The Impact of Potassium Dynamics on Cardiomyocyte Beating in Hemodialysis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Mathematical Modeling
2.2. Mathematical Analysis of Cardiomyocyte Beating
3. Results
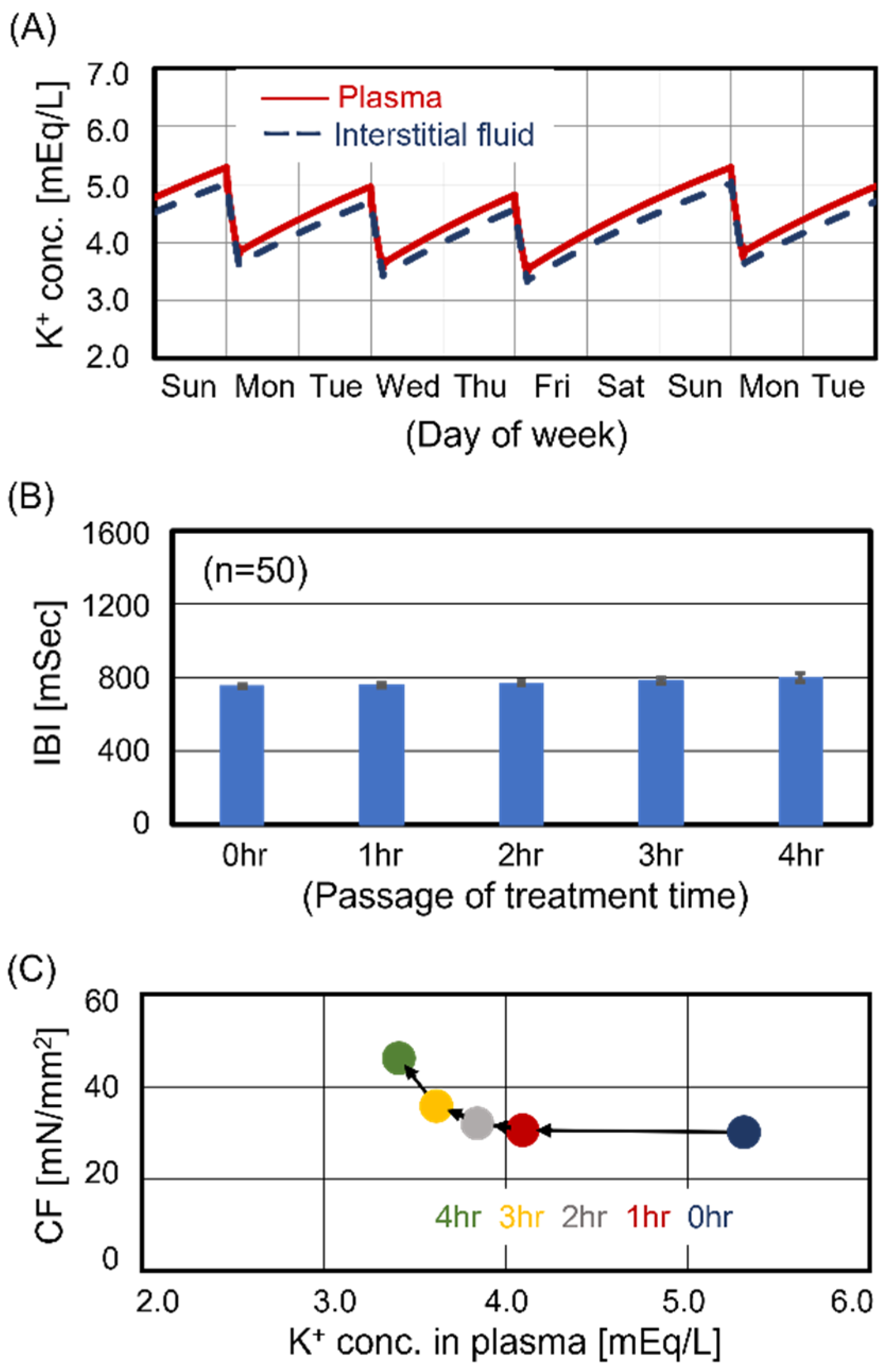
3.1. Cardiomyocyte Beating Observed in Case #1
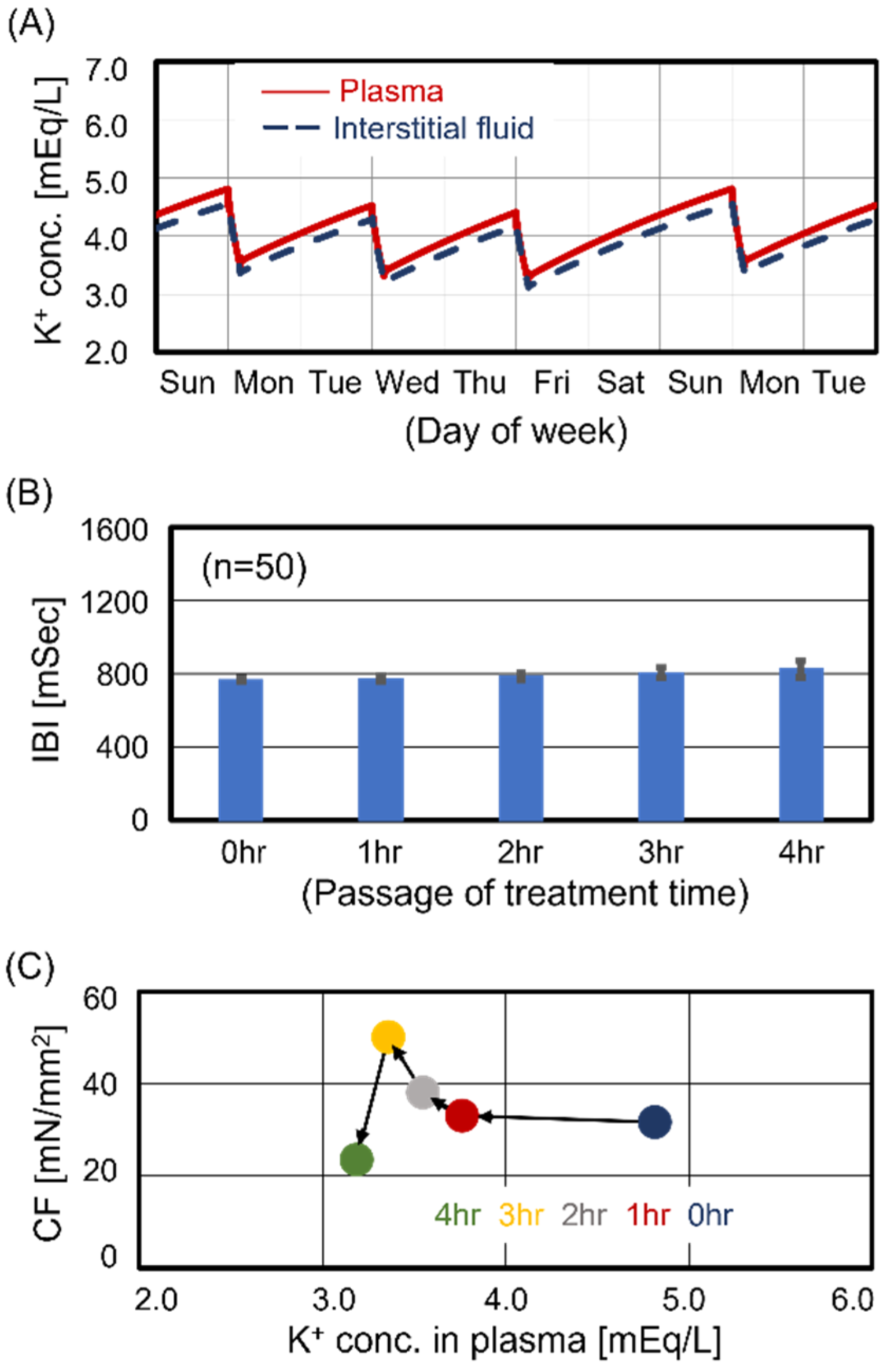
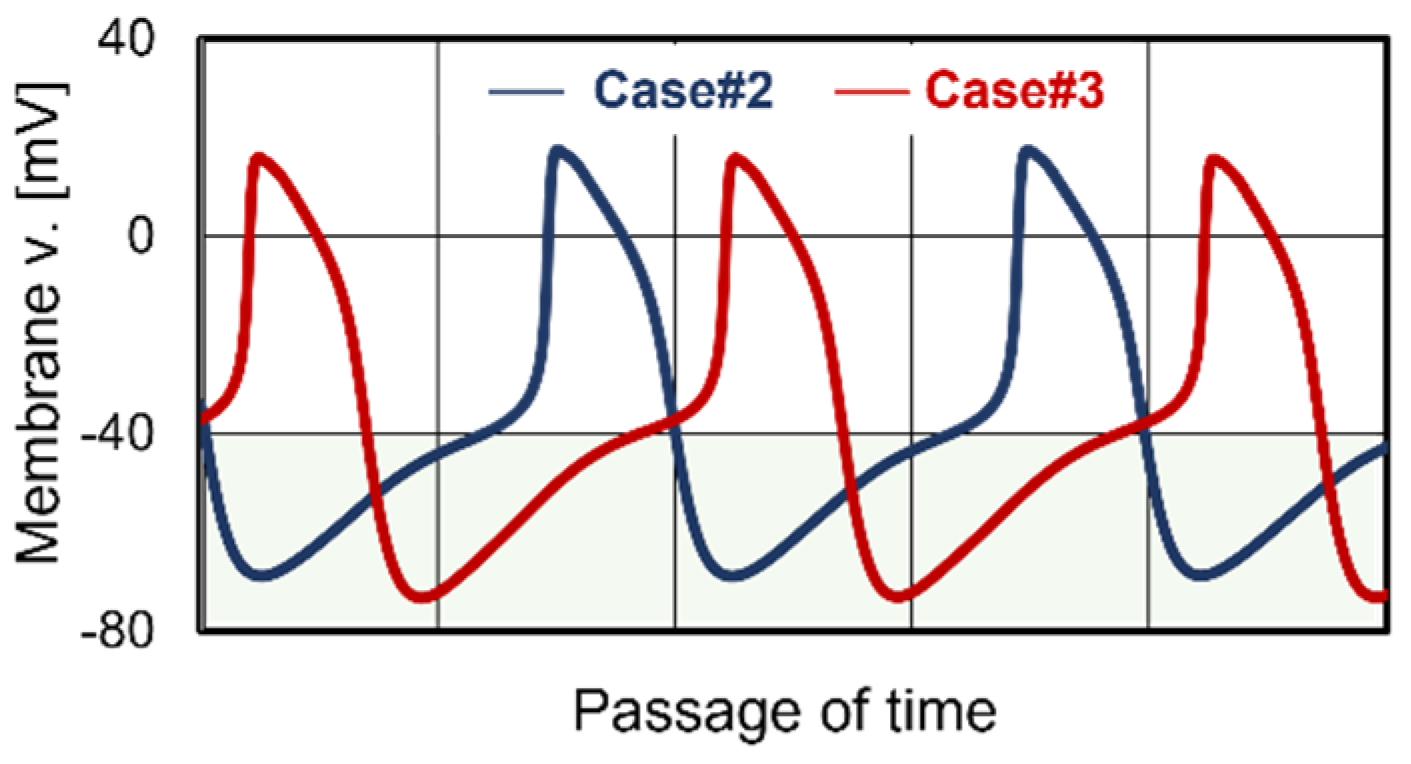
3.2. Cardiomyocyte Beating Observed in Case #2
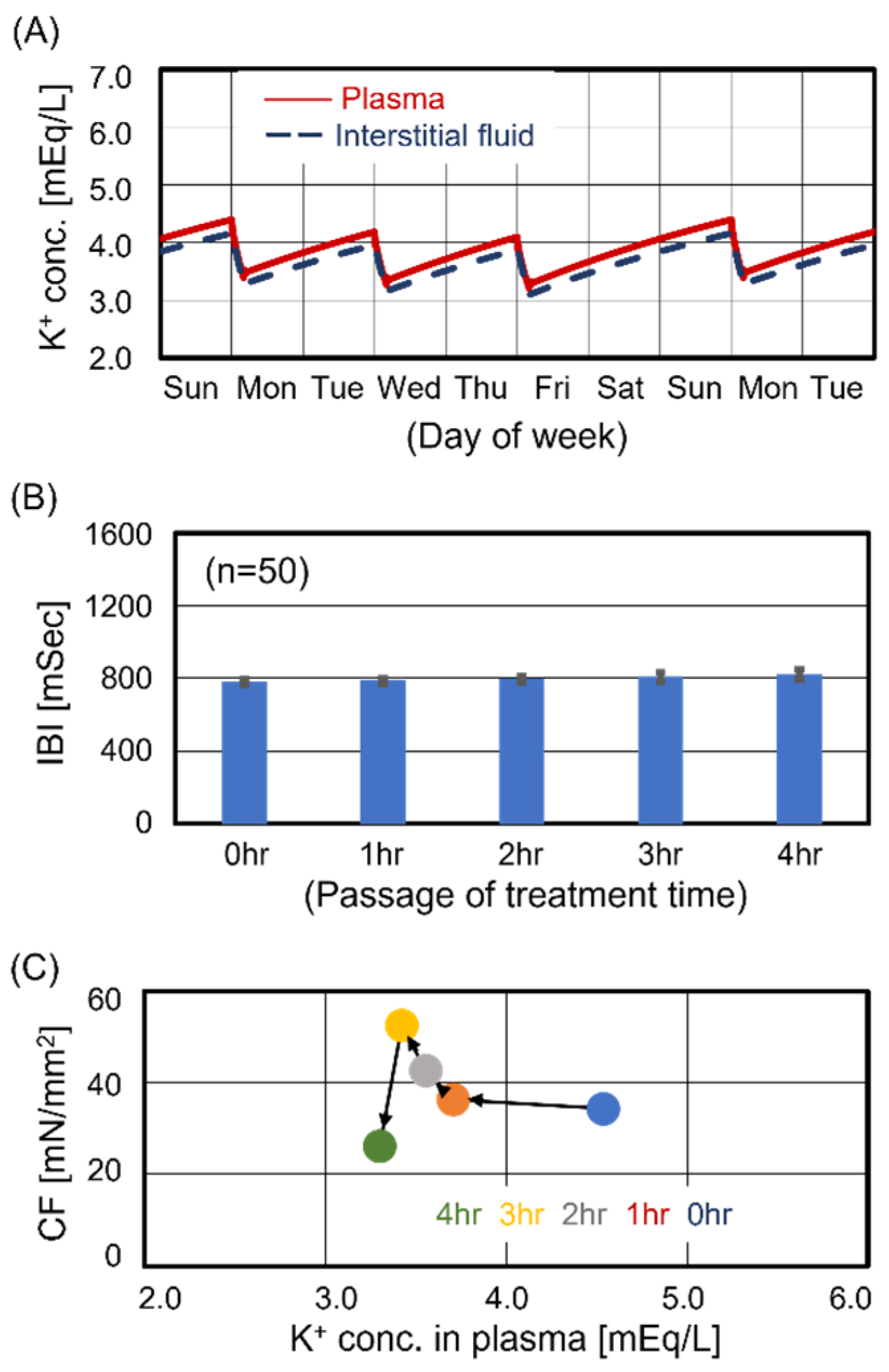
3.3. Cardiomyocyte Beating Observed in Case #3
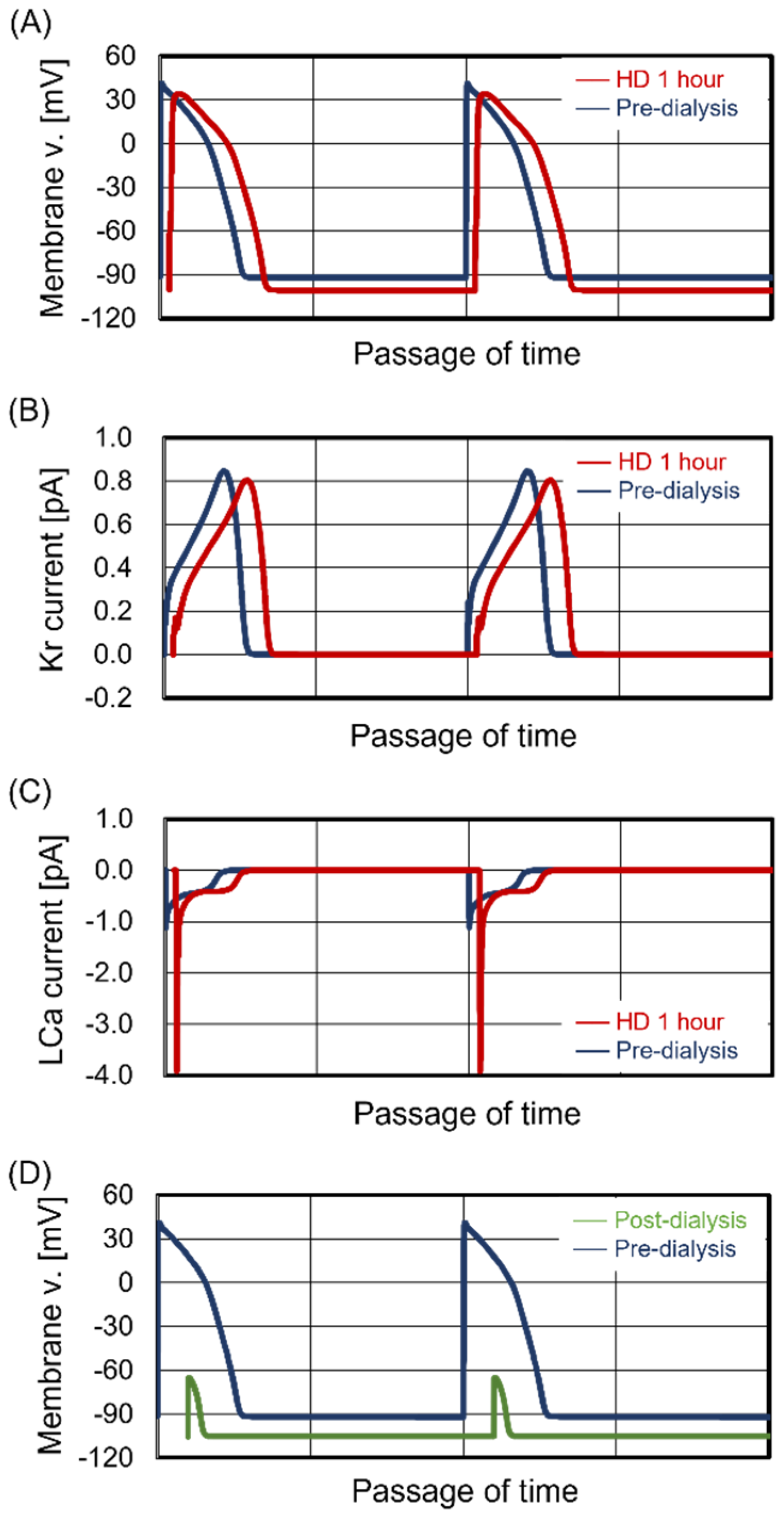
3.4. Unstable Beating Rhythm
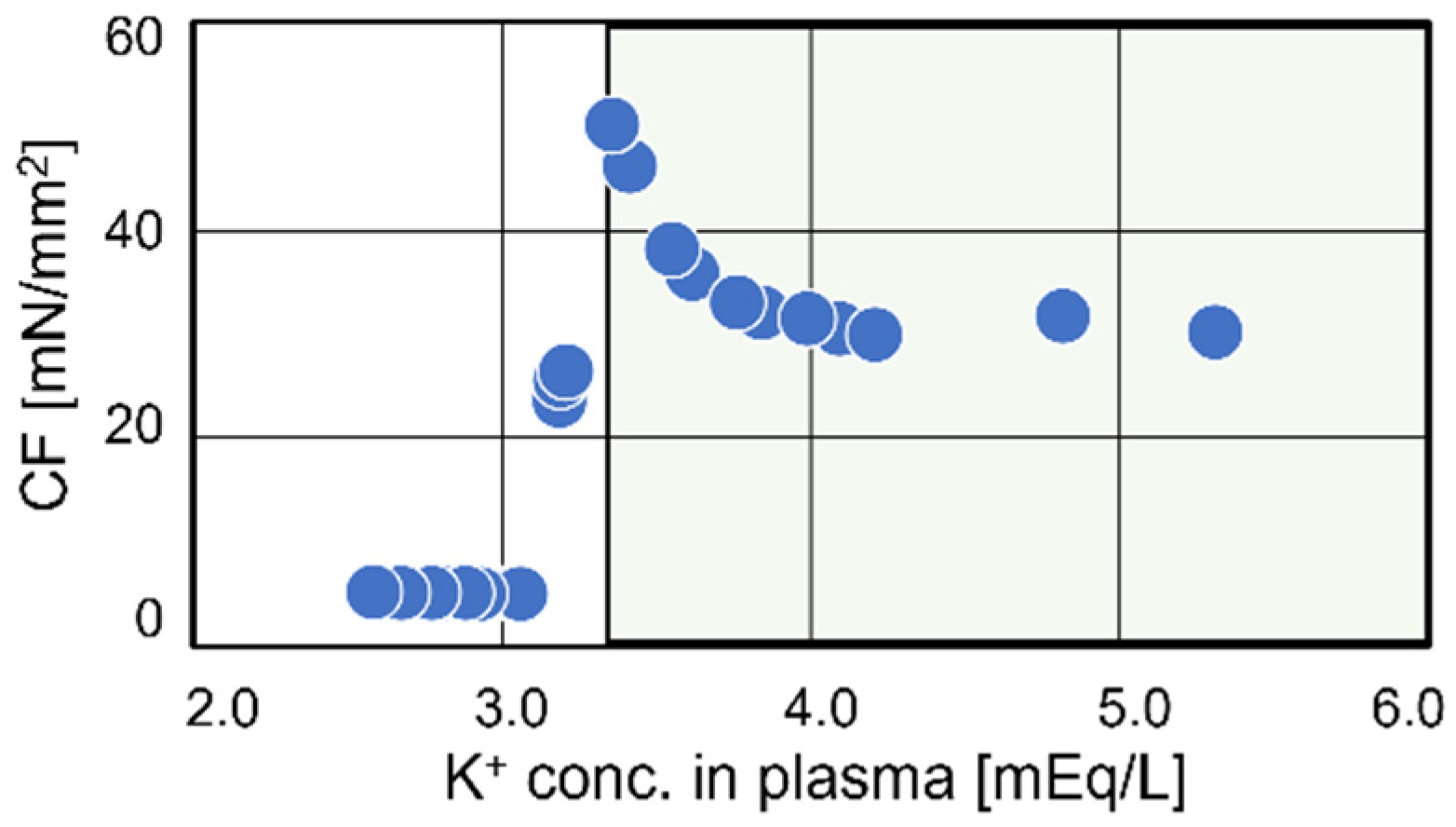
3.5. Transition of Contraction Force
3.6. Stabilization of Cardiomyocyte Beating in Case #3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, N.; Dalal, P.; Panesar, M. Dialysis disequilibrium syndrome: A narrative review. Semin. Dial. 2008, 21, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Negoianu, D.; Zhang, H.; Casper, S.; Hsu, J.Y.; Kotanko, P.; Raimann, J.; Dember, L.M. Dynamics of Plasma Refill Rate and Intradialytic Hypotension During Hemodialysis: Retrospective Cohort Study with Causal Methodology. Kidney360 2023, 4, e505–e514. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.H.; Lehrich, R.W.; Smith, S.R.; Middleton, J.P. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin. J. Am. Soc. Nephrol. 2007, 2, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, M.; Thumma, J.; Fuller, D.S.; Tentori, F.; Li, Y.; Morgenstern, H.; Mendelssohn, D.; Tomo, T.; Ethier, J.; Port, F.; et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin. J. Am. Soc. Nephrol. 2012, 7, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Makar, M.S.; Pun, P.H. Sudden Cardiac Death Among Hemodialysis Patients. Am. J. Kidney Dis. 2017, 69, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Enderle, J. Bioelectric Phenomena. In Introduction to Biomedical Engineering, 2nd ed.; Academic Press: Boston, MA, USA, 2005; pp. 627–691. [Google Scholar]
- Karaboyas, A.; Zee, J.; Brunelli, S.M.; Usvyat, L.A.; Weiner, D.E.; Maddux, F.W.; Nissenson, A.R.; Jadoul, M.; Locatelli, F.; Winkelmayer, W.C.; et al. Dialysate Potassium, Serum Potassium, Mortality, and Arrhythmia Events in Hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2017, 69, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Colatsky, T.; Fermini, B.; Gintant, G.; Pierson, J.B.; Sager, P.; Sekino, Y.; Strauss, D.G.; Stockbridge, N. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods 2016, 81, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Tomo, T.; Kim, S.T.; Hanai, T.; Okamoto, M.; Yamashita, A.C. Electrophysiological insights into the relationship between calcium dynamics and cardiomyocyte beating function in chronic hemodialysis treatment. J. Artif. Organs. 2021, 24, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gotch, F.; Levin, N.W.; Kotanko, P. Calcium balance in dialysis is best managed by adjusting dialysate calcium guided by kinetic modeling of the interrelationship between calcium intake, dose of vitamin D analogues and the dialysate calcium concentration. Blood Purif. 2010, 29, 163–176. [Google Scholar] [CrossRef]
- Hamada, H.; Nomura, F.; Kaneko, T.; Yasuda, K.; Okamoto, M. Exploring the implicit interlayer regulatory mechanism between cells and tissue: Stochastic mathematical analyses of the spontaneous ordering in beating synchronization. Biosystems 2013, 111, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Hisatome, I.; Imanishi, S.; Shibamoto, T. Dynamical description of sinoatrial node pacemaking: Improved mathematical model for primary pacemaker cell. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2074–H2101. [Google Scholar] [CrossRef] [PubMed]
- Wilders, R. Computer modelling of the sinoatrial node. Med. Biol. Eng. Comput. 2007, 45, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, V.A.; Lakatta, E.G. A novel quantitative explanation for the autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2010–H2023. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, T.; Virág, L.; Varró, A.; Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol. 2011, 7, e1002061. [Google Scholar] [CrossRef] [PubMed]
- Negroni, J.A.; Lascano, E.C. A cardiac muscle model relating sarcomere dynamics to calcium kinetics. J. Mol. Cell. Cardiol. 1996, 28, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Tomo, T. History of dialysis fluid. Jpn. J. Artif. Organ. 2017, 46, 55–59. [Google Scholar]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Bowling, C.B.; Pitt, B.; Ahmed, M.I.; Aban, I.B.; Sanders, P.W.; Mujib, M.; Campbell, R.C.; Love, T.E.; Aronow, W.S.; Allman, R.M.; et al. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: Findings from propensity-matched studies. Circ. Heart Fail. 2010, 3, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, E.; Yoo, K.D.; Choi, Y.; Kim, D.K.; Joo, K.W.; Yang, S.H.; Kim, Y.L.; Kang, S.W.; Yang, C.W.; et al. Lower serum potassium associated with increased mortality in dialysis patients: A nationwide prospective observational cohort study in Korea. PLoS ONE 2017, 12, e0171842. [Google Scholar] [CrossRef]
- Endo, M. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 2009, 89, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Baruscotti, M.; Difrancesco, D. Pacemaker channels. Ann. N. Y. Acad. Sci. 2004, 1015, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T. Clearance of extracellular K+ during muscle contraction—Roles of membrane transport and diffusion. J. Gen. Physiol. 2008, 131, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Alderman, M.H.; Piller, L.B.; Ford, C.E.; Probstfield, J.L.; Oparil, S.; Cushman, W.C.; Einhorn, P.T.; Franklin, S.S.; Papademetriou, V.; Ong, S.T.; et al. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 2012, 59, 926–933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.; Brunelli, S.M.; Jensen, D.E.; Yang, A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin. J. Am. Soc. Nephrol. 2016, 11, 90–100. [Google Scholar] [CrossRef]
- Hayes, J.; Kalantar-Zadeh, K.; Lu, J.L.; Turban, S.; Anderson, J.E.; Kovesdy, C.P. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: The role of race. Nephron Clin. Pract. 2012, 120, c8–c16. [Google Scholar] [CrossRef] [PubMed]
- Krogager, M.L.; Torp-Pedersen, C.; Mortensen, R.N.; Køber, L.; Gislason, G.; Søgaard, P.; Aasbjerg, K. Short-term mortality risk of serum potassium levels in hypertension: A retrospective analysis of nationwide registry data. Eur. Heart J. 2017, 38, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L. SHIFT Investigators, Ivabradine and outcomes in chronic heart failure (SHIFT): A randomized placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, N.; Turitto, G. Electrolyte disorders and arrhythmogenesis. Cardiol. J. 2011, 18, 233–245. [Google Scholar]
- White, E.; Terrar, D.A. Action potential duration and the inotropic response to reduced extracellular potassium in guinea-pig ventricular myocytes. Exp. Physiol. 1991, 76, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Pogwizd, S.M.; Schlotthauer, K.; Li, L.; Yuan, W.; Bers, D.M. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ. Res. 2001, 88, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Trenor, B.; Cardona, K.; Romero, L.; Gomez, J.F.; Saiz, J.; Rajamani, S.; Belardinelli, L.; Giles, W. Pro-arrhythmic effects of low plasma [K+] in human ventricle: An illustrated review. Trends Cardiovasc. Med. 2018, 28, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Clase, C.M.; Carrero, J.J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, B.; Limido, D.; Beretta, P.; Viganò, M.R. Hemodialysis using a constant potassium gradient: Rationale of a multicenter study. Int. J. Artif. Organs 1995, 18, 731–734. [Google Scholar] [CrossRef] [PubMed]


| Central SA Nodal Cell | Ventricular Cell | Transport Mechanism |
|---|---|---|
| Funny ch. (Na+, K+) | Fast Na ch. (Na+) | Voltage-dependent |
| L-type Ca ch. (Ca2+) | Late Na ch. (Na+) | Voltage-dependent |
| T-type Ca ch. (Ca2+) | L-type Ca ch. (Ca2+) | Voltage-dependent |
| Ks ch. (K+) | Ks ch. (K+) | Voltage-dependent |
| Kr ch. (K+) | Kr ch. (K+) | Voltage-dependent |
| Sus ch. (K+) | to ch. (K+) | Voltage-dependent |
| 4Ap ch. (K+) | K1 ch. (K+) | Voltage-dependent |
| Na/Ca exchanger (Na+, Ca2+) | Na/Ca exchanger (Na+, Ca2+) | Ca2+ conc.-dependent |
| Na/K pump (Na+, K+) | Na/K pump (Na+, K+) | Constitutive |
| Ca pump (Ca2+) | Ca pump (Ca2+) | Constitutive |
| Ryanodine receptor (Ca2+) | Ryanodine receptor (Ca2+) | Ca2+ conc.-dependent |
| SERCA pump (Ca2+) | SERCA pump (Ca2+) | Constitutive |
| Leak (Na+, Ca2+) | Leak (Na+, Ca2+) | Constitutive |
| Case # | Electrolytes Concentration in Dialysis Fluid [mEq/L] | Predialysis Electrolytes Concentration in Plasma [mEq/L] | Japanese Data in DOPPS Phase 5 * | ||||
|---|---|---|---|---|---|---|---|
| Ca2+, $ | Na+, $ | K+, $ | Ca2+, $ | Na+, $ | K+, $ | Percentile | |
| 1 | 2.75 | 140.0 | 2.0 | 2.5 | 140.0 | 5.2 * | 75 (High) |
| 2 | 4.8 * | 50 (Average) | |||||
| 3 | 4.2 * | 25 (Low) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, H.; Tomo, T.; Kim, S.-T.; Yamashita, A.C. The Impact of Potassium Dynamics on Cardiomyocyte Beating in Hemodialysis Treatment. J. Clin. Med. 2024, 13, 2289. https://doi.org/10.3390/jcm13082289
Hamada H, Tomo T, Kim S-T, Yamashita AC. The Impact of Potassium Dynamics on Cardiomyocyte Beating in Hemodialysis Treatment. Journal of Clinical Medicine. 2024; 13(8):2289. https://doi.org/10.3390/jcm13082289
Chicago/Turabian StyleHamada, Hiroyuki, Tadashi Tomo, Sung-Teh Kim, and Akihiro C. Yamashita. 2024. "The Impact of Potassium Dynamics on Cardiomyocyte Beating in Hemodialysis Treatment" Journal of Clinical Medicine 13, no. 8: 2289. https://doi.org/10.3390/jcm13082289
APA StyleHamada, H., Tomo, T., Kim, S.-T., & Yamashita, A. C. (2024). The Impact of Potassium Dynamics on Cardiomyocyte Beating in Hemodialysis Treatment. Journal of Clinical Medicine, 13(8), 2289. https://doi.org/10.3390/jcm13082289






