen, J.; Bode, C. Patients with underuse or overuse of inhaled corticosteroids have different perceptions and beliefs regarding COPD and inhaled medication. Patient Prefer. Adherence 2018, 12, 1777–1783. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, M.H.; Yu, X.Q.; Li, S.Y.; Xie, Y. Development and validation of a patient reported outcome instrument for chronic obstructive pulmonary diseases. Chin. J. Integr. Med. 2015, 21, 667–675. [Google Scholar] [CrossRef]
- Plaza, V.; Fernández-Rodríguez, C.; Melero, C.; Cosio, B.G.; Entrenas, L.M.; DeLlano, L.P.; Gutiérrez-Pereyra, F.; Tarragona, E.; Palomino, R.; López-Viña, A.; et al. Validation of the “test of the adherence to inhalers” (TAI) for asthma and COPD patients. J. Aerosol Med. Pum. Drug Deliv. 2016, 29, 142–152. [Google Scholar] [CrossRef]
- Baiardini, I.; Rogliani, P.; Santus, P.; Corsico, A.G.; Contoli, M.; Scichilone, N.; Di Marco, F.; Lessi, P.; Scognamillo, C.; Molinengo, G.; et al. Disease awareness in patients with COPD: Measurement and extend. Int. J. COPD 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Cobos, F.; Calleja Carton, L.A.; Colacicchi, P.; Rivera Ríos, I.; Polo Barrero, P.; Leiva-Fernández, F. Diseño de un cuestionario de creencias sobre tratamiento inhalado (CCTI) en pacientes con EPOC. Med. Fam. Andal. 2020, 3, 145–155. [Google Scholar]
- Nakken, N.; Janssen, D.J.A.; Van den Bogaart, E.H.A.; Muris, J.W.M.; Vercoulen, J.H.; Custers, F.L.; Bootsma, G.P.; Gronenschild, M.H.M.; Wouters, E.F.M.; Spruit, M.A. Knowledge gaps in patients with COPD and their proxies. BMC Pulm. Med. 2017, 17, 136. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis; Management; and Prevention of Chronic Obstructive Pulmonary Disease (2021 Report). Global Initiative for Chronic Obstructive Lung Disease. 2021. Available online: https://goldcopd.org/ (accessed on 26 November 2022).
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Barnestein-Fonseca, P.; Leiva-Fernández, J.; Vidal-España, F.; García-Ruiz, A.; Prados-Torres, D.; Leiva-Fernández, F. Is it possible to diagnose the therapeutic adherence of patients with COPD in clinical practice? A cohort study. BMC Pulm. Med. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Bonett, D.G.; Wright, T.A. Cronbach’s alpha reliability: Interval estimation; hypothesis testing; and sample size planning. J. Organ. Behav. 2015, 36, 3–15. [Google Scholar] [CrossRef]
- Conway, J.M.; Huffcutt, A.I. A Review and Evaluation of Exploratory Factor Analysis Practices in Organizational Research. Organ. Res. Methods 2003, 6, 147–168. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Amirrudin, M.; Nasution, K.; Supahar, S. Effect of Variability on Cronbach Alpha Reliability in Research Practice. J. Mat. Stat. Dankomputasi 2021, 17, 223–230. [Google Scholar] [CrossRef]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Taber, K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Salman, A.A.; Kopp, B.J.; Thomas, J.E.; Ring, D.; Fatehi, A. What Are the Priming and Ceiling Effects of One Experience Measure on Another? J. Patient Exp. 2020, 7, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, L. t-Test and ANOVA for data with ceiling and/or floor effects. Behav. Res. Methods 2021, 53, 264–277. [Google Scholar] [CrossRef]
- Rosenstock, I.M. Historical origins of the health belief model. Health Educ. Monogr. 1974, 2, 328–335. [Google Scholar] [CrossRef]
- Plaza, V.; López-Viña, A.; Entrenas, L.M.; Fernández-Rodríguez, C.; Melero, C.; Pérez-Llano, L.; Gutiérrez-Pereyra, F.; Tarragona, E.; Palomino, R.; Cosio, B.G. Differences in Adherence and Non-Adherence Behaviour Patterns to Inhaler Devices Between COPD and Asthma Patients. COPD 2016, 13, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Brandstetter, S.; Finger, T.; Fischer, W.; Brandl, M.; Böhmer, M.; Pfeifer, M.; Apfelbacher, C. Differences in medication adherence are associated with beliefs about medicines in asthma and COPD. Clin. Transl. Allergy 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Duarte-de-Araújo, A.; Teixeira, P.; Hespanhol, V.; Correia-de-Sousa, J. COPD: Understanding patients’ adherence to inhaled medications. Int. J. Chron. Obs. Pulmon Dis. 2018, 13, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Krauskopf, K.; Federman, A.D.; Kale, M.S.; Sigel, K.M.; Martynenko, M.; O’Conor, R.; Wolf, M.S.; Leventhal, H.; Wisnivesky, J.P. Chronic Obstructive Pulmonary Disease Illness and Medication Beliefs are Associated with Medication Adherence. COPD 2015, 12, 151–164. [Google Scholar] [CrossRef] [PubMed]
| Health Center | Environment | N | n | Total Included n = (np + nt) | Physical Interview np (%) | Telephone Interview nt (%) | Percentage of the Total Sample |
|---|---|---|---|---|---|---|---|
| El Palo | Urban | 925 | 76 | 77 | 53 (69) | 24 (31) | 29.4 |
| Alameda-Perchel | Urban | 634 | 53 | 53 | 11 (21) | 42 (79) | 20.2 |
| Rincón de la Victoria | Urban | 936 | 77 | 78 | 9 (12) | 69 (88) | 29.8 |
| Puerto de la Torre | Urban | 532 | 44 | 44 | 12 (27) | 32 (73) | 16.8 |
| Colmenar | Rural | 109 | 10 | 10 | 7 (70) | 3 (30) | 3.8 |
| 3136 | 260 | 262 | 92 (35.1) | 156 (64.9) | 100 |
| Variable | Result |
|---|---|
| Age: mean (SD), median, years | 71.5 (8.1), 72 |
| Gender: male, n (%) | 198 (75.6) |
| Urban environment: n (%) | 249 (95) |
| Education level: n (%) | |
| Lower to primary level | 19 (7.3) |
| Primary level | 124 (47.3) |
| Secondary level | 69 (26.3) |
| Higher education | 43 (16.4) |
| Missing values | 7 (2.7) |
| Smoking habit: n (%) | |
| Never | 7 (2.7) |
| Ex-smoker | 193 (73.9) |
| Active smoker | 55 (21.1) |
| Passive smoker | 6 (2.3) |
| Tobacco use packs/year: mean (SD), median | 48.4 (31.6), 41 |
| Severity a n (%) | |
| Mild | 51 (19.5) |
| Moderate | 133 (50.8) |
| Severe | 64 (24.4) |
| Very severe | 7 (2.7) |
| Missing values | 7 (2.7) |
| Exacerbations last year: mean (SD), median | 0.6 (1.05), 0.0 |
| COPD evolution time (years since diagnosis): mean (SD), median | 8.9 (5.11), 8.0 |
| Time with inhalers: n (%) | |
| Less 1 year | 7 (2.7) |
| Between 1 and 5 years | 86 (32.8) |
| Between 6 and 10 years | 72 (27.5) |
| More than 10 years | 96 (36.6) |
| Missing Values | 1 (0.4) |
| Inhaled drug used: n (%) | |
| SABA | 70 (26.7) |
| SAMA | 51 (19.5) |
| LABA | 16 (6.1) |
| LAMA | 103 (39.3) |
| CORTICOIDE | 13 (5.0) |
| LAMA + LABA | 91 (34.7) |
| LABA + CORTICOID | 97 (37.0) |
| LAMA + LABA + CORTICOID | 19 (7.3) |
| SABA + CORTICOID | 1 (0.4) |
| Number of inhaled drugs: mean (SD), median | 1.79 (0.73), 2.0 |
| Number of inhalation devices: mean (SD), median | 1.73 (0.67), 2.0 |
| Type of inhalation devices: n (%) | |
| Pressurized metered-dose inhalers | 95 (36.6) |
| Turbuhaler | 84 (32.1) |
| Handihaler | 61 (23.3) |
| Breezhaler | 61 (23.3) |
| Novolizer | 46 (17.6) |
| Respimat | 39 (14.9) |
| Accuhaler | 14 (5.3) |
| Genuhair | 12 (4.6) |
| Spiromax | 8 (3.1) |
| Nexthaler | 6 (2.3) |
| Easyhaler | 4 (1.5) |
| Zonda | 3 (1.1) |
| Twisthaler | 1 (0.4) |
| Aerolizer | 1 (0.4) |
| Statements of the Questions of the CCTI Questionnaire | % Success * | % Error ** | % Do Not Know/No Answer |
|---|---|---|---|
| 1. The disease is treated by using inhalers daily | 85.5 | 11.1 | 3.4 |
| 2. The effect of inhalers is to make the mucus more liquid | 32.4 | 39.7 | 27.9 |
| 3. For the inhaler to be effective, I must feel that it enters my bronchial tubes. | 10.7 | 78.2 | 10.7 |
| 4. The inhaler should only be used if I am breathlessness and I have a cold | 71.4 | 24.4 | 3.8 |
| 5. It is better not to use the inhaler every day | 67.2 | 22.9 | 9.5 |
| 6. Inhalers are used to open the bronchial tubes and let in more air | 90.8 | 2.7 | 5.7 |
| 7. If I use the inhaler every day, I will get used to it and it will not work | 68.7 | 13 | 18.3 |
| 8. The inhaler should be used as little as possible | 64.5 | 29 | 6.5 |
| 9. When I get better, I have to stop using the inhaler | 71.8 | 22.1 | 5.3 |
| 10. If I use inhalers daily, breathlessness will decrease | 80.2 | 12.6 | 7.3 |
| Statements of the Questions of the CCTI Questionnaire | Mean of Scale If Item Is Removed | Scale Variance If Item Is Removed | Adjusted Item-Total Correlation | Cronbach’s Alpha If Element Is Removed |
|---|---|---|---|---|
| 1. Inhalers taken daily are the treatment of the disease | 7.50 | 5.87 | 0.29 | 0.59 |
| 2. The effect of inhalers is to make the mucus more liquid | 7.54 | 4.98 | 0.24 | 0.61 |
| 3. For the inhaler to take effect, I must feel that it enters my bronchial tubes | 8.10 | 5.29 | 0.26 | 0.59 |
| 4. The inhaler should only be used if I am breathlessness and I have a cold | 7.63 | 5.51 | 0.33 | 0.57 |
| 5. It is better not to use the inhaler every day | 7.56 | 5.40 | 0.32 | 0.57 |
| 6. Inhalers are used to open the bronchial tubes and let in more air | 7.39 | 6.18 | 0.18 | 0.57 |
| 7. If I use the inhaler every day, I will get used to it and it will not work | 7.37 | 5.38 | 0.32 | 0.57 |
| 8. The inhaler should be used as little as possible | 7.65 | 5.35 | 0.34 | 0.57 |
| 9. When I get better, I have to stop using the inhaler | 7.59 | 5.25 | 0.45 | 0.55 |
| 10. If I use inhalers daily, breathlessness will decrease | 7.47 | 6.01 | 0.15 | 0.61 |
| Statements of the Questions of the CCTI Questionnaire | Factor 1: Beliefs Regarding Inhaler Use | Factor 2: Beliefs Regarding the Effect of Inhalers | Factor 3: Beliefs Regarding the Goal of Treatments |
|---|---|---|---|
| 1. Inhalers taken daily are the treatment of the disease | 0.58 | ||
| 2. The effect of inhalers is to make the mucus more liquid | 0.56 | ||
| 3. For the inhaler to take effect, I must feel that it enters my bronchial tubes | 0.64 | ||
| 4. The inhaler should only be used if I am breathlessness and I have a cold | 0.68 | ||
| 5. It is better not to use the inhaler every day | 0.58 | ||
| 6. Inhalers are used to open the bronchial tubes and let in more air | 0.70 | ||
| 7. If I use the inhaler every day, I will get used to it and it will not work | 0.48 | ||
| 8. The inhaler should be used as little as possible | 0.66 | ||
| 9. When I get better, I have to stop using the inhaler | 0.74 | ||
| 10. If I use inhalers daily, breathlessness will decrease | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Cobos, F.; Aguiar-Leiva, V.P.; Argüello-Suárez, C.; Colacicchi, P.; Calleja-Cartón, L.A.; Leiva-Fernández, F. Validation of an Inhaled Therapy Beliefs Questionnaire in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2024, 13, 2281. https://doi.org/10.3390/jcm13082281
Muñoz-Cobos F, Aguiar-Leiva VP, Argüello-Suárez C, Colacicchi P, Calleja-Cartón LA, Leiva-Fernández F. Validation of an Inhaled Therapy Beliefs Questionnaire in Patients with Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2024; 13(8):2281. https://doi.org/10.3390/jcm13082281
Chicago/Turabian StyleMuñoz-Cobos, Francisca, Virginia P. Aguiar-Leiva, Carmen Argüello-Suárez, Paula Colacicchi, Luis Antonio Calleja-Cartón, and Francisca Leiva-Fernández. 2024. "Validation of an Inhaled Therapy Beliefs Questionnaire in Patients with Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 13, no. 8: 2281. https://doi.org/10.3390/jcm13082281






