Using Sequence Analyses to Quantitatively Measure Oropharyngeal Swallowing Temporality in Point-of-Care Ultrasound Examinations: A Pilot Study
Abstract
:1. Introduction
1.1. Swallowing Assessment Modalities
1.2. Swallowing Sequencing: The Status Quo
1.3. Ultrasound Swallowing Assessment and Quantitative Medical Image Analysis
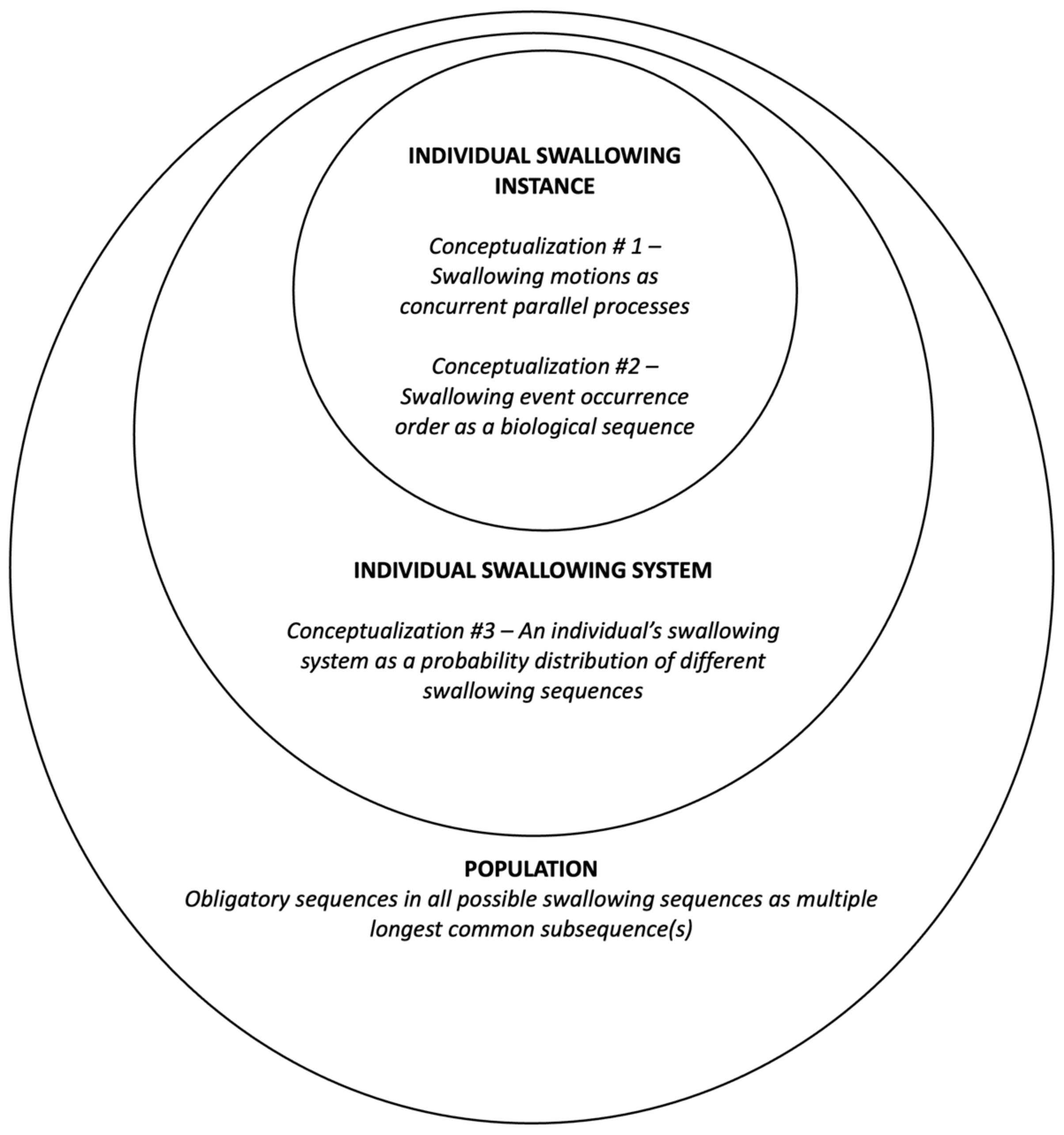
1.4. The Proposed Framework: A Systemic Conceptualization
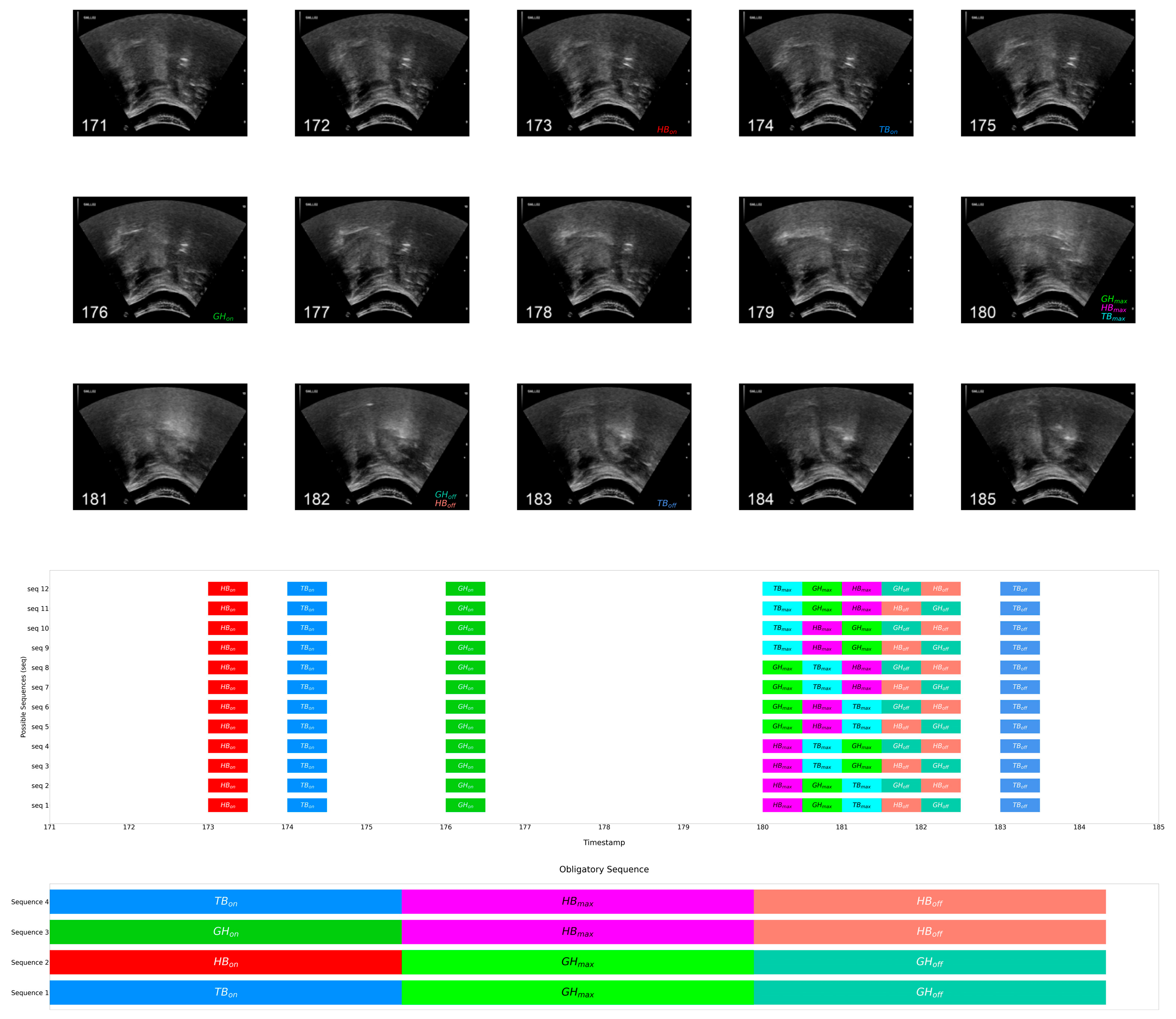
1.4.1. Swallowing Sequence Instances
1.4.2. Swallowing Sequencing System
1.4.3. Obligatory Swallowing Sequences
1.5. Research Objectives
2. Materials and Methods
2.1. Participants
2.2. Materials and Equipment
2.3. USI Acquisition
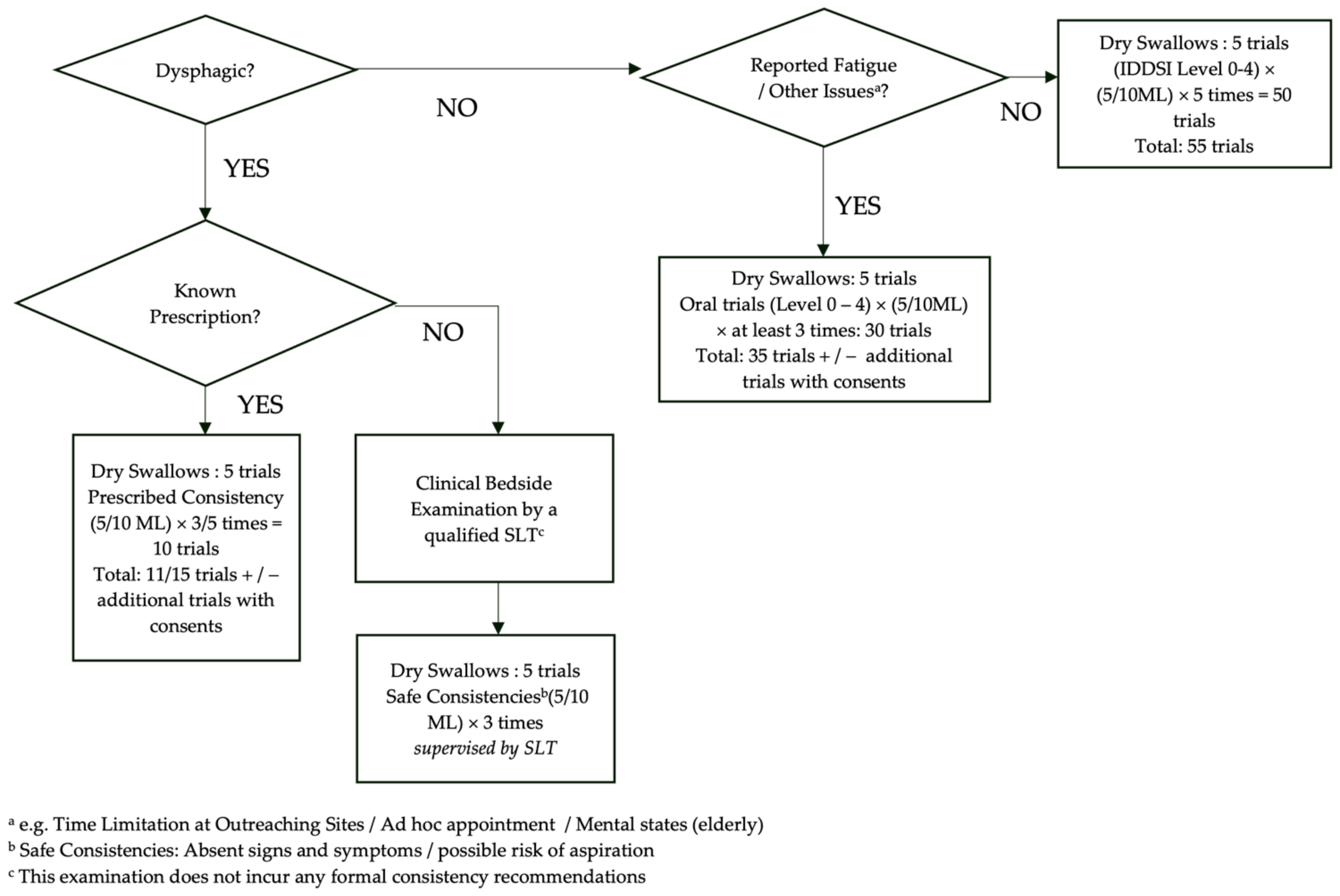
2.4. Swallow Trial Protocol
2.5. Data Annotation
- Event onset: the first frame where the SOI begins leaving its at-rest position, thus the commencement of the movement (for onset of HB, commencement of anterior-superior displacement was considered);
- Event maximum: the first frame where the SOI reaches its maximum displacement or contraction;
- Event offset: the frame at which the SOI begins returning to the at-rest position.
- An event state is denoted with subscripts: e.g., HBon as the onset, HBmax as the maximum, and HBoff as the offset of hyoid bone displacement.
- The duration from onset to maximum was defined as the initiating period using subscripted init and that from maximum to offset as the sustaining period using subscripted sus: e.g., HBinit denotes the onset to maximum duration/transition and HBsus the maximum to offset duration/transition of hyoid bone displacement, respectively.
- The overlapping transitions of two events are denoted by hyphenating the abbreviations of the events with a subscripted abbreviation of transition: for instance, HB-TBinit denotes the overlapping period of the onset-to-maximum transitions of hyoid bone displacement and tongue base retraction.
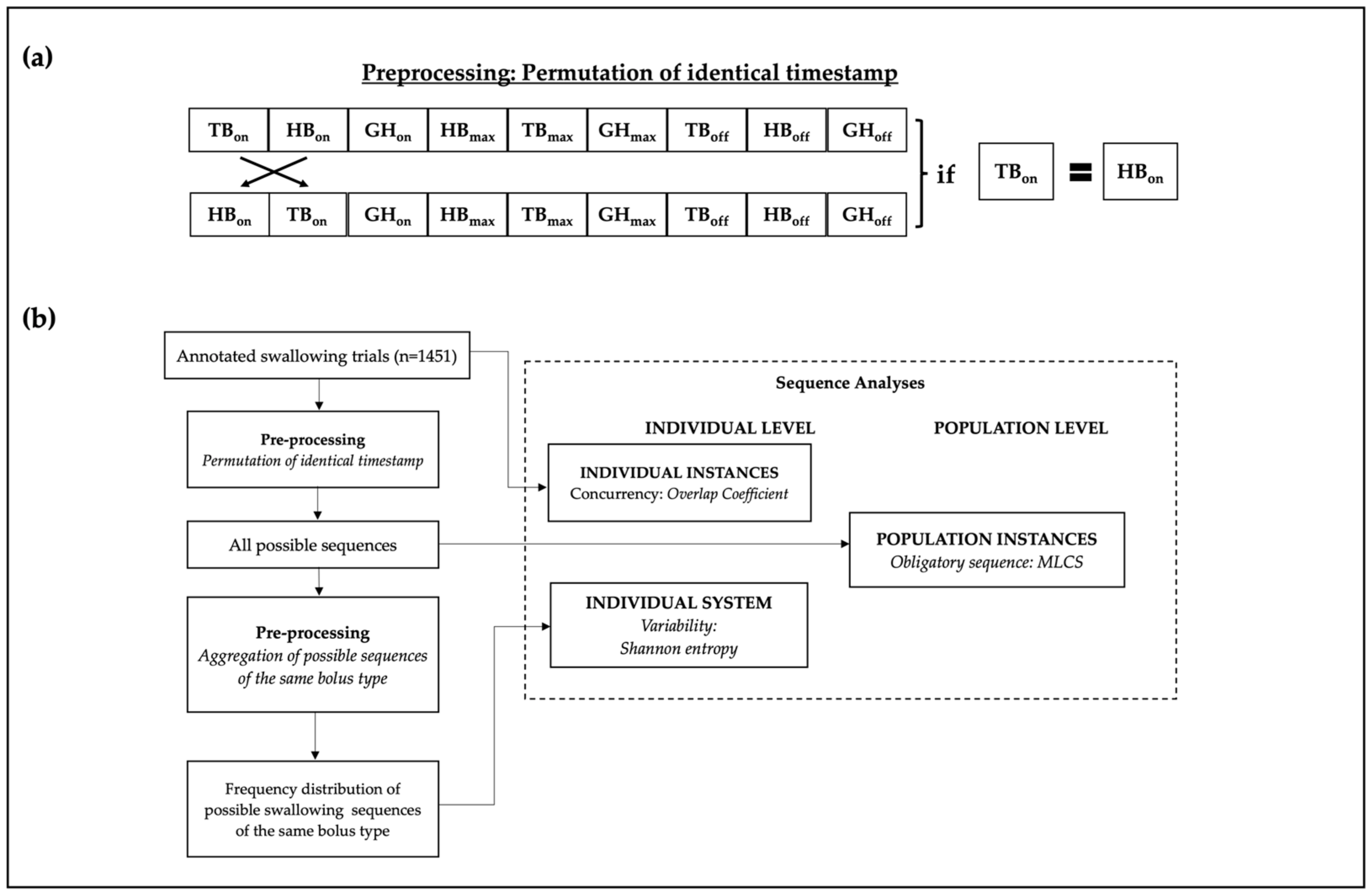
2.5.1. Data Preprocessing
2.6. Data Extraction and Statistical Analysis
2.6.1. Concurrency Analysis: Overlap Coefficient
2.6.2. Variability Analysis: Shannon Entropy
2.6.3. Obligatory Sequence: Multiple Longest Common Subsequence
2.6.4. Statistical Analysis
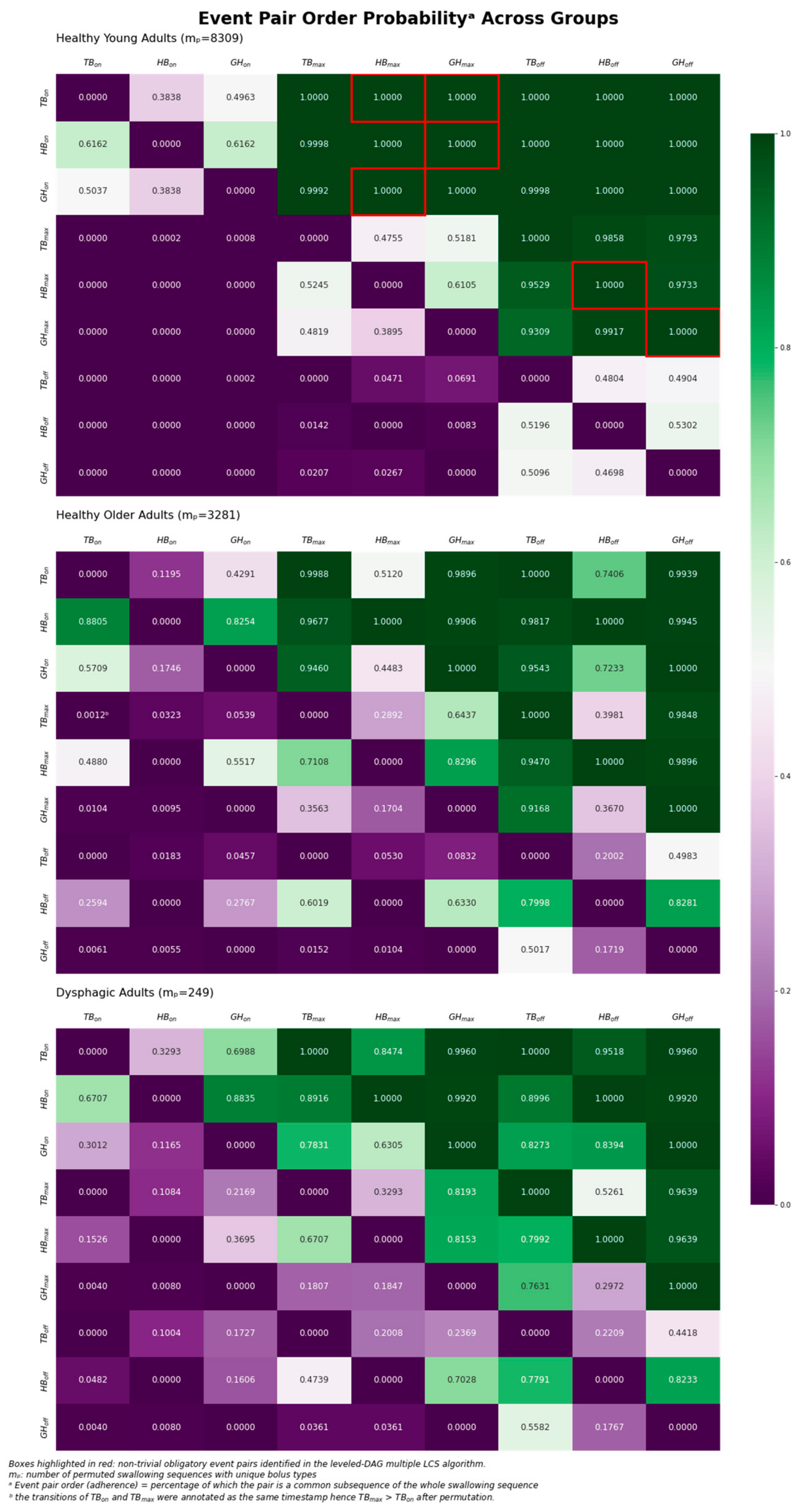
3. Results
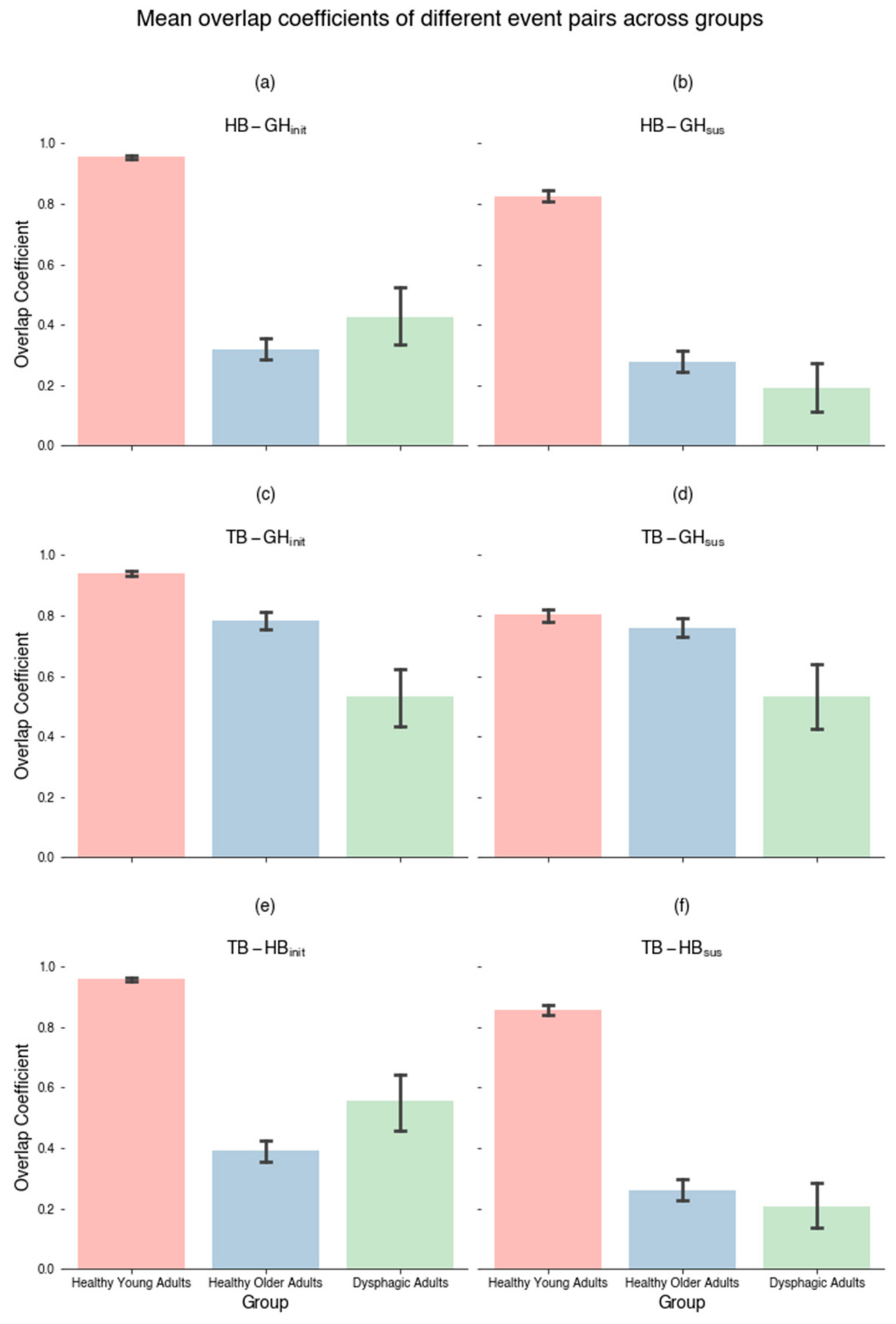
3.1. Concurrency Analysis: Overlap Coefficient (SSC)
3.2. Variability Analysis: Shannon Entropy
3.3. Obligatory Sequence: Multiple Longest Common Subsequence
- TBon prior to GHmax prior to GHoff (Sequence 1);
- HBon prior to GHmax prior to GHoff (Sequence 2);
- GHon prior to HBmax prior to HBoff (Sequence 3);
- TBon prior to HBmax prior to HBoff (Sequence 4).
4. Discussion
4.1. Concurrency
4.2. Variability
4.3. Obligatory Pairs in Healthy Young Adults
- (i)
- Tongue base retraction onset prior to geniohyoid muscle contraction maximum, prior to geniohyoid muscle contraction offset (Sequence 1);
- (ii)
- Hyoid bone displacement onset prior to geniohyoid muscle contraction maximum, prior to geniohyoid muscle contraction offset (Sequence 2);
- (iii)
- Geniohyoid muscle contraction onsets prior to hyoid bone displacement maximum, prior to hyoid bone displacement offset (Sequence 3);
- (iv)
- Tongue base retraction onsets prior to hyoid bone displacement maximum, prior to hyoid bone displacement offset (Sequence 4).
4.4. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pongpipatpaiboon, K.; Inamoto, Y.; Matsuo, K.; Aoyagi, Y.; Shibata, S.; Kagaya, H. Physiological models of swallowing. In Dysphagia Evaluation and Treatment: From the Perspective of Rehabilitation Medicine; Springer: Singapore, 2018; pp. 17–25. [Google Scholar]
- Martin-Harris, B.; Brodsky, M.B.; Michel, Y.; Castell, D.O.; Schleicher, M.; Sandidge, J.; Maxwell, R.; Blair, J. MBS measurement tool for swallow impairment—MBSImp: Establishing a standard. Dysphagia 2008, 23, 392–405. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Robbins, J.A.; Roecker, E.B.; Coyle, J.L.; Wood, J.L. A penetration-aspiration scale. Dysphagia 1996, 11, 93–98. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Di Luciano, C.; Chiaramonte, I.; Serra, A.; Bonfiglio, M. Multi-disciplinary clinical protocol for the diagnosis of bulbar amyotrophic lateral sclerosis. Acta Otorrinolaringol. (Engl. Ed.) 2019, 70, 25–31. [Google Scholar]
- Vaiman, M.; Eviatar, E. Surface electromyography as a screening method for evaluation of dysphagia and odynophagia. Head Face Med. 2009, 5, 9. [Google Scholar] [CrossRef]
- Diaz, K.; Stegemöller, E.E. Electromyographic measures of asymmetric muscle control of swallowing in Parkinson’s disease. PLoS ONE 2022, 17, e0262424. [Google Scholar] [CrossRef]
- Geddes, D.T.; Chadwick, L.M.; Kent, J.C.; Garbin, C.P.; Hartmann, P.E. Ultrasound imaging of infant swallowing during breast-feeding. Dysphagia 2010, 25, 183–191. [Google Scholar] [CrossRef]
- Casas, M.J.; McPherson, K.A.; Kenny, D.J. Durational aspects of oral swallow in neurologically normal children and children with cerebral palsy: An ultrasound investigation. Dysphagia 1995, 10, 155–159. [Google Scholar] [CrossRef]
- Shawker, T.H.; SONIES, B.; Hall, T.E.; BAUM, B.F. Ultrasound analysis of tongue, hyoid, and larynx activity during swallowing. Investig. Radiol. 1984, 19, 82–86. [Google Scholar] [CrossRef]
- Kuhl, V.; Eicke, B.; Dieterich, M.; Urban, P. Sonographic analysis of laryngeal elevation during swallowing. J. Neurol. 2003, 250, 333–337. [Google Scholar] [CrossRef]
- Yabunaka, K.; Sanada, H.; Sanada, S.; Konishi, H.; Hashimoto, T.; Yatake, H.; Yamamoto, K.; Katsuda, T.; Ohue, M. Sonographic assessment of hyoid bone movement during swallowing: A study of normal adults with advancing age. Radiol. Phys. Technol. 2011, 4, 73–77. [Google Scholar] [CrossRef]
- Feng, X.; Cartwright, M.S.; Walker, F.O.; Bargoil, J.H.; Hu, Y.; Butler, S.G. Ultrasonographic evaluation of geniohyoid muscle and hyoid bone during swallowing in young adults. Laryngoscope 2015, 125, 1886–1891. [Google Scholar] [CrossRef]
- Stone, M.; Shawker, T.H. An ultrasound examination of tongue movement during swallowing. Dysphagia 1986, 1, 78. [Google Scholar] [CrossRef]
- Söder, N.; Miller, N. Using ultrasound to investigate intrapersonal variability in durational aspects of tongue movement during swallowing. Dysphagia 2002, 17, 288–297. [Google Scholar] [CrossRef]
- Ohkubo, M.; Scobbie, J.M. Tongue shape dynamics in swallowing using sagittal ultrasound. Dysphagia 2019, 34, 112–118. [Google Scholar] [CrossRef]
- Kwong, E.; Shek, P.T.-C.; Leung, M.-T.; Zheng, Y.-P.; Lam, W.Y.S. Temporal measures of oropharyngeal swallowing events identified using ultrasound imaging in healthy young adults. PLoS ONE 2022, 17, e0270704. [Google Scholar] [CrossRef]
- Mendell, D.A.; Logemann, J.A. Temporal Sequence of Swallow Events During the Oropharyngeal Swallow. J. Speech Lang. Hear. Res. 2007, 50, 1256–1271. [Google Scholar] [CrossRef]
- Kendall, K.A.; Leonard, R.J.; McKenzie, S.W. Sequence Variability During Hypopharyngeal Bolus Transit. Dysphagia 2003, 18, 85–91. [Google Scholar] [CrossRef]
- Molfenter, S.M.; Leigh, C.; Steele, C.M. Event Sequence Variability in Healthy Swallowing: Building on Previous Findings. Dysphagia 2014, 29, 234–242. [Google Scholar] [CrossRef]
- Herzberg, E.G.; Lazarus, C.L.; Steele, C.M.; Molfenter, S.M. Swallow Event Sequencing: Comparing Healthy Older and Younger Adults. Dysphagia 2018, 33, 759–767. [Google Scholar] [CrossRef]
- Chen, H.; Gomez, C.; Huang, C.-M.; Unberath, M. Explainable medical imaging AI needs human-centered design: Guidelines and evidence from a systematic review. npj Digit. Med. 2022, 5, 156. [Google Scholar] [CrossRef]
- Logemann, J.A. Evaluation and Treatment of Swallowing Disorders, 2nd ed.; PRO-ED: Austin, TX, USA, 1998. [Google Scholar]
- Suiter, D.M.; Leder, S.B. Clinical utility of the 3-ounce water swallow test. Dysphagia 2008, 23, 244–250. [Google Scholar] [CrossRef]
- Cichero, J.A.Y.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J.; et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef]
- Nakajima, N.; Hayashi, T.; Fujiki, K.; Shirahige, K.; Akiyama, T.; Akutsu, T.; Nakato, R. Codependency and mutual exclusivity for gene community detection from sparse single-cell transcriptome data. Nucleic Acids Res. 2021, 49, e104. [Google Scholar] [CrossRef]
- Chanda, P.; Costa, E.; Hu, J.; Sukumar, S.; Van Hemert, J.; Walia, R. Information theory in computational biology: Where we stand today. Entropy 2020, 22, 627. [Google Scholar] [CrossRef]
- Deorowicz, S.; Debudaj-Grabysz, A.; Gudyś, A. FAMSA: Fast and accurate multiple sequence alignment of huge protein families. Sci. Rep. 2016, 6, 33964. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y. A novel efficient graph model for the multiple longest common subsequences (MLCS) problem. Front. Genet. 2017, 8, 104. [Google Scholar] [CrossRef]
- Mckinney, W. Pandas: A Foundational Python Library for Data Analysis and Statistics. Python High Perform. Sci. Comput. 2011, 14, 1–9. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Terpilowski, M.A. scikit-posthocs: Pairwise multiple comparison tests in Python. J. Open Source Softw. 2019, 4, 1169. [Google Scholar] [CrossRef]
- Pearson, W.G.; Davidoff, A.A.; Smith, Z.M.; Adams, D.E.; Langmore, S.E. Impaired swallowing mechanics of post radiation therapy head and neck cancer patients: A retrospective videofluoroscopic study. World J. Radiol. 2016, 8, 192–199. [Google Scholar] [CrossRef]
- Mori, T.; Izumi, S.; Suzukamo, Y.; Okazaki, T.; Iketani, S. Ultrasonography to detect age-related changes in swallowing muscles. Eur. Geriatr. Med. 2019, 10, 753–760. [Google Scholar] [CrossRef]
- Teismann, I.K.; Suntrup, S.; Warnecke, T.; Steinsträter, O.; Fischer, M.; Flöel, A.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Cortical swallowing processing in early subacute stroke. BMC Neurol. 2011, 11, 34. [Google Scholar] [CrossRef]
- Pearson, W.G.; Langmore, S.E.; Zumwalt, A.C. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia 2011, 26, 345–351. [Google Scholar] [CrossRef]
- Gawryszuk, A.; Bijl, H.P.; Holwerda, M.; Halmos, G.B.; Wedman, J.; Witjes, M.J.H.; van der Vliet, A.M.; Dorgelo, B.; Langendijk, J.A. Functional Swallowing Units (FSUs) as organs-at-risk for radiotherapy. PART 1: Physiology and anatomy. Radiother. Oncol. 2019, 130, 62–67. [Google Scholar] [CrossRef]


| Group | Mean Age in Years (SD) | Gender (M/F) | Etiology |
|---|---|---|---|
| Healthy Young Adult (n = 17) | 41.18 (17.37) | 7/10 | - |
| Healthy Older Adult (n = 15) | 69.46 (5.45) | 6/9 | - |
| Dysphagic Adult (n = 11) | 63.55 (12.32) | 4/7 | neurological (e.g., stroke): 8 structural (e.g., head and neck cancer): 3 |
| Event Transition Pair | Y | O | D | df | H | p-Value | η2 | Dunn’s Test | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Y vs. O p-Value | Y vs. D p-Value | O vs. D p-Value | ||||||
| Initiating | HB-GHinit | 0.955 (0.088) | 0.319 (0.428) | 0.427 (0.416) | 2 | 622.700 | <0.0001 *** | 0.429 | <0.0001 *** | <0.0001 *** | 0.598 |
| TB-GHinit | 0.938 (0.122) | 0.785 (0.353) | 0.533 (0.441) | 2 | 99.907 | <0.0001 *** | 0.068 | <0.0001 *** | <0.0001 *** | <0.0001 *** | |
| TB-HBinit | 0.959 (0.092) | 0.391 (0.434) | 0.556 (0.450) | 2 | 526.413 | <0.0001 *** | 0.362 | <0.0001 *** | <0.0001 *** | 0.004 ** | |
| Sustaining | HB-GHsus | 0.825 (0.263) | 0.277 (0.439) | 0.190 (0.364) | 2 | 456.167 | <0.0001 *** | 0.314 | <0.0001 *** | <0.0001 *** | 0.175 |
| TB-GHsus | 0.802 (0.287) | 0.759 (0.373) | 0.534 (0.485) | 2 | 16.745 | <0.001 *** | 0.011 | 0.99 | <0.001 ** | <0.001 ** | |
| TB-HBsus | 0.856 (0.239) | 0.260 (0.423) | 0.207 (0.341) | 2 | 571.850 | <0.0001 *** | 0.394 | <0.0001 *** | <0.0001 *** | 0.539 | |
| Bolus Types | Y | O | D | df | H | p-Value | η2 | Dunn’s Test | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Y vs. O p-Value | Y vs. D p-Value | O vs. D p-Value | |||||
| Matched—Dry and 5 mL of IDDSI Levels 0–4 | 5.119 (1.095) | 4.428 (0.950) | 3.038 (0.872) | 2 | 52.253 | <0.0001 *** | 0.260 | <0.001 ** | <0.0001 *** | <0.0001 *** |
| All bolus types | 5.07 (1.075) | 4.286 (1.095) | 2.896 (1.011) | 2 | 78.472 | <0.0001 *** | 0.224 | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| TBon | HBon | GHon | TBmax | HBmax | GHmax | TBoff | HBoff | GHoff | |
|---|---|---|---|---|---|---|---|---|---|
| TBon | 0.3838 | 0.4963 | 1.0 | 1.0 * | 1.0 * | 1.0 | 1.0 | 1.0 | |
| HBon | 0.6162 | 0.9998 ^ | 1.0 | 1.0 * | 1.0 | 1.0 | 1.0 | ||
| GHon | 0.9992 | 1.0 * | 1.0 | 0.9998 | 1.0 | 1.0 | |||
| TBmax | 0.4755 | 0.5181 | 1.0 | 0.9858 ^ | 0.9793 | ||||
| HBmax | 0.6105 | 0.9529 | 1.0 * | 0.9733 | |||||
| GHmax | 0.9309 | 0.9917 | 1.0 * | ||||||
| TBoff | 0.4804 | 0.4904 | |||||||
| HBoff | 0.5302 | ||||||||
| GHoff |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, W.Y.S.; Kwong, E.; Chan, H.W.T.; Zheng, Y.-P. Using Sequence Analyses to Quantitatively Measure Oropharyngeal Swallowing Temporality in Point-of-Care Ultrasound Examinations: A Pilot Study. J. Clin. Med. 2024, 13, 2288. https://doi.org/10.3390/jcm13082288
Lam WYS, Kwong E, Chan HWT, Zheng Y-P. Using Sequence Analyses to Quantitatively Measure Oropharyngeal Swallowing Temporality in Point-of-Care Ultrasound Examinations: A Pilot Study. Journal of Clinical Medicine. 2024; 13(8):2288. https://doi.org/10.3390/jcm13082288
Chicago/Turabian StyleLam, Wilson Yiu Shun, Elaine Kwong, Huberta Wai Tung Chan, and Yong-Ping Zheng. 2024. "Using Sequence Analyses to Quantitatively Measure Oropharyngeal Swallowing Temporality in Point-of-Care Ultrasound Examinations: A Pilot Study" Journal of Clinical Medicine 13, no. 8: 2288. https://doi.org/10.3390/jcm13082288






